Abstract
Mathematical modelling provides mechanistic insight of an infirmity by emulating the course of disease on individual or group level under various interventions and hence makes pragmatic contribution to complement conventional biomedical research modalities. Subsequently, real-world effectiveness is observed in the prediction of experimental outcome that leads to optimized clinical therapies. This article addresses with the aid of mathematical modelling, the drug pharmacokinetic-pharmacodynamic along with treatment responses. This article uses an agent based model to discuss the effects of chemotherapy on angiogenesis as well as tumor microenvironment and establishes pertinency between numerical and experimental results. This study supports the emerging “discrete analysis ” which, in the near future, is anticipated to be a promising major tool, for designing rational dosage regimes and effective dosage forms.

Similar content being viewed by others
Introduction
Angiogenesis is a phenomenon associated with formation of systematic and functional capillary network from pre-existing vasculature through sprouting and branching. It is a central component in physiological and many pathological conditions. Under several physiological conditions such as embryogenesis, wound healing and tissue repair, controlled angiogenesis takes place to recruit new blood vessels in multicellular organisms in order to provide nutrients and oxygen to mitotically active cells. Uncontrolled blood vessel formation is observed in tumorogenesis and some inflammatory diseases such as duodenal ulcers, proliferative retinopathy, arthritis and myocardial infarction (Folkman 1985). In each case though the order of events remains the same, starting with disassembly of endothelial cells from parent vessel, rearrangement and formation of new vascular plexus (Madri and Pratt 1986).
The objective of this article is to discuss the chemotherapy treatment effects on the angiogenesis (subsequently on tumor microenvironment) using an agent based model (Wilensky and Reisman 2006) and to provide a relevance between numerical and experimental findings. Studies have shown that tumor growth is dramatically diminished by limiting microvasculature that results in tumor necrosis via cell apoptosis. Avascular tumor remains dormant and has a restricted growth upto 1–2 cm (Folkman 2002). Angiogenic switch or transformation of a dormant tumor into an angiogenic phenotype requires sufficient vascular supply to satisfy high oxygen and nutrients requirements (Bergers and Benjamin 2003). Release of many angiogeneic growth factors, collectively called tumor angiogenic factors (TAF), by rapidly proliferating neoplastic cells marks the first step of tumor induced angiogenesis. Quite a few isolated pro-angiogenic molecules include fibroblast growth factor (aFGF, bFGF), vascular endothelial growth factor (VEGF) and angiogenin (Relf et al. 1997). VEGF is identified as most potent TAF molecule as beside neovascular growth it facilitates remodelling processes in extra cellular matrix (Folkman 1971). Up regulated expression of VEGF stimulates the chemotaxis response of endothelial cells in response to chemical gradient generated by cancerous cells. Consequently several released matrix degradrative enzymes such as proteinaises and collagenases detach endothelial cells from parent vessel through degradation of venule basement membrane and surrounding extra cellular matrix (Kubis and Levy 2004). Disassembled endothelial cells begin to ooze out of the parent capillary and coalescence in a finger like vascular projection (Ausprunk and Folkman 1977). Leading front of the sprout is composed of tip cell while rest of the body is made up of mitotically active stalk cells. Without survival signals, endothelial cells undergo apoptosis in young vasculature. VEGF maintains the viability of immature neovasculature by generating anti apoptosis factors such as survivin and Bcl-2. Extra cellular matrix (ECM), a mesh like network of protein, provides mechanical hold and chemical signals for endothelial cell adhesion and migration. Major components of ECM are collagen, fibronectin and interstitial tissues. Fibronectin, an insoluble macromolecular component of ECM, directs movement of endothelial cells through adhesion. Prime function of fibronectin is to act as a ligand and adhere endothelial cells to ECM to stimulate directional drive towards tumor. Thus endothelial cells show haptotaxis response towards concentration gradient of bound ECM fibronectin, complementary to chemotactic response to TAF (Woodley et al. 1988). Initially capillary sprouts move parallel to each other to a definite distance from parent vessel and then start inclining towards each other. Several tip to tip and tip to sprout mergers or anastomoses takes place forming asymmetrical loops and arcades. This irregularly shaped vessel network is functionalized by initiation of blood circulation. Further extension in the capillary network takes place by repeated sequence of angiogenic events. Dramatically enhanced branching or brush border effect of sprouts takes place near the tumor and eventually these capillaries penetrate the neoplasm to vascularize it. Since tumor induced angiogenesis is the essential process for conversion of a tumor into lethal malignant form, anti-angiogenesis strategies are targeted as potential therapeutic strategy to deal with this pathological condition.
