Abstract
Background
Heavy metals like Cu, Cd, Pb, Ni, Co and Cr can naturally be found almost all over this planet in various amounts. Urban activities such as heavy metal industry, traffic and waste can rapidly increase the metal concentrations in a fresh water ecosystem.
Methods
This study was done in natural conditions to capture as many aspects in heavy metals pollution and bioremediation of Nicolina River, Romania considered a stream model which is under anthropogenic pressure. Water, sediment and leaves samples of Typha latifolia L. were collected during October 2013 and analyzed in order to assess certain heavy metals (Cu, Cd, Pb, Ni, Co and Cr) from each sampling site using GF-HR-CS-AAS with platform. Heavy metals in significant concentrations in cattail samples were correlated with the water parameters to show the possibility to use the cattail leaves as indicators in heavy metals pollution with potential in bioremediation because they can be easily harvested in autumn and this species is spread worldwide.
Results
The levels of metals concentrations in leaves were: Cu > Ni > Cr > Pb > Co knowing that copper is an essential element for plants. The sampling time was important to draw the river diagnosis for heavy metal pollution. The samples were collected, from river, after more than 60 days without rain same as a “human patient” prepared for blood test. Cobalt was considered the metal marker because it was an element with the lowest level of usage in the city. Compared with it only lead, cadmium and copper were used intensively in the industrial activities.
Conclusions
T. latifolia L. can be use as an indicator for the health of the studied stream and it was noticed that the heavy metals were not accumulated, although the metal uptake was influenced by sediments and water parameters. The alkalinity of the studied river acts as an inhibitor in the bioremediation process of cattail for cadmium and copper. Lead was uptake by leaves and the water parameters influenced it but it wasn’t concentrated enough in leaves to propose this species in lead bioremediation process for Nicolina River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Human activities have a high pressure against the environment with damages of all ecosystems. Each living being deserves a healthy and a clean environment to be borne, rise, to reproduce and die. The organisms react different when the concentration of chemical compounds is increasing in the environment, some of them tolerating very fast the changes but the others suffering so much because of this [1]. Food can be easy contaminated with toxic compounds and this problem can produce damage to human health if it is not well monitored [2]. A stream that crosses a city or a small urban area may negatively interact with the anthropogenic activities, with a significant amount of pollutants. Some of these pollutants are represented by heavy metals from the industrial activities [3], the illegal landfills, traffic and industrial wastewaters released into the environment, without a prior treatment.
Heavy metals can persist for a long time in plant and animal tissues, even if they are in small amounts in the environment [4]. The water chemistry has an important role in heavy metal absorption by organisms within aquatic environments and it can be easily influenced by urban activities [5]. The heavy metals pollution in a river can be analyzed for water, sediments and biota. The seston in a river is in a direct relationship with the rainwater that transports these compounds from atmosphere and terrestrial environment very fast [6]. Sediments can directly influence the heavy metal pollution if they are mixed by the water streams [7]. In the aquatic environments, some parameters like pH, redox potential, salinity, other metals and ionic bounds affect the metal absorption in organisms [8]. Biological absorption of heavy metals is a friendly environment technology with large applications in the future. The plants have the capacity in removing toxic metals from water; also some fungus species have high potential in this process [9].
Typha latifolia L. is a wide spread macrophyte that grows very fast in biomass [10], with a high capacity of absorption and accumulation of heavy metals from the environment [11-15]; thus can be used as a bioindicator of heavy metal pollution [16] and in bioremediation processes. Typha sp. has the capacity to degraded fast the organic pollutants [17], which is very important in the treatment of the domestic water.
The study was conducted on the analysis and interpretation of the connection between the change of water parameters (pH, salinity, conductivity, ORP, TDS) and heavy metals concentrations (Cu, Cd, Pb, Ni, Co, Cr) in water, sediments and upper parts of Typha latifolia L. from Nicolina River, along its course through Iasi City in different areas affected by urban activities like heavy industry. It was studied the capacity of this species in bioremediation of heavy metals and the role as bioindicator for health of studied environment.
