Abstract
Background
Although great success has been achieved, schistosomiasis remains a major public health concern in China, and the remaining core endemic regions are concentrated along the middle and lower reaches of the Yangtze River. In this longitudinal study, we evaluated the effectiveness of a multidisciplinary, integrated approach for schistosomiasis elimination in a historically hyper-endemic region in the lower reaches of the Yangtze River, China over the 10-year period from 2005 through 2014.
Methods
A three-step roadmap for schistosomiasis elimination was designed in the study site, and multidisciplinary, integrated interventions were implemented by the health, agriculture, water resources development, land and resources, and forestry sectors from 2005 to 2014, including chemotherapy for infected individuals, health education, management of the source of Schistosoma japonicum infection, and intermediate host snail control. The annual number of schistosomiasis patients, S. japonicum infection in humans, bovines and Oncomelania hupensis snails, and water infectivity were observed to assess the effectiveness of the multidisciplinary, integrated approach for the elimination of schistosomiasis.
Results
There was a tendency towards a gradual decline in both the number of schistosomiasis cases and the prevalence of S. japonicum human infection across the study period from 2005 through 2014. No S. japonicum human infection was detected since 2012, and no acute infection was seen since 2006. During the study period, no infection was found in bovines, and a 0.03% overall infection rate was observed in O. hupensis snails. Since 2009, no infected snails were identified, and the area of both snail habitats and infected snail habitats appeared a reduction over the study period. Following the 3-year multidisciplinary, integrated control, infection control was achieved, and transmission control was achieved after 6-year implementation, with all infected snails and water infectivity eliminated; in addition, the 10-year implementation resulted in interruption of schistosomiasis transmission in the study site in 2014.
Conclusions
The results of the present 10-year longitudinal study demonstrate that the multidisciplinary, integrated approach is effective for the elimination of schistosomiasis as a public health problem in the lower reaches of the Yangtze River, China.
Similar content being viewed by others
Multilingual abstract
Please see Additional file 1 for translations of the abstract into the five official working language of the United Nations.
Background
Schistosomiasis is a neglected tropical disease caused by the blood fluke of the genus Schistosoma, which remains a major public health concern worldwide [1]. The disease is estimated to affect 240 million people in 78 countries, with a further 800 million at risk of infection [2]. Worldwide, the total number of disability adjusted life years (DALY) lost due to schistosomiasis is estimated at 1.532 million per year [3], in which 77% are measured in sub-Saharan Africa [4–6]. In addition, meta-analyses estimated 280 000 schistosomiasis-attributable deaths annually in sub-Saharan Africa alone [7, 8]. With the advent of praziquantel in 1970s, a highly effective and lowly toxic schistosomicide with easy administration and competitive cost [9–11], the World Health Organization (WHO) Expert Committee on the Control of Schistosomiasis recommended a shift of the global schistosomiasis control strategy from transmission control to morbidity control [12]. Since then, mass drug administration (MDA) with praziquantel has become the predominant strategy for schistosomiasis control in this wormy world [13–15], and such a strategy has been proved to be effective to greatly reduce both the prevalence and intensity of schistosome infections, which facilitates the progress towards the global elimination of the disease [16–19]. In 2013, the agenda was set for the global schistosomiasis elimination based on the global status of schistosomiasis [20], with 2025 defined as the target date for global elimination as a public health concern [21].
Three major species of the trematode worm Schistosoma cause human schistosomiases, S. mansoni, S. haematobium and S. japonicum [1]. Two more species, S. intercalatum and S. mekongi, are of public health interest but their distribution is geographically limited, while S. malayensis is currently not perceived as a human problem even if cases have been reported [22]. S, japonicum, S. mekongi and S. malayensis are zoonoses, the former being the only species in China [1]. Following the control efforts for more than half a century in China [23], notably the implementation of the new integrated strategy with emphasis on the control of infectious sources since 2004 [24–27], the number of cases with S. japonicum infection has dramatically reduced from over 11 million at the initiation of the national schistosomiasis control program in 1950s to 77.2 thousand in 2015, and transmission control for schistosomiasis (less than 1% S. japonicum infection in humans and bovines, no local acute cases, and no infected snails detected for successive 2 years) has been achieved in the country by 2015 [28]. A two-step roadmap for schistosomiasis elimination was therefore proposed in China in 2015, based on the endemic status of schistosomiasis, with aims to achieve transmission interruption (no local S. japonicum infections in humans, bovines and snails for successive 5 years, and establishment of a sensitive, effective surveillance system for schistosomiasis) in the country by 2020 and elimination of the disease (no local S. japonicum infections in humans, bovines and snails for successive 5 years after transmission interruption) by 2025 [29].
