Abstract
Background
Pegylated-interferon alpha (PegINFα) treatment of patients with polycythemia vera (PV) and essential thrombocythemia (ET) has resulted in long-term clinical response, decreased JAK2 V617F allelic burden and restoration of polyclonal hematopoiesis. The mechanisms of the beneficial effects of PegINFα are not clear, but available evidence suggests direct suppression of JAK2-mutated clone, induction of dormant stem cells to proliferation, and augmentation of an immune effect against PV and ET clones.
Methods
We analyzed the phenotype and frequency of peripheral blood lymphocytes (PBL) from PegINFα treated patients and compared them to patients treated with hydroxyurea (HU). Samples collected at various time points before and during treatment were analyzed using multicolor flow cytometry.
Results
We found that PegINFα increased the frequency of peripheral blood CD4+ Foxp3+ regulatory T cells (Treg). Highly suppressive Treg, characterized by co-expression of CD39 and HLA-DR, were also increased in PBL from PegINFα treated patients. We observed an augmentation of cycling CD8+ T cells, NK cells, and of poorly activated CD38+CD8+ T cells. Our results also suggest that PegINFα increased the frequency of PD-1+ CD4+ helper cells and PD-1+ CD4+ Foxp3+ Treg cells. None of these changes were present in HU treated patients. We analyzed the correlation between changes in different T cell populations in the peripheral blood with the changes in JAK2 V617F allelic burden in clonal granulocytes. Augmentation of Ki-67+ Treg, HLA-DR+ CD39+ Treg, Helios+ Treg and HLA-DR+ CD38+ CD8+ T cells correlated with an increase in JAK2 V617F allelic burden. We also found a positive correlation between PD-1+ Treg and JAK2 V617F allelic burden; however, the number of available patients was small (n = 7).
Conclusions
We report marked changes in frequencies of PBL subsets after PegINFα treatment, suggesting an immunomodulatory effect by PegINFα. Generation of a more suppressive immune response, as measured by an increase in highly suppressive Treg and poorly activated CD8+ T cells, correlated with a poor molecular response. In this study, we have not identified changes in the PBL that would indicate the presence of an effective anti-tumor response.Trial registration NCT01259856, December 7. 2010 and NCT01259817, December 6. 2010, Grant #1P01CA108671-O1A2, July 17. 2006, Sponsor: MPDRC/NIH, NCI-2012-00269, January 12. 2011 and NCI-2012-00268, January 12. 2011
Similar content being viewed by others
Background
Polycythemia vera (PV) and essential thrombocytosis (ET) are myeloproliferative neoplasms (MPN) characterized by the excessive proliferation but unhindered differentiation of a hematopoietic stem cell clone. The JAK2 V617F mutation is present in more than 95 % of patients with PV and more than 50 % of patients with ET [1–4]. Chemotherapy decreases cell counts and thrombotic events by suppressing proliferation of PV and ET clones, but only interferon alpha (INFα) has occasionally restored polyclonal hematopoiesis [5] and often reduced JAK2 V617F allelic burden [6]. However, there are multiple mechanisms by which INFα promotes this response, including a direct suppression of JAK2 mutated hematopoietic progenitors [7, 8], stimulation of quiescent residual normal cells by promoting cell division, differentiation of hematopoietic stem and progenitor cells [9], or beneficial alteration of humoral and cellular immune responses.
The role of T cells in tumor immunity is well established. The recent successes of immunomodulatory therapies such as antibodies targeting checkpoint molecules or adaptive T cell therapy have clearly established the role of immunotherapy in the treatment of a variety of cancers, such as melanoma, renal carcinoma and non-small lung cancer [10, 11]. It was previously reported that INFα increases function of CD4 effector T-cells, CD8+ T-cells and B-cells and that it may exert anti-tumor effects. [12–14]. INFα effects on Treg are not fully understood. However, some studies have shown an activating effect of INFα on Treg [15, 16]. Thus, in melanoma patients, INFα treatment is used as an adjuvant therapy [17] and the systemic immune effects of INFα in these patients have been described [18].