Materials and methods
Sampling area description
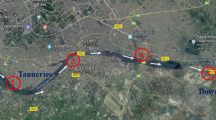
Nicolina River crosses the Iasi City situated in the eastern side of Romania, along its way to Bahlui River, from the direction South to North. For this study five sampling sites (Figure 1) were selected and they were used as a model in this study. Site_1 is considered the reference sampling site and it is situated outside the city in Dumbrava village, an area without any industrial activities but with the traffic as the main pollution source. Site_2 is situated to the entrance in the city, close to the industrial area called in the past Heavy Equipment Works (CUG), at present days Fortus. Its activity was intensive between 1976 and 2003 period focused on construction of iron and steel heavy parts for nuclear reactors, cargo boats and others. Today only a small part is still active. Site_3 is situated 1 km downstream the industrial complex in a residential area with an intensive traffic. Site_4, located in a residential area and it is surrounded by vegetation and neighborhoods. Site_5 is situated 400 m upstream of the confluence with the Bahlui River. The sampling was conducted in October 2013 after more than 60 days with no rainfall.
The pH, salinity, conductivity, total dissolved solids (TDS) and redox potential (ORP) were measured at each sampling site in situ using a HI 9828 produced by Hanna Instruments, calibrated in the laboratory, 24 h before sampling. The period between the measurements at each sampling site was 20–30 minutes.
Water samples preparation
The samples were collected from each sampling site in replicates (n = 3) in 250 ml polyethylene (PE) sterile bottles, prewashed with sampling water and acidified to a pH < 2 with HNO3 65% Suprapur, Merck for preservation [18,19]. In the laboratory the samples were prepared for microwave mineralization in TFM pressure vessels, carefully mixing 25 ml of water sample with 1.5 ml HNO3 65% Suprapur. The mixture needed 15 minutes to react before mineralization in 3 steps program of the MWS-2, Berghof following the protocol recommended by producer. After mineralization, the samples were kept in sterile 30 ml PE bottles for metal analysis.
Sediment samples preparation
The sediments were collected in replicates (n = 3) from each sampling site, in a 20 cm column using a polycarbonate (PC) corer, 7 cm diameter. Each sample was stored in PE bags in a cooling box until the preparation for the next step. In laboratory they were slowly dried at 75°C, grinded in fine powder and sorted the small stones. The fine powder was stored in PE bags and shacked for 5 minutes. 0.5 g from each sample were weighted and mixed in TFM pressure vessels with 2 ml HCl 37%, 2 ml HNO3 65% Suprapur and 1 ml H2O2 30% Merck. The mixture needed 25 minutes to react before mineralization in 3 steps program for sediments. After mineralization, the samples were filtered using filter paper for quantitative analysis (ashless) IDL GMBH &CO in 100 ml flasks and washed with ultrapure water until it reached the 100 ml volume for each sample. After filtration and dilution, the samples were kept in the flasks for heavy metal analysis.
Typha latifolia L. tissues samples preparation
The most important for study were the cattail leaves because they can be harvested every year in the same sampling period (October-November, 2013) and they may clean the environment by concentrating the heavy metals. This macrophyte was present in the highest biomass and abundance in the end of vegetation season. It was absent at Site_5. This species was identified in the field as Typha latifolia (L.) and they were sampled leaves in replicates (n = 3) from biomass above the water surface from each sampling site. The samples were stored in PE bags in a cooling box. In the laboratory, the samples were washed with ultrapure water, chopped with plastic tools, dried at 85°C and grinded. 0.15 g of each sample were weighted and mixed in TFM pressure vessels with 1 ml HCl 37%, 2 ml HNO3 65% Suprapur and 0.5 ml H2O2 30% Merck. The mixture needed 25 minutes to react before mineralization in 3 steps program for dried plants. After mineralization, the samples were filtered using filter paper for quantitative analysis (ashless) in 50 ml flasks and washed with ultrapure water until was reached a 50 ml volume. After filtration and dilution, the samples were kept in the flasks for metal analysis.