Currently in China, the remaining core endemic regions are predominantly located along the middle and lower reaches of the Yangtze River, in which more than 92% of the national schistosomiasis patients and over 96% of the total snail habitats are detected [28, 30, 31]. Since 2005, a multidisciplinary, integrated approach was implemented for elimination of schistosomiasis in Yangzhou City, a historically hyper-endemic region for schistosomiasis along the middle and lower reaches of the Yangtze River, China [32]. In this study, we evaluated the effectiveness of the multidisciplinary, integrated approach for schistosomiasis elimination in Yangzhou locating in the lower reaches of the Yangtze River, China over the 10-year period from 2005 through 2014.
Methods
Ethical statement
This study was approved by the Ethical Review Committee of Jiangsu Institute of Parasitic Diseases (permission number: IRB00004081). All animal experiments were performed in accordance with the 3R rules for animal experiments and the Guidelines for the Care and Use of Laboratory Animals, and signed informed consent was obtained from all participants included in the study.
Study site
Yangzhou City is located in the lower reaches of the Yangtze River in the east of China, which has a population of 4.66 million, and covers an area of 6.6 thousand km2. Historically, Yangzhou City was highly endemic for S. japonicum, and there were 55 townships detected with infections in the city, with more than 300 million people at risk of infection [33]. There were 336 thousand accumulated schistosomiasis cases and accumulated snail habitats of about 0.2 billion m2 detected in Yangzhou City [34].
Roadmap of the multidisciplinary, integrated approach
During the 10-year study period between 2005 and 2014, a three-step roadmap of the multidisciplinary, integrated approach was designed for schistosomiasis elimination in Yangzhou City (Fig. 1). From 2005 to 2007, a total of 17 villages reporting the persistent presence of infected Oncomelania hupensis snails or acute schistosomiasis, were selected and subject to the integrated control, including snail control, chemotherapy, health education, replacement of bovines with machines, improved sanitation and access to clean water [35]. Between 2008 and 2010, a total of 31 marshlands with repeated emergence of infected snails were selected and given interventions including prohibition of grazing on marshlands, and snail control with molluscicide treatment and environmental improvment [36]. During the period from 2009 through 2014, 15 to 20 sentinel sites with the detection of positive sentinel mice or frequent human and animal activities were selected in the marshlands along the middle and lower reaches of the Yangtze River and were given a package of interventions consisting of cercarial killing, allocation of excrement collector to boatmen and fishermen, construction of public latrines at assembly centers for mobile boatmen and fishermen and chemotherapy of mobile boatmen and fishermen [37–39].
Multidisciplinary, integrated approach for schistosomiasis elimination
The multidisciplinary, integrated approach for schistosomiasis elimination consisted of routine control interventions, measures to control the source of S. japonicum infection, and integrated snail control. Routine control interventions included chemotherapy for infected individuals, snail survey and control, and health education implemented by health sectors. The measures to control the source of S. japonicum infection involved replacing cattle with small farm machines, raising livestock in pens, and examination of schistosomiasis in livestock and chemotherapy for infected livestock implemented by the agriculture sectors, as well as construction of public latrines with three-cell septic tanks and household sanitary toilets completed by the health sectors. Integrated snail control interventions consisted of hardening river banks with concrete, building sluices for prevention of snail spread and digging ditches implemented by the water resources development sectors, constructing fish ponds by the agriculture sectors, land improvement by the departments of land and resources, and building trees in marshlands by the forestry sectors.
Detection of S. japonicum infection in humans and bovines
From 2005 to 2014, 17 villages were selected using the clustering sampling, and all residents living in the enrolled villages were detected for specific IgG antibodies against S. japonicum with a dipstick dye immunoassay (DDIA) kit (Wuxi Saide Sci & Tech Development Co., Ltd.; Wuxi, China) during the schistosomiasis non-transmission period in each year [40–42]. Then, all seropositives were subject to miracidium hatching testing for identification of S. japonicum infections [43]. At spring and autumn of each year, all bovines in the study villages were detected for S. japonicum infection with a miracidium hatching test [44]. The prevalence of S. japonicum infection was estimated in both humans and bovines.
Snail survey
At spring in each year during the period from 2005 through 2014, a snail survey was performed in historical snail habitats using a systematic sampling method [45]. Briefly, a snail collection device, a 0.1 m2 square frame made of iron wire, was placed every 20 m along the survey line. All snails within the frame were collected, transferred to the laboratory, counted, and identified for S. japonicum infection under a microscope. The area of snail habitats, area with infected snails and snail infection rate were estimated.
Monitoring of water contamination with S. japonicum
Between May and September from 2009 to 2014, S. japonicum infection was detected using a mouse bioassay in the sites with detection of acute infections, frequent human and livestock activities, or assembly centers for mobile boatmen and fishermen [46].
Data management and analysis
A descriptive epidemiological method was employed in this study [47]. All data were processed in Microsoft Excel version 2007 (Microsoft Corporation; Redmond, WA, USA) and all statistical analyses were performed using the statistical software SPSS version 13.0 (SPSS, Inc.; Chicago, IL, USA).