Failure of the immune system to control clonal growth is very often due to immunological imbalance. In many cancers, Treg are increased and contribute to the failure of the immune system to clear the tumor. Several types of Treg with distinct functional differences have been recognized. Treg that co-express CD39 and HLA-DR are more suppressive than Treg that do not express these two surface markers [19]. Another highly suppressive Treg subset is characterized by expression of the Ikaros family member transcription factor Helios [20]. A decrease in a highly suppressive CD39+ and HLA-DR+ Treg subset has been correlated with a decreased tolerance, resulting in kidney graft rejection or pre-term pregnancy loss [21, 22]. Increased Helios+ Treg have been observed in tumor infiltrating lymphocytes [23].
To be fully functional, CD8+ T cells need to be stimulated along a well-defined pathway [24]. First, a T cell receptor is engaged by its cognate MHC-peptide complex. Then a second signal is delivered when CD28 expressed at the surface of activated T cells is engaged by B7 present at the surface of antigen presenting cells. To fully activate T cells, a third signal is necessary and is provided by the engagement of OX40 by its ligand OX40 ligand. OX40 is a member of the tumor necrosis superfamily expressed at the surface of recently activated T cells [25]. Failure to accomplish these three steps leads to poorly activated, hypofunctional T cells characterized by co-expression of CD38 and HLA-DR. Non-specific activation, or bystander activation, is often observed in cancer or acquired immunodeficiency syndrome [26]. In patients infected with human immunodeficiency virus, the presence of CD38+ HLA-DR+ CD8+ T cells correlates with early progression of disease [27].
To prevent overactivation of the immune system, several checkpoint molecules control T cell activation and prevent accumulation of activated T cells. CTLA-4, a member of the immunoglobulin superfamily, upon binding of its ligand (CD80 or CD86), inhibits proliferation of effector T cells. PD-1, a member of the CD28 family, is expressed on T cells upon activation, and its engagement by PDL-1 or PDL-2, results in T cell death, anergy and exhaustion. High levels of CTLA-4 or PD-1 expression on tumor infiltrating T cells contributes to dysregulation of the immune response in cancer patients.
In this study, we attempted to determine whether PegINFα stimulation of the immune system contributes to the beneficial response to PegINFα therapy. PegINFα is a form of INFα with a longer half-life, less toxicity and more convenient administration. Riley and colleagues have reported an increase in circulating NK cells and Treg in MPN patients treated with PegINFα [28–30]; however, no correlation with clinical, cytological, or molecular response to therapy was reported. This study confirms increased circulating NK cells and Treg after PegINFα therapy and provides a more detailed characterization of PBL in patients treated with PegINFα. We report a positive correlation between changes in JAK2 V617F allelic burden and changes in lymphocyte populations in the peripheral blood. These changes are independent of myelosuppression, as they were not observed in PV and ET patients treated with HU. We hypothesize that the increase in CD39+ HLA-DR+ Treg contributes to suppression of the immune response, resulting in treatment failure. We suggest that increased CD39+ HLA-DR+ Treg could be used as a biomarker to predict if Peginfα treatment will have beneficial effects in MPN patients.
Methods
Study subjects
This study included the following three groups of subjects: (1) patients who participated in MPD-RC 112, a Phase 3 randomized trial in high-risk PV and ET using PegINFα versus HU; (2) patients who participated in MPD-RC 111 for PV and ET patients intolerant or refractory to hydroxyurea and patients with Budd Chiari syndrome (both trials are part of the Myeloproliferative Disorders Research Consortium); and (3) patients who did not participate in these protocols. Peripheral blood samples were collected prior to and at different time points during treatment, after patients signed an approved Institutional Review Board informed consent. Patient’s characteristics are described in Table 1.
Trial registration
NCT01259856, December 7. 2010, NCT01259817, December 6. 2010, IRB_00017793, grant #1P01CA108671-O1A2, July 17. 2006, Sponsor: MPDRC/NIH, IRB_ 45342, NCI-2012-00269, January 12. 2011 and IRB_36791, NCI-2012-00268, January 12. 2011.