Apparatus optimization for metal analysis and method validation
Metals measurement was performed using a GF-HR-CS-AAS with platform contrAA600, AnalytikJena. For each metal, the AAS was optimized and calibrated using stock solutions for Cu, Cd, Pb, Ni, Co and Cr diluted from standard certificated solutions 1000 mg L−1 (Merck) in ultrapure water with 0.05% HNO3. The samples needed Palladium matrix modifier. It was studied the signal and the spectrum of each sample to avoid the interferences and possible contaminations. During the measurements there were conducted QC (Quality Control) analyzes for each metal using certificated solutions. The linear calibration and the method validation results are presented in Tables 1, 2, 3, 4, 5 and 6 for each studied metal.
Metal concentration factor
The heavy metal concentration factor was calculated according to formula: CF = [M]plant/[M]environment [20], where M is the metal concentration. This factor had been calculated as the report between leaf and sediment concentrations at each sampling site. Its value expressed metal’s bioconcentration and level of the pollution.
Statistical analysis
For Normality Test it was performed the Shapiro-Wilk test. Statistical analyses for sediment, biota and Typha latifolia L. were performed using the One-Way ANOVA, followed by Tukey HSD test, in order to explain the influence of urban activities on river course. Pearson correlation followed by 2-tailed test of significance was performed between heavy metal from environment and cattail, water parameters and the significant metal concentrated in the cattail samples from all sampling sites. All the statistical analyses were carried out using OriginPro 8 software.
Results
Water analysis
Water parameters analysed provided the first image about the influence of the city. The values of pH (Figure 2) were between 8.17-8.6 different for each sampling site which is characteristic for this area rich in limestone. The lowest value was recorded outside the city, at Site_1 while in the city the pH easily increased with the highest value at Site_4 - area with fine sediments and stones. There was recorded an increasing pattern for salinity 0.45-0.63 PSU, total dissolved solids (TDS) 453-625 ppm (Figure 3) and conductivity 907-1250 μS cm−1 (Figure 4) that suggested a strong variation of these starting from Site_2 near industrial area. The redox potential (ORP) was 13.55-28.8 with the highest value between Site_2 and Site_4, around the Heavy Equipment Works (CUG), at present Fortus, that appeared to have the highest influence in variation of this parameters. The lowest value was recorded at Site_5, which suggested once more how urban activities can fast change some parameters of the water.
There were identified and measured in water samples Cr, Pb and Ni (Figure 5). The other analyzed metals (Cu, Cd, Co) were under detection limit of the GF-HR-CS-AAS. The metal that was present in the highest concentration was nickel followed by lead and chromium. Nickel concentration did not exceed significant according to ANOVA (p > 0.05, df = 4, F = 1.61) even the averages were between 4.47-5.9 μg L−1. This result is explained by the absence of an active pollution source with Ni at present. Same explanation was in case of lead (p > 0.05, df = 4, F = 0.91) 1.28-2.1 μg L−1 but the chromium concentration in water had a significant difference (**p < 0.01, df = 4, F = 8.89) with averages 0.11-0.54 μg L−1. The highest values were recorded at Site_1, outside the city, in the area with the lowest anthropogenic activity; the water pH may influence the concentration of this metal, even if its value was closer to 8.
Cattail analysis
In samples of T. latifolia (L.) tissues, the levels of cadmium were not detectable, but the others analyzed metals were quantified (Figure 6) in this order of the concentration: Cu > Ni > Cr > Pb > Co. Copper concentrations in samples were 1.67-7.17 μg g−1 (d.w., p > 0.05, df = 3, F = 1.86) with no significant variations between the sampling sites. There were not any significant variations for Ni 1.74-4.4 μg g−1 (d.w., p > 0.05, df = 3, F = 3.03), Cr 0.26-1.47 μg g−1(d.w., df = 3, F = 1.28) and Co 0.04-0.35 μg g−1 (d.w., p > 0.05, df = 3, F = 0.75). The only significant difference (p < 0.001, df = 3, F = 29.49) was for Pb 0.062-0.86 μg g−1 (d.w.).