Results
Implementation of multidisciplinary integrated interventions
During the 10-year study period from 2005 through 2014, the health departments performed snail survey at 168 542.18 hm2, and molluscicide treatment with niclosamide formulations at 32 391.35 hm2; in addition, 3 143.645 thousand information, education and communication (IEC) materials were given to high-risk populations, and 1065.2 thousand people received chemotherapy with praziquantel at a single oral dose of 40 mg/kg (Table 1). The health sectors also built 221 public latrines, and 546.6 thousand household sanitary toilets, and the agriculture departments built 5.29 hm2 fens to raise livestock, eliminated 402 bovines and treated 101 259 bovines with praziquantel at single dose of 30 mg/kg, aiming to control the source of S. japonicum infection (Table 2). Moreover, the water resources development sectors hardened river banks with concrete at 205.25 km, built 68 sluices and dug 182.51 km ditches; the agriculture sectors built 221 fish ponds; the land and resources sectors completed land improvements at 8 704.35 hm2, and the forestry sectors built trees at 3 446.06 hm2, with attempts to control the intermediate host snails (Table 3).
Overall status of schistosomiasis control from 2005 to 2014
In 2005, there were three out of the eight schistosomiasis-endemic districts and seven out of the 55 endemic townships with uncontrolled transmission in Yangzhou City. Following the implementation of the multidisciplinary, integrated approach, infection control of schistosomiasis (less than 5% S. japonicum infection in humans and bovines, and no outbreak of acute schistosomiasis) was achieved in the study site in 2007, transmission control achieved in 2010, and transmission interruption achieved in 2014 (Figs. 2 and 3).
S. japonicum infection in humans and bovines from 2005 to 2014
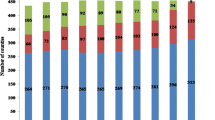
During the study period, a total of 954477 individuals received serological examinations with DDIA, and all seropositives were then subject to the miracidium hatching test. Finally, a total of 313 egg-positive individuals were identified, with 0.03% overall prevalence of S. japonicum infection. Since 2012, no S. japonicum human infection was detected, and no acute infection was seen since 2006. There was a tendency towards a gradual decline seen in both the number of schistosomiasis cases and the prevalence of S. japonicum infection across the study period from 2005 through 2014 (Figs. 4 and 5). A total of 4 481 bovines were detected for S. japonicum infection with the miracidium hatching test between 2005 and 2014, and no infection was identified (Fig. 5).
Outcomes of snail control
From 2005 to 2014, integrated snail control was employed, which were implemented by health, water resources development, agriculture, land and resources, and forestry sectors. During the 10-year study period, a total of 282079 snails were captured and examined for S. japonicum infection, and 95 snails were identified with infection, with a 0.03% overall infection rate. Since 2009, no infected snails were identified (Fig. 5). In addition, the area of both snail habitats and infected snail habitats appeared a reduction over the study period, and infected snail habitats were eliminated in the study site since 2009 (Fig. 6).
Water infectivity
During the period from 2009 through 2014, a total of 351 sentinel sites were assigned, and 5 sites were identified positive, with an overall positive rate of 1.42%. Of the totally 6 507 mice examined, 14 mice were detected positive, with a 0.22% overall positive rate. Since 2010, neither positive sites nor positive mice were detected in the study site (Table 4).
Discussion
Schistosomiasis has been widely recognized as a disease that is socially determined [48], and the transmission and control of this disease of poverty is considered to be strongly linked to multiple social, economic and behavioral factors [49–53]. In addition, it is indicated that an integrated, multi-sectoral control approach is necessary for sustainable schistosomiasis control and progressively moving towards elimination [54].
The national schistosomiasis control program was initiated in China at early 1950s [55–57]. At the initial stage of the national schistosomiasis control program, extensive farming and undeveloped water conservancy facilities resulted in the wide distribution of the intermediate host snails. Farmers lived close to water, and had a high possibility to get S. japonicum infection [58–60]. With the socio-economic development, the increase in the frequency of human activities may also lead to a rise in the likelihood of the parasite infection [61]. Based on the epidemiological profiles and status of schistosomiasis and the national social and economic situation, integrated strategies have been proposed for schistosomiasis control in China [62], aiming to eliminate this public health concern in the country through integration of multi-sectoral resources and multidisciplinary tools [63–65]. Until late 1990s, schistosomiasis elimination had been achieved in 5 out of the 12 endemic provinces in China [66–68]. Notably, the wide implementation of the integrated strategy with emphasis on infectious source control throughout the main endemic foci of China since 2004 has been proved to greatly facilitate the progress towards the elimination of schistosomiasis in the country [26, 27, 69–80].