Blood sample processing
10 mL of peripheral blood were obtained by venipuncture. Granulocyte and mononuclear cell fractions were isolated according to previously published protocols [31, 32]. After isolation, PBMC were frozen and stored prior to analysis.
Flow cytometry
Single cell suspensions of leukocytes from peripheral blood were stained using the following monoclonal antibodies: anti-CD3 APC-H7 (BD, 641397), anti-CD4 Alexa Fluor 700 (BD, 557922), anti-CD8 Alexa Fluor 700 (BD, 557945), anti-CD25 APC (Miltenyi 130-092-858), anti-CD28 BV421(BD, 562613), anti-CD38 BV605 (Biolegend 303532), anti-HLA-DR PE-Cy7 (BD Biosciences, 335795), anti-CD39 BV421 (BD 563679), anti-CD45 RO BV510 (BD, 563215), anti-CD56 APC (Biolegend 318310), anti-CCR7 PE (BD 561008), anti-FoxP3 PE (eBioscience, 12-4777-42), anti-Ki-67 FITC (BD 556026), anti-HELIOS (Biolegend, 137214), anti-CTLA-4 APC (BD 560938), anti-OX40 FITC (Biolegend 35006), and anti-PD1 BV510 (Biolegend 329932). Cells were stained in FACs buffer (1 % FBS in PBS with 0.01 % NaN3) and fixed according to the eBioscience FoxP3 Fix-Perm kit protocol (eBioscience, 00-5521-00). All samples were analyzed on a BD X20 Fortessa Flow cytometer. We used Fluorescence Minus One to discriminate between positive and negative cells for each antibody [33].
DNA isolation and JAK2 V617F analysis
Genomic DNA was isolated from granulocytes using Puregene DNA purification kits (Gentra, MN, USA). The JAK2 V617F mutational burden was determined by quantitative real-time allele specific PCR (qRT-PCR) employing a sequence 7500 detection system (Applied Biosystems, Foster City CA, USA) in which an allele-specific primers, containing both a mismatched nucleotide and a locked nucleic acid, are used to enhance discrimination between polymorphic nucleotides, as previously described [34].
PBL and JAK2 V617F correlation analysis
Correlation matrices were created to identify potential bivariate linear relationships between changes in peripheral blood Treg populations and changes in JAK2 V617 allelic burden. Significantly correlated variables, with corresponding Pearson’s product-moment correlation coefficient (r) and p value, are presented in Fig. 6. A generalized linear model (GLM) was calculated for each pair as well. However, for interpretation, r was preferable to the beta coefficients to include in Fig. 6. Analysis was performed adapting the cor.test and corrplot functions using R software version 3.2.3 (Copyright (C) 2015 The R Foundation for Statistical Computing).
Results
PegINFα increases the frequency of CD4+ Foxp3+ Treg and highly suppressive Treg in peripheral blood
Under the approval of our institute’s Institutional Review Board, 32 patients with MPN were enrolled in the study. As described in Table 1, 25 patients were treated with PegINFα and 7 with HU.
Peripheral blood samples were received and processed as described in “Methods” section. To determine the effects of PegINFα on peripheral blood Treg, we performed multicolor flow cytometry analysis with antibodies that recognize CD3, CD4, CD25, Foxp3, CD39, HLA-DR, Helios and Ki-67. Changes in T-cell subpopulations were analyzed by prism using fluorescence minus one as internal controls. Peripheral blood mononuclear cells (PBMC) were gated on CD3 and CD4 and then further analyzed for Ki-67, a marker of cell proliferation, as well as Foxp3 and CD25, markers of Treg. Treg were further analyzed for co-expression of CD39 and HLA-DR, and expression of Helios. Treg expressing these markers represent Treg that are more suppressive. An example of flow cytometry analysis is shown in Additional file 1: Fig. S1. As shown in Fig. 1a, CD4+ Foxp3+ Treg increased from 6.4 to 9.9 %, p = 0.0017, in patients receiving PegINFα, while no change was observed in HU treated patients. This increase continued with time and was higher after 52 weeks of treatment at 11.4 % (Fig. 1b).