Sediment analysis
The highest variation of the concentrations was in the sediments samples. Copper (17.63–33.95 μg g−1, p < 0.001, df = 4, F = 48.05) increased from Site_2 in the industrial area and concentrated to Site_4 and Site_5. The concentrations for cadmium (0.26–0.71 μg g−1, p < 0.001, df = 4, F = 225.19) increased from Site_1 (Figure 7) to Site_5 where was the highest concentration. At Site_4 there was recorded a decrease in cadmium because of the sediment mixed with stones.
The lead (14.50–41.70 μg g−1, p < 0.01, df = 4, F = 9.3) was concentrated at Site_5 with a slow decrease at Site_4. Downstream of the industrial area there was a significant increase of lead (Figure 8), possibly caused by the intensive traffic as well. In case of nickel (66.5–91.9 μg g−1, p < 0.01, df = 4, F = 4.35), the highest concentration was at Site_1 outside the city compared to the sites inside the city. For this metal, the city’s activities had no influence. The concentration with the lowest significant value was at Site_4 the area with the most stones in the substrate.
Values for cobalt (4.57–5.72 μg g−1, p < 0.01, df = 4, F = 6.86) were highest in the samples outside (Figure 9) the city and they were not increased at the rest of the sampling stations; Site_4 had the lowest amount because of the substrate. Chromium (27.4–56 μg g−1, p < 0.01, F = 8.68) was the only analyzed element with high levels in samples both outside and inside the city.
Discussions
Heavy metal concentration factor
Present study used as a bioindicator for heavy metal pollution T. latifolia L. leaves. The level of pollution and transfer capacity were expressed by calculating the metal concentration factor. Sasmaz et al. [13] used the same report to express the metal transfer factor. This factor indicated low capacity of heavy metal absorption in this macrophyte in our study (Table 7). Heavy metal concentration in leaves tissues may depends on multiple variables, like water parameters. Their influence was well studied in toxicological laboratory experiments. The results provided the understanding of the mechanism of toxicity in case of heavy metals from aquatic solutions. The problem in this type of experiment is the reduction of the variables. Between the variable they exists interactions: environment - organism, organism-organism and inside the organism. The studied sites from the Nicolina River have different parameters for water, metal content from sediment and water. They were correlated with the uptake of the studied metals from environment. Copper is an essential micronutrient for plants and it has important role physiological functions of the plant [21]. In this study the copper was uptake in leaves in the highest concentration. This element increased in concentration from Site_1 to Site_4 for sediments but it had not significant differences in cattail leaves.
In Sasmaz et al. [13] the transfer factor was highest for cadmium and lead. In the present study, copper and nickel (considered to be another important micronutrient for plants) had the greatest values and the lowest one was for cobalt, chromium and lead (Table 7).
Heavy metal uptake from water in leaves
Chromium was the only heavy metal from water samples with a significant variation over the studied sites. It was applied the first correlation between the chromium from water and from cattail leaves. It was necessary to demonstrate and to show the uptake capacity of chromium from water by this macrophyte. It had an insignificant negative correlation between water and analyzed biota (Figure 10). In this case chromium from the water was not significantly uptake even if the concentration was higher for this aquatic macrophyte. Lead was the only metal with different concentrations in leaves from Site_1 to Site_2, but with no significance in water samples. We correlated its concentrations between water and cattail leaves. The lead from water was not the main source for cattail leaves. There was no significant correlation to show chromium and lead uptake by this macrophyte from water.
Heavy metal uptake from sediments in leaves
The main source of lead in cattail samples was not from water. Sediments were the main source of lead in leaves samples and this was revealed by the positive correlation between sediments and leaves concentrations which were different for each sampling site (Figure 11). It uptakes the lead from sediments by roots to leaves. According to the heavy metal concentration factor, this macrophyte did not accumulated lead in leaves (MCF < 1), but the sediments enrichment with lead increased the uptake. The macrophytes from the urban area have more lead in significant concentrations than those located outside the city. Bioremediation processes are necessary inside the city.