Currently, China is moving from transmission control towards transmission interruption and elimination of schistosomiasis [29], and the schistosomiasis control programs require a shift from “extensive control” to “precision control” [81]. Implementation of a highly effective and precise roadmap and approach, which tailors to the intensity of transmission, has been recognized as a key factor that determines the sustainable schistosomiasis control [82–84].
In this study, a three-step roadmap for schistosomiasis elimination was designed in Yangzhou City, a historically hyper-endemic region in the lower reaches of the Yangtze River, China, and multi-sectoral resources were mobilized through integration of multidisciplinary, integrated interventions implemented by the health, agriculture, water resources development, land and resources, and forestry sectors, including chemotherapy for infected individuals, health education, integrated control of the source of S. japonicum infection, and integrated snail control. During the 10-year study period from 2005 through 2014, the number of schistosomiasis cases appeared a tendency towards a gradual decline year by year, and the infection rates in both humans and snails, as well as the area of both snail habitats and infected snail habitats showed a reduction over the study period. Following 3-year multidisciplinary, integrated control, infection control was achieved, and transmission control was achieved after 6 years, with all infected snails and water infectivity eliminated in the study site; in addition, the 10-year implementation of this multidisciplinary, integrated approach resulted in interruption of schistosomiasis transmission in the study site in 2014. Our data indicate that the multidisciplinary, integrated approach mobilizing multi-sectoral resources is an effective approach leading to schistosomiasis elimination in marshland and lake regions.
Currently, the Kato-Katz technique and miracidium hatching test remain the gold standard for the diagnosis of S. japonicum human infection [85]. However, these two techniques exhibit a high missing rate in detecting S. japonicum infections, notably in low-intensity regions [43]. Recently, a variety of immunodiagnostics and molecular biological assays have been developed, which shows a high sensitivity and specificity for the detection of S. japonicum human infections [86–88]. A combination of parasitologic techniques and immunodiagnostics/molecular biological assays may greatly reduce the missing rate for detecting S. japonicum infections, which facilitates the national schistosomiasis elimination program in China.
Of the six types of human schistosomiasis, the transmission cycle and epidemiological factors linked to schistosomiasis japonica seem more complicated than other five types [1]. O. hupensis snail, the only intermediate host of S. japonicum, is widely distributed along the Yangtze River basin, and the annual flood results in extensive snail spread in the middle and lower reaches of the Yangtze River, China [45]. In addition to humans, over 40 species of wild and domestic animals may serve as reservoir hosts for S. japonicum [9], which complicates the control efforts [89–92]. Currently, China is facing rapid socio-economic development and large eco-environmental changes. It is suggested that the schistosomiasis elimination program should be developed tailored to the socio-economic development plan and the natural and environmental factors affecting the transmission of schistosomiasis in the endemic regions. In addition, a highly effective, sensitive surveillance-response system is of great importance for the rapid identification and elimination of the source of S. japonicum infection, which is effective to sustain the control achievements and facilitate the progress towards schistosomiasis elimination [93–96].
Conclusions
The current study presents a multidisciplinary, integrated approach for schistosomiasis elimination in the lower reaches of the Yangtze River, China, and results of the 10-year longitudinal study between 2005 and 2014 demonstrate that this approach is effective to eliminate schistosomiasis as a public health problem in the marshland and lake regions, which provides new insights into the development of the national schistosomiasis elimination program in China. Currently, China is transferring its successful experiences on schistosomiasis control to Southeast Asia and Africa [97, 98], our multidisciplinary, integrated approach may provide valuable experiences for the global schistosomiasis elimination programs.
Abbreviations
- DALY:
-
Disability adjusted life years
- DDIA:
-
Dipstick dye immunoassay
- IEC:
-
Information, education and communication
- MDA:
-
Mass drug administration
- WHO:
-
World Health Organization
References
Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–64.
Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25.
Bhutta ZA, Sommerfeld J, Lassi ZS, Salam RA, Das JK. Global burden, distribution, and interventions for infectious diseases of poverty. Infect Dis Poverty. 2014;3:21.
Stothard JR, Chitsulo L, Kristensen TK, Utzinger J. Control of schistosomiasis in sub-Saharan Africa: progress made, new opportunities and remaining challenges. Parasitology. 2009;136:1665–75.
Lai YS, Biedermann P, Ekpo UF, Garba A, Mathieu E, Midzi N, et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect Dis. 2015;15:927–40.
Mazigo HD, Nuwaha F, Wilson S, Kinung’hi SM, Morona D, Waihenya R, et al. Epidemiology and interactions of human immunodeficiency virus - 1 and Schistosoma mansoni in sub-Saharan Africa. Infect Dis Poverty. 2013;2:2.
King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–9.
van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke JD, Habbema JD, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–39.
Fenwick A, Webster JP. Schistosomiasis: challenges for control, treatment and drug resistance. Curr Opin Infect Dis. 2006;19:577–82.
Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–67.
Wu W, Wang W, Huang YX. New insight into praziquantel against various developmental stages of schistosomes. Parasitol Res. 2011;109:1501–7.