Since tumor induced angiogenesis is the vital process for the transition of a tumor into lethal malignant form, anti-angiogenesis strategies are targeted as potential therapeutic strategy to deal with this pathological condition. Folkman and Dr. Ferrara established and transformed this research into therapeutic modality and developed Avastin the first FDA approved (2004) anti-angiogenesis VEGF targeted cancer therapy (Ferrara 2004). Now a days it has been prescribed as first-line or adjuvant neocancer therapy in numerous types of cancers in clinical practice. The paradoxically limiting factor for the efficacy of anti-angiogenesis therapy is the hindrance in the supply of anticancer agent to the whole tumor body without appropriate supply of blood. Currently more than 13 FDA approved different anti-angiogenic drugs such as monoclonal antibodies, tyrosine kinase inhibitors and mTOR inhibitors are being used worldwide to prolong the survival of cancer patients (Gacche and Meshram 2014).
Numerical analysis of complex engineering and biological problems has always remained a great challenge (Riaz et al. (2016; Farooq and Hussain 2016). The discrete mathematical modelling of cancer has remained a topic of debate during the past decade (Sun and Hu 2017). In this article, we have discussed the effects of chemotherapy, on angiogenesis and on the associated tumor microenvironment with the help of a computational tool. Different combinations of drug applications are analyzed in order to predict the best dose candidate to eliminate the tumor. The article is organised as follows: In the next section, the technique is described, and the results are discussed afterwards, followed by conclusions and future work.
Methods
Discrete approach
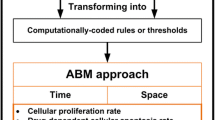
Drug delivery schedules are key factors in the efficacy of cancer therapies, and mathematical modeling of population dynamics and treatment responses can be applied to identify better drug administration regimes as well as provide mechanistic insights (Pillis and Radunskaya 2001). In recent years, theoretical models are efficaciously applied to elucidate the extremely variable and unusual outcome of anti-angiogenic chemotherapy in multiple clinical trials (Cao 2016). For the last 2 decades, agent-based modelling (ABM), a discrete-based hybrid modelling approach, has emerged as a valuable tool in the field of computational biology owing to its diverse modelling rules. In ABM each cell is often represented as an agent. While simulating a problem, each agent follows certain rules, defined for its independent behavior along with its interactions with other agents. Literature reveals that, ABM models have been used extensively, for discussing the biology of angiogenesis and its role in tumor morphology and metastasis (Olsen and Siegelmann 2013).
Agent based modelling
The agent based modelling has earned fame in almost all disciplines of sciences (Xiong et al. 2016; Farooqui and Niazi 2016). In this research, agent based modelling (ABM) is used to simulate the effect of chemotherapy on the angiogenesis by understanding the spatiotemporal extension/reduction of the microvessels associated with the untreated/treated tumour cells. Different platforms have been used in the literature for ABM of tumour including Objective-C versions, MASON, Repast, NetLogo, Java and Swarm (An et al. (2009; Kang et al. 2017). NetLogo has proved to be one of the highest-level platforms, for simulating tumour dynamics (Wilensky and Evanston 1999; Chaudhry 2016) since it is a simple yet powerful programming language.