Water parameters and heavy metal uptake in biota samples
The water parameters values were different at each sampling site. Prasad et al. [8] explained the importance of these in heavy metal uptake from the environment by macrophytes. The present study showed, using the correlations, the influence of water parameters in the lead uptake by cattail leaves. For the first two parameters (pH and salinity) it was a strong positive correlation. Acidic medium increases the metals uptake into the plants parts [7]. In our study, an increasing of alkalinity was correlated with a higher amount of lead in cattail leaves (Figure 12). This can be possible as an adaptation of this species to an alkaline environment. The salinity was very strongly positively correlated with lead from the sample. The importance of salinity in metal uptake was well studied. Furthermore, it is possible that same species to uptake differently the metal at same salinity because of the interaction between other factors (physical, chemical, biological).
The redox potential represents an important factor in metal uptake from sediments. In our study, this parameter had not a significant role (Figure 13) in metal uptake from sediments to leaves. It had the highest value between Site_2 and Site_3, the industrial area and it did not influence the metal uptake by leaves. The values were positive typical for an oxidative environment. Total dissolved solids (TDS) had a strong influence in metal uptake from sediments. It is possible that other compounds dissolved in water body (calcium, magnesium, carbonates, chloride) to increase the metal absorption capacity in leaves biomass even this did not accumulate the metal.
The connection between conductivity and lead in cattail leaf had a strong positive correlation that made the city activity to affect even more the lead uptake from the environment (Figure 14). Though there was a strong relation of heavy metal through biota-sediment-water parameters, the alkalinity of Nicolina River determinates a possible relative inactivation, being less harmful for the plants, concentrating into the sediments. The urban activities had an important role in uptake of the lead by T. latifolia L. They modified the water parameters and increased the capacity to absorb the metal from environment into the leaves.
Conclusions
Urban activities from Iasi City had a direct impact upon water parameters from Nicolina River. These activities like steel and iron industry from the Heavy Equipment Works (CUG), at present Fortus, traffic and wastes increased the salinity, conductivity, TDS, pH and redox potential in the water body. There was not recorded a significant increase of the lead and nickel in water body inside the city, but a significant decrease of the chromium inside the city and possible of other different parameters was noticed. T. latifolia L. were used as a bioindicator for the health of this ecosystem and it was noticed that the heavy metals were not accumulated, although the metal uptake was influenced by sediments and water parameters. An increase of Cd, Pb and Cu in the city area was recorded for sediments and a decrease of Co, Cr and Ni. The water alkalinity, combined with other factors can reduce the toxic activity of these metals. T. latifolia L. cannot be use in the process of bioremediation in Nicolina River because it didn’t accumulated the studied metals.
This study represents a beginning in understanding the action of anthropogenic activities upon the environment at different levels and factors. These interactions will provide new data for future bioremediation experiments and in understanding of the chemical behavior (heavy metals uptake) of different species. There are many of variables in a stream that are influencing the organism’s behavior. To understand all the processes the measurement technology must to develop more and more.
References
Welch EB, Jacoby JM. Pollutant effects in freshwater. Appl Limnol, Third Edition. 2004;89–94:271–353.
Shekoohiyan S, Ghoochani M, Mohagheghian A, Mahvi AH, Yunesian M, Nazmara S. Determination of lead, cadmium and arsenic in infusion tea cultivated in north of Iran. Iranian J Environ Health Sci Eng. 2012;9:37.
Hu X, Sun Y, Ding Z, Zhang Y, Jichun W, Lian H, et al. Lead contamination and transfer in urban environmental compartments analyzed by lead levels and isotopic compositions. Environ Pollut. 2014;187:42–8.
Speak AF, Rothwell JJ, Lindley SJ, Smith CL. Metal and nutrient dynamics on an aged intensive green roof. Environ Pollut. 2014;184:33–43.
Juckers M, Watmough SA. Impacts of simulated drought on pore water chemistry of peatlands. Environ Pollut. 2014;184:73–80.
Gunawardana C, Egodawatta P, Goonetilleke A. Role of particle size and composition in metal absorption by solids deposited on urban road surfaces. Environ Pollut. 2014;184:44–53.
Yu K-C, Tsai L-J, Chen S-H, Ho S-T. Chemical binding of heavy metals in anoxic river sediments. Wat Res. 2000;35(17):4086–94.