WHO. The control of schistosomiasis. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1985;728:1–113.
Mutapi F, Maizels R, Fenwick A, Woolhouse M. Human schistosomiasis in the post mass drug administration era. Lancet Infect Dis. 2017;17:e42–8.
Wang W, Liang Y. Mass drug administration (MDA) for schistosomiasis. J Infect Dis. 2015;211:848–9.
Olveda DU, McManus DP, Ross AG. Mass drug administration and the global control of schistosomiasis: successes, limitations and clinical outcomes. Curr Opin Infect Dis. 2016;29:595–608.
Cleland CR, Tukahebwa EM, Fenwick A, Blair L. Mass drug administration with praziquantel reduces the prevalence of Schistosoma mansoni and improves liver morbidity in untreated preschool children. Trans R Soc Trop Med Hyg. 2014;108:575–81.
Wu W, Huang Y. Application of praziquantel in schistosomiasis japonica control strategies in China. Parasitol Res. 2013;112:909–15.
Webster JP, Molyneux DH, Hotez PJ, Fenwick A. The contribution of mass drug administration to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130434.
Ross AG, Olveda RM, Li Y. An audacious goal: the elimination of schistosomiasis in our lifetime through mass drug administration. Lancet. 2015;385:2220–1.
Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–40.
Fenwick A, Jourdan P. Schistosomiasis elimination by 2020 or 2030? Int J Parasitol. 2016;46:385–8.
Latif B, Heo CC, Razuin R, Shamalaa DV, Tappe D. Autochthonous human schistosomiasis, Malaysia. Emerg Infect Dis. 2013;19:1340–1.
Yang GJ, Liu L, Zhu HR, Griffiths SM, Tanner M, Bergquist R, et al. China’s sustained drive to eliminate neglected tropical diseases. Lancet Infect Dis. 2014;14:881–92.
Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–8.
Wang LD, Guo JG, Wu XH, Chen HG, Wang TP, Zhu SP, et al. China’s new strategy to block Schistosoma japonicum transmission: experiences and impact beyond schistosomiasis. Trop Med Int Health. 2009;14:1475–83.
Wang X, Wang W, Wang P. Long-term effectiveness of the integrated schistosomiasis control strategy with emphasis on infectious source control in China: a 10-year evaluation from 2005 to 2014. Parasitol Res. 2017;116:521–8.
Liu R, Dong HF, Jiang MS. The new national integrated strategy emphasizing infection sources control for schistosomiasis control in China has made remarkable achievements. Parasitol Res. 2013;112:1483–91.
Zhang LJ, Xu ZM, Qian YJ, Dang H, Lu S, Xu J, et al. Endemic situation of schistosomiasis in People’s Republic of China. Chin J Schisto Control. 2016;28:611–7 (in Chinese).
Lei ZL, Zhou XN. Eradication of schistosomiasis: a new target and a new task for the National Schistosomiasis Control Porgramme in the People’s Republic of China. Chin J Schisto Control. 2015;27:1–4 (in Chinese).
Zhang SQ, Sun CS, Wang M, Lin DD, Zhou XN, Wang TP. Epidemiological features and effectiveness of schistosomiasis control programme in lake and marshland region in the People’s Republic of China. Adv Parasitol. 2016;92:39–71.
Zou L, Ruan S. Schistosomiasis transmission and control in China. Acta Trop. 2015;143:51–7.
Zuo YP, Zhu DJ, Du GL, Tang K, Zhang ZQ, Chen SZ, et al. Surveillance and risk assessment system of schistosomiasis in Jiangsu Province III Risk of schistosomiasis transmission in the area along the Yangtze River in Yangzhou City. Chin J Schisto Control. 2016;28:353–7 (in Chinese).
Zhang XB. Investigation of acute schistosomiasis in Yangzhou City from 1985 to 1990. Chin J Schisto Control. 1992;4:297 (in Chinese).
Zuo YP, Gao Y, Hong QB. Endemic situation of schistosomiasis in surveillance sites of Yangzhou City, 2006. Chin J Schisto Control. 2007;19:404 (in Chinese).
Yang K, Li W, Sun LP, Huang YX, Zhang JF, Wu F, et al. Spatio-temporal analysis to identify determinants of Oncomelania hupensis infection with Schistosoma japonicum in Jiangsu province, China. Parasit Vectors. 2013;6:138.
Sun LP, Tian ZX, Yang K, Hong QB, Gao Y, Gao Y, et al. Strategy of comprehensive control for schistosomiasis and its effect in key areas of Jiangsu Province. Chin J Schisto Control. 2011;23:626–33 (in Chinese).
Yang K, Sun LP, Liang YS, Wu F, Li W, Zhang JF, et al. Schistosoma japonicum risk in Jiangsu province, People’s Republic of China: identification of a spatio-temporal risk pattern along the Yangtze River. Geospat Health. 2013;8:133–42.