During ABM, the agents receive and convey the signals. They collect the input signals ‘from’ other agents and provide output ‘to’ the environment and their neighboring agents. The agents make decisions which are based on the input from around them and their internal, sub-cellular decision making rules. These rules control the growth, proliferation and change in state (such as apoptosis or necrosis) of the agents in response to surrounding environmental conditions. In this model, the drug delivery is mimicked, in a manner similar to Wilensky (1999), we have introduced the durg into the agent based modelling, by targeting the transitory cells.
Results and discussion
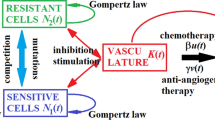
Maturation of nascent tumor involves a spatio-temporal sequence of interactions with its microenvironment. Initial step involves compression and mechanical displacement of surrounding tissue including the basement membrane. Next vital stage is hypoxia induced angiogenesis caused by disassembly and reassembly of endothelial cells from pre-existing blood vessels. This results in fabrication of new vasculature to fulfil the nutrient supply of sprouting neoplasm for rapid expansion. Hypoxia-induced factors accumulated in hypoxic cells release many tumor angiogeneic growth factors (TAFs) that are disseminated from the hypoxic regions of tumor to nearby blood vessels. Under the influence of TAFs endothelial cells from the pre-existing blood vessels are detached and chemotactically march towards the hypoxic region of the tumor to form a network of neo-vasculature whose configuration is dependent on equilibrium of pro- and anti-angiogenic growth factors, flow stress inside the emerging blood vessels and compression exerted by the expanding tumor. The fresh nutrient supply helps the budding tumor to flourish rapidly into the surrounding tissue. However, resulting neovasculature tends to be much more convoluted and malformed than regular vascular network owing to perforations by reason of large gaps between endothelial cells and does not possess stiffness and rigidity of mature blood vessels. As a result it is often collapsed by tissue stress, specifically exerted by fast growing tumor itself. Deformity and fragility of neo-vasculature hampers the drug delivery to the interior regions of tumor and hence accelerates the rate of neo-angiogenesis by creating new hypoxic regions within the tumor.
Figures 1 and 2 present the two staged schematic of the discrete modelling. We can see that under favourable circumstances (supply of oxygen and nutrients via angiogenesis), the tumour cells metastasize. The chemotherapy is applied by killing the young cells as shown in stage-II of the algorithm. Tumour invasion, angiogenesis and advancement in metastasis are presented in Fig. 3.
From Fig. 4 and Table 1 it is obvious that for frequent treatments, the tumour is least dense and the tumour stem cells resist to the treatment in a cyclic order. As the frequency is reduced, the maximum number of cells increases, at the lowest frequencies, there is a fluctuation after each cycle as shown by arrow in Fig. 4.
From Fig. 5, we can see that at the intermediate and high doses, the chemotherapy significantly reduced microvessel spatial extension in terms of the vascularized area (VA) by 20 and 70%, furthermore, the overall angiogenic response in terms of the total microvascular length (TMVL) was reduced. From the numerical experiments, it is evident that the difference in VA between treatments with the low and either the intermediate or the high dose was significant. These numerical results obtained are in close agreement with the results obtained by (Lennernas et al. 2003). Lennernäs et al. reported that Paclitaxel (one of the few chemotherapeutics that have been shown to affect angiogenesis in vivo), strongly suppressed angiogenesis and affected several variables relating to the MV network formation.
Conclusions and future work
Besides the positive role of the immense vascularization system, by controlling the growth of tumor cells, by delivering tyrosine kinase inhibitors, it is believed that tumour induced angiogenesis causes inefficiency, due to its pathological nature. This inefficiency includes hinderance to drug delivery within tumors and also lead to the development of new hypoxic regions within the tumor and additional sessions of angiogenesis. During this discussion, dynamic response of tumour cells to different rates of drug dosage has been observed. The change in the vascularized area (VA) and the overall angiogenic response in terms of the total microvascular length (TMVL) are reported with the help of graphical analysis.
Furthermore, in this article, we have considered the discrete modelling to demonstrate the effect of chemotherapy on tumour growth and on the corresponding angiogenesis process inhibition. In future, we will consider the combined effect of chemotherapy and anti-angiogenesis drug using a multiple discrete approach.