Prasad MNV, Greger M, Smith BN. Aquatic Macrophytes. Metals in the Environment - Analysis by Biodiversity. 2001. p. 259–88.
Mohsenzadeh F, Shahrokhi F. Biological removing of Cadmium from contaminated media by fungal biomass of Trichoderma species. J Environ Health Sci Eng. 2014;12:102.
Rajaei GE, Aghaie H, Zare K, Aghaie M. Adsorption of Cu(II) and Zn(II) ions from aqueous solutions onto fine powder of Typha latifolia L. root: kinetics and isotherm studies. Res Chem Intermed. 2013;39:3579–94.
Ye ZH, Baker AJM, Wong MH, Willis AJ. Copper and nickel uptake, accumulation and tolerance in Typha latifolia with and without iron plaque on the root surface. New Phytol. 1997;136:481–8.
Carranza-Álvarez C, Alonso-Castro AJ, Alfaro-De La Torre MC, García-De La Cruz RF. Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosí, México. Water Air Soil Pollut. 2008;188:297–309.
Sasmaz A, Obek E, Hasar H. The accumulation of heavy metals in Typha latifolia L. grown in a stream carring secondary effluent. Ecol Eng. 2008;33:278–84.
Ye ZH, Baker AJM, Wong MH, Willis AJ. Zinc, lead and cadmium tolerance, uptake and accumulation by Typha latifolia. New Phytol. 1997;136:468–80.
Leto C, Tuttolomondo T, La Bella S, Leone R. Effects of plants species in a horizontal subsurface flow constructed wetland – phytoremedation of treated urban wastewaster with Cyperus alternifolius L. and Typha latifolia L. in the west of Sicily (Italy). Ecol Eng. 2013;61:282–91.
Klink A, Macicoł A, Wisłock A, Krawczyk J. Metal accumulation and distribution in the organs of Typha latifolia L.(cattail) and their potential use in bioindication. Limmnologica. 2013;43:164–8.
Jamshidi S, Akbarzadeh A, Woo K-S, Valipour A. Wastewater treatment using integrated anaerobic baffled reactor and Bio-rack wetland planted with Phragmites sp. and Typha sp. J Environ Health Sci Eng. 2014;12:131.
Marcovecchio JE, Botté SE, Freije RH. Heavy metals, major metals, trace elements. Handbook of water analysis, second edition. 2007. p. 275–311.
Biziuk M, Beyer A, Zukowska J. Preservation and storage of water samples. In: Analytical Measurements in Aquatic Environments. 2010. p. 19–39.
Coelho JP, Lillebø AI, Pacheco M, Pereira ME, Pardal MA, Duarte AC. Biota analysis as a source of information on the state of aquatic environments. In: Analytical Measurements in Aquatic Environments. 2010. p. 103–20.
Thomas G, Stärk H-J, Wellenreuther G, Dickinson BC, Küpper H. Effects of nanomolar copper on water plants – Comparison of biochemical and biophysical mechanisms of deficiency and sublethal toxicity under environmentally relevant conditions. Aquat Toxicol. 2013;140–141:27–36.
Acknowledgements
This study was financial supported by the project Resources pilot center for cross-border preservation of the aquatic biodiversity of Prut River MIS-ETC 1150 Romania – Ukraine – Republic of Moldova. The support of strategic grant POSDRU/159/1.5/S/133391, Project “Doctoral and Post-doctoral programs of excellence for highly qualified human resources training for research in the field of Life sciences, Environment and Earth Science” cofinanced by the European Social Fund within the Sectorial Operational Program Human Resources Development 2007 – 2013 is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The first author and the corresponding author did the primary plan of this research. All authors of this research paper have directly participated in the planning, execution, and analysis of this study. All authors read and approved the final manuscript.
Oana Jitar and Gabriel Plavan contributed equally to this work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strungaru, SA., Nicoara, M., Jitar, O. et al. Influence of urban activity in modifying water parameters, concentration and uptake of heavy metals in Typha latifolia L. into a river that crosses an industrial city. J Environ Health Sci Engineer 13, 5 (2015). https://doi.org/10.1186/s40201-015-0161-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40201-015-0161-7