Sun LP, Liang YS, Dai JR, Hong QB, Xu M, Wang W, et al. Integration and demonstration of key techniques in surveillance and forecast of schistosomiasis in Jiangsu Province I Layout and effect of the demonstration sites for schistosomiasis surveillance and forecast. Chin J Schisto Control. 2015;27:221–8 (in Chinese).
Liang YS, Huang YX, Hong QB, Yang K, Sun LP, Dai JR, et al. Novel strategies and technologies to achieve the transmission control of schistosomiasis in Jiangsu Province. Chin J Schisto Control. 2012;24:119–22.
Zhu Y, He W, Liang Y, Xu M, Yu C, Hua W, et al. Development of a rapid, simple dipstick dye immunoassay for schistosomiasis diagnosis. J Immunol Methods. 2002;266:1–5.
Li YF, Wang HS, Chen XP, Shen XH, Hou HG, Wu FX, et al. Investigation on infection of schistosomiasis japonica in school children by dipstick dye immunoassay. Chin J Schisto Control. 2005;17:60–1 (in Chinese).
Wang XY, Yang K. Serological diagnosis methods of schistosomiasis japonica at different prevalence: a meta-analysis. Chin J Schisto Control. 2016;28:18–25. 29, (in Chinese).
Zhu HQ, Xu J, Zhu R, Cao CL, Bao ZP, Yu Q, et al. Comparison of the miracidium hatching test and modified Kato-Katz method for detecting Schistosoma japonicum in low prevalence areas of China. Southeast Asian J Trop Med Public Health. 2014;45:20–5.
Li H, Dong GD, Liu JM, Gao JX, Shi YJ, Zhang YG, et al. Elimination of schistosomiasis japonica from formerly endemic areas in mountainous regions of southern China using a praziquantel regimen. Vet Parasitol. 2015;208:254–8.
Li ZJ, Ge J, Dai JR, Wen LY, Lin DD, Madsen H, et al. Biology and control of snail intermediate host of Schistosoma japonicum in the People’s Republic of China. Adv Parasitol. 2016;92:197–236.
Sun LP, Dai JR, Hong QB, Li HJ, Gao Y, Zhang LH, et al. Surveillance and forecast system of schistosomiasis in Jiangsu Province V Temporal and spatial distribution of water infectivity of Yangtze River. Chin J Schisto Control. 2010;22:446–51 (in Chinese).
Maremmani C, Rossi G, Bonuccelli U, Murri L. Descriptive epidemiologic study of epilepsy syndromes in a district of northwest Tuscany, Italy. Epilepsia. 1991;32:294–8.
Bruun B, Aagaard-Hansen J. The social context of schistosomiasis and its control: an introduction and annotated bibliography. Geneva: World Health Organization; 2008. p. 1–227.
Huang YX, Manderson L. The social and economic context and determinants of schistosomiasis japonica. Acta Trop. 2005;96:223–31.
Jiang MS, Liu R, Zhao QP, Dong HF, Guo Y. Social epidemiological thinking about schistosomiasis. Chin J Schisto Control. 2010;22:201–5 (in Chinese).
Deng Y, Zhou XN. Social factors of schistosomiasis transmission in China. Chin J Schisto Control. 2007;19:393–7 (in Chinese).
Kloos H. Human behavior, health education and schistosomiasis control: a review. Soc Sci Med. 1995;40:1497–511.
Huang Y, Manderson L. Schistosomiasis and the social patterning of infection. Acta Trop. 1992;51:175–94.
Utzinger J, Brattig NW, Leonardo L, Zhou XN, Bergquist R. Progress in research, control and elimination of helminth infections in Asia. Acta Trop. 2015;141:135–45.
Chen MG. Assessment of morbidity due to Schistosoma japonicum infection in China. Infect Dis Poverty. 2014;3:6.
Collins C, Xu J, Tang S. Schistosomiasis control and the health system in P.R. China. Infect Dis Poverty. 2012;1:8.
Wang W, Dai JR, Liang YS. Apropos: factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors. 2014;7:408.
Mao SP, Shao BR. Schistosomiasis control in the People’s Republic of China. Am J Trop Med Hyg. 1982;31:92–9.
Zhou D, Li Y, Yang X. Schistosomiasis control in China. World Health Forum. 1994;15:387–9.
Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, et al. The public health significance and control of schistosomiasis in China--then and now. Acta Trop. 2005;96:97–105.
Watts S, Khallaayoune K, Bensefia R, Laamrani H, Gryseels B. The study of human behavior and schistosomiasis transmission in an irrigated area in Morocco. Soc Sci Med. 1998;46:755–65.
Xu J, Steinman P, Maybe D, Zhou XN, Lv S, Li SZ, et al. Evolution of the national schistosomiasis control programmes in the People’s Republic of China. Adv Parasitol. 2016;92:1–38.