References
An G, Mi Q, Dutta-Moscato J, Vodovotz Y (2009) Agent-based models in translational systems biology. Wiley Interdisciplinary Rev 1(2):159–171
Ausprunk DH, Folkman J (1977) Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 14(1):53–65
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3(6):401–410
Cao Y (2016) Future options of anti-angiogenic cancer therapy. Chin J Cancer 35(1):21
Chaudhry QA (2016) An introduction to agent-based modeling modeling natural, social, and engineered complex systems with NetLogo: a review. Complex Adapt Syst Model 4 (11)
De Pillis LG, Radunskaya A (2001) A mathematical tumor model with immune resistance and drug therapy: an optimal control approach. Comput Math Method Med 3(2):79–100
Farooq K, Hussain A (2016) A novel ontology and machine learning driven hybrid cardiovascular clinical prognosis as a complex adaptive clinical system. Complex Adapt Syst Model 4(1):12
Farooqui AD, Niazi MA (2016) Game theory models for communication between agents: a review. Complex Adapt Syst Model 4(1):13
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Revi 25(4):581–611
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186
Folkman J (1985) Tumor angiogenesis. Adv Cancer Res 43:175–203
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis, in seminars in oncology, Vol 29. Elsevier, Amsterdam, pp 15–18
Gacche RN, Meshram RJ (2014) Angiogenic factors as potential drug target: efficacy and limitations of anti-angiogenic therapy. Biochimica Biophysica Acta Rev Cancer 1846(1):161–179
Kang G, Marquez C, Barat A, Byrne AT, Prehn JH, Sorribes J, Cesar E (2017) Colorectal tumour simulation using agent based modelling and high performance computing. Fut Gen Comput Syst 67:397408
Kubis N, Levy B (2004) Understanding angiogenesis: a clue for understanding vascular malformations. J Neuroradiol 31(5):365368
Lennernas B, Albertsson P, Lennernas H, Norrby K (2003) Chemotherapy and antiangiogenesis. Acta Oncol 42(4):294–303
Madri JA, Pratt BM (1986) Endothelial cell-matrix interactions: in vitro models of angiogenesis. J Histochem Cytochem 34(1):85–91
Olsen MM, Siegelmann HT (2013) Multiscale agent-based model of tumor angiogenesis. Proceed Comput Sci 18:1016–1025
Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL (1997) Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 57(5):963–969
Riaz S, Chaudhry QA, Siddiqui S (2016) Mathematical modeling and optimization of complex structures: a review. Complex Adapt Syst Model 4(19)
Sun X, Hu B (2017) Mathematical modeling and computational prediction of cancer drug resistance. Briengs Bioinformatics
Wilensky U (1999) Netlogo tumour model. (ccl. northwestern. edu/netlogo), center for connected learning and computer based modeling. Northwestern University, Evanston
Wilensky U, Evanston I (1999) Netlogo: center for connected learning and computerbased modeling. Northwestern University, Evanston, p 4952
Wilensky U, Reisman K (2006) Thinking like a wolf, a sheep, or a firefly: learning biology through constructing and testing computational theoriesan embodied modeling approach. Cogn Instr 24(2):171–209
Woodley DT, Bachmann PM, OKeefe EJ (1988) Laminin inhibits human keratinocyte migration. J Cell Physiol 136(1):140–146
Xiong H, Kinsella S, Payne D (2016) Self-enforcing agreement in cooperative teams: an agent-based modeling approach. Complex Adapt Syst Model 4(1):21
Authors' contributions
LS and AS conceived the manuscript. AS collected materials and methods. ZL and AS did the analytic modelling. AS, ZL and QAC did numerical modelling. All the authors did the literature review. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
No such research is conducted.
Funding
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sohail, A., Sherin, L., Li, Z. et al. Embodied modeling approach to explore tumour cells drug resistance. Complex Adapt Syst Model 6, 3 (2018). https://doi.org/10.1186/s40294-018-0055-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40294-018-0055-5