PegINFα increases Treg and highly suppressive Treg cells in peripheral blood of PV and ET patients. PBMC were collected from patients with PV or ET treated with PegINFα or HU for at least 70 days (range 70–616, median 112 for PegINFα and range 113–2422, median 175 for HU treated patients). Lymphocytes were analyzed by flow cytometry using surface markers, CD3, CD4, CD25, CD39, HLA-DR and intracellular markers Foxp3, Ki-67 and Helios. a represents CD4+ CD25+ Foxp3+ Treg cells. In b the frequency of Treg was analyzed at different time points after initiation of PegINFα treatment. c–e show frequency of Ki-67+ Treg, Helios+ Treg, and CD39+/HLA-DR+) Treg
For 18 of 25 patients treated with PegINFα and 5 of 7 HU treated patients, we were able to perform a more detailed analysis of the Treg population. The proliferation of Treg as measured by Ki-67 was not significantly increased in PegINFα treated patients (12.3–14.4 % p = 0.5509) (Fig. 1c). When analyzing the more suppressive phenotype of Treg, we observed that PegINFα significantly increased the proportion of CD39+ HLA-DR+ Treg from 10.6 to 16.7 %, p = 0.0139 (Fig. 1d). No significant increase in the proportion of Helios+ Treg (63.15–64.2 % p = 0.2188) was observed in PegINFα treated patients (Fig. 1e). There was no statistically significant change in the absolute number of CD39+ HLA-DR+, Ki-67+ or Helios+ Treg in Peginfa treated patients (Additional file 2: Fig. S2). We did not observe any significant changes in Treg subpopulations in HU treated patients.
Proliferation and phenotypic changes in CD8+ T cells induced by PegINFα
Next, we analyzed CD8+ CD3+ cytotoxic T cells for expression of CD28, Ki-67, CD38 and HLA-DR before and after therapy with PegINFα or HU. The frequency of CD8+ T cells was not affected by PegINFα (20.3 and 20.1 %) or HU (21.8 and 13.8 %), respectively (Fig. 2a). However, in PegINFα treated patients, a higher proportion of CD8+ T cells co-expressed Ki-67, indicating increased proliferation (4.6–7.5 %, p = 0.001). HU did not induce proliferation of CD8+ T cells (Fig. 2b). Moreover, we observed phenotypic differences in CD8+ T cells, including a significant increase in the CD38+ HLA-DR+ CD8+ subset, representing activated cells, in patients receiving PegINFα (10.2 % before treatment and 21.2 % during treatment, p = 0.0015; Fig. 2c). No changes were observed in HU treated patients.
Increased proliferation and activation of CD8+ T cells in peripheral blood of PegINFα treated patients. PBMC collected prior to and after at least 10 weeks of treatment were analyzed using anti-CD3, -CD8, -CD38, -HLA-DR and Ki-67 antibodies. Percentage of CD8+ T cells, cycling (Ki-67+) CD8+ T cells and CD38+/HLA-DR+ CD8+ T cells are shown in (a–c) respectively
PegINFα induces expression of checkpoint molecules on T cells
We investigated the expression of checkpoint molecules PD-1 and CTLA-4 on CD3+ T cells in peripheral blood in 14 of 25 PegINFα treated patients and 4 of 7 HU treated patients. The frequency of peripheral blood CD4+ Treg and CD4+ effector T cells (Teff) expressing PD-1 increased in PegINFα-treated patients (0.18 % before treatment and 0.44 % during treatment for Treg, p = 0.016; and 1.2–3.1 % Teff, p = 0.0029) (Fig. 3c, d). The proportion of CTLA-4 expressing Treg was also augmented in patients treated with PegINFα (Fig. 3a, b). HU did not affect the expression of either PD-1 or CTLA-4.
Increased CD3− CD56+ NK cell proliferation
Analysis of