Zhu H, Yap P, Utzinger J, Jia TW, Li SZ, Huang XB, et al. Policy support and resources mobilization for the national schistosomiasis control programme in the People’s Republic of China. Adv Parasitol. 2016;92:341–83.
Yi P, Peng ZZ, Li XS, Luo ZH, Cai KP, Li YY, et al. Endemic features and control strategies of schistosomiasis in Dongting Lake area, Hunan Province, P. R China. Chin J Schisto Control. 2012;24:123–6 (in Chinese).
Yang Y, Zhou YB, Song XX, Li SZ, Zhong B, Wang TP, et al. Integrated control strategy of schistosomiasis in the People’s Republic of China: projects involving agriculture, water conservancy, forestry, sanitation and environmental Modification. Adv Parasitol. 2016;92:237–68.
Yuan H, Jiang Q, Zhao G, He N. Achievements of schistosomiasis control in China. Mem Inst Oswaldo Cruz. 2002;97:187–9.
Wu XH, Chen MG, Zheng J. Surveillance of schistosomiasis in five provinces of China which have reached the national criteria for elimination of the disease. Acta Trop. 2005;96:276–81.
Wen LY, Yan XL, Zhang JF, Li LS, Lin LJ, Huang SY, et al. Strategy of solidification and surveillance for schistosomiasis in transmission-interrupted provinces in China. Chin J Schisto Control. 2011;23:18–21. 31, (in Chinese).
Qian YL, Wang W, Hong QB, Liang YS. Bibliometric analysis of literature regarding integrated schistosomiasis control strategy with emphasis on infectious source control. Chin J Schisto Control. 2014;26:626–31 (in Chinese).
Chen HG, Zeng XJ, Xiong JJ, Jiang WS, Hong XL, Hu SZ, et al. Study on comprehensive schistosomiasis control strategy with emphasis on infectious source control in Poyang Lake areas. Chin J Schisto Control. 2009;21:243–9 (in Chinese).
Yi DH, Yi P, Liu ZC, Li YS, Quan MZ, Xiao SY. Practice and thought of schistosomiasis contorl with emphasis on control sources of infection in Dongting Lake area. Chin J Schisto Control. 2009;21:161–4 (in Chinese).
Zhu SP, Li SM, Wei CJ, Yang QY, Lu BK, Liao YZ, et al. Evaluation of schistosomiasis control effect of buffalo removal in Anxiang County. Chin J Schisto Control. 2011;23:546–50 (in Chinese).
Hong XC, Xu XJ, Chen X, Li YS, Yu CH, Yuan Y, et al. Assessing the effect of an integrated control strategy for schistosomiasis japonica emphasizing bovines in a marshland area of Hubei Province, China: a cluster randomized trial. PLoS Negl Trop Dis. 2013;7:e2122.
Chen YY, Liu JB, Huang XB, Cai SX, Su ZM, Zhong R, et al. New integrated strategy emphasizing infection source control to curb Schistosomiasis japonica in a marshland area of Hubei Province, China: findings from an eight-year longitudinal survey. PLoS One. 2014;9:e89779.
Yang K, Li HJ, Yang WC, Shi XW, Qi YL. Effect of comprehensive schistosomiasis control measures with emphasis on infectious source control in dam areas of mountainous region, Yunnan Province. Chin J Schisto Control. 2009;21:272–75 (in Chinese).
Chen SR, Li BG, Luo JJ, Li WB, Mu LX, Tian SH, et al. Effect of comprehensive schistosomiasis control measures based on infection source control in mountainous areas of Yunnan Province. Chin J Schisto Control. 2015;27:11–6 (in Chinese).
Yihuo WL, Zhou YB, Liu GM, Wu ZS, Wang SA, Xu L, et al. Effect of four-year comprehensive schistosomiasis control in Puge County, Sichuan Province. Chin J Schisto Control. 2009;21:276–9 (in Chinese).
Chen Z, Rao XL, Li YF, Gu XN, Xu MX, Lin DD. Effect of schistosomiasis control strategy based on infection source control of Poyang Lake region in Yongxiu County promotion zone. Chin J Schisto Control. 2015;27:579–82 (in Chinese).
Zeng XJ, Chen HG, Hong XL, Hu ZH, Jiang WS, Hu SZ, et al. Evaluation on medium-term effect of schistosomiasis comprehensive control strategy based on infectious source control in Poyang Lake area. Chin J Schisto Control. 2012;24:382–6 (in Chinese).
He LC, Wang JS, Rong XB, Peng XW, Fu ZY, He ZW, et al. Effect of comprehensive schistosomiasis control strategies with emphasis on infection source control in marshland and lake regions. Chin J Schisto Control. 2010;22:278–80 (in Chinese).
Zhou XN. Implementation of precision control to achieve the goal of schistosomiasis elimination in China. Chin J Schisto Control. 2016;28:1–4 (in Chinese).
Zhou XN, Jiang QW, Guo JG, Lin DD, Zhu R, Yang GJ, et al. Road map for transmission interruption of schistosomiasis in China. Chin J Schisto Control. 2012;24:1–4 (in Chinese).
Utzinger J, Raso G, Brooker S, De Savigny D, Tanner M, Ornbjerg N, et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136:1859–74.
Utzinger J, Bergquist R, Shu-Hua X, Singer BH, Tanner M. Sustainable schistosomiasis control--the way forward. Lancet. 2003;362:1932–4.
Zhang JF, Xu J, Bergquist R, Yu LL, Yan XL, Zhu HQ, et al. Development and application of diagnostics in the National Schistosomiasis Control Programme in the People’s Republic of China. Adv Parasitol. 2016;92:409–34.
Zhu YC. Immunodiagnosis and its role in schistosomiasis control in China: a review. Acta Trop. 2005;96:130–6.
You H, McManus DP. Vaccines and diagnostics for zoonotic schistosomiasis japonica. Parasitology. 2015;142:271–89.
He P, Song LG, Xie H, Liang JY, Yuan DY, Wu ZD, Lv ZY. Nucleic acid detection in the diagnosis and prevention of schistosomiasis. Infect Dis Poverty. 2016;5:25.
Wang TP, Vang Johansen M, Zhang SQ, Wang FF, Wu WD, Zhang GH, et al. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop. 2005;96:198–204.
Zhou YB, Liang S, Jiang QW. Factors impacting on progress towards elimination of transmission of schistosomiasis japonica in China. Parasit Vectors. 2012;5:275.
Wang QZ, Wang TP, Zhang SQ. Research progress on transmission capacity of reservoir host of Schistosoma japonicum. Chin J Schisto Control. 2013;25:86–9 (in Chinese).
Yang GJ, Utzinger J, Zhou XN. Interplay between environment, agriculture and infectious diseases of poverty: case studies in China. Acta Trop. 2015;141:399–406.
Bergquist R, Yang GJ, Knopp S, Utzinger J, Tanner M. Surveillance and response: tools and approaches for the elimination stage of neglected tropical diseases. Acta Trop. 2015;141:229–34.
Zhou XN, Bergquist R, Tanner M. Elimination of tropical disease through surveillance and response. Infect Dis Poverty. 2013;2:1.
Tambo E, Ai L, Zhou X, Chen JH, Hu W, Bergquist R, et al. Surveillance-response systems: the key to elimination of tropical diseases. Infect Dis Poverty. 2014;3:17.
Zhou X, Yap P, Tanner M, Bergquist R, Utzinger J, Zhou XN. Surveillance and response systems for elimination of tropical diseases: summary of a thematic series in infectious diseases of poverty. Infect Dis Poverty. 2016;5:49.
Xu J, Bergquist R, Qian YJ, Wang Q, Yu Q, Peeling R, et al. China-Africa and China-Asia collaboration on schistosomiasis control: A SWOT Analysis. Adv Parasitol. 2016;92:435–66.
Xu J, Yu Q, Tchuenté LA, Bergquist R, Sacko M, Utzinger J, et al. Enhancing collaboration between China and African countries for schistosomiasis control. Lancet Infect Dis. 2016;16:376–83.
Acknowledgements
We would like to thank the staff from Yangzhou Municipal and Hanjiang District Centers for Disease Control and Prevention for their kind participation in the field work.
Funding
This study was supported by the grants from the National Science & Technology Pillar Program of China (grant no. 2009BAI78B06), Jiangsu Provincial Department of Science and Technology (grant no. BL2014021), Jiangsu Provincial Young Talents in Medical Sciences (grant no. QNRC2016621) and Jiangsu Department of Health (grant no. Q201410).
Availability of data and materials
All data described in the study can be provided for free by contact with the corresponding author.
Authors’ contributions
LPS, YSL and WW conceived and designed the study. YPZ, QBH, GLD, YCM, JW, GJY and DJZ performed the field experiments. LPS collected and analyzed the data. LPS prepared the first version of the manuscript. WW revised and finalized the manuscript. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval
This study was approved by the Ethical Review Committee of Jiangsu Institute of Parasitic Diseases (permission number: IRB00004081). All animal experiments were performed in accordance with the 3R rules for animal experiments and the Guidelines for the Care and Use of Laboratory Animals, and signed informed consent was obtained from all participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
Multilingual abstract in the five official working languages of the United Nations. (PDF 1100 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sun, LP., Wang, W., Zuo, YP. et al. A multidisciplinary, integrated approach for the elimination of schistosomiasis: a longitudinal study in a historically hyper-endemic region in the lower reaches of the Yangtze River, China from 2005 to 2014. Infect Dis Poverty 6, 56 (2017). https://doi.org/10.1186/s40249-017-0270-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40249-017-0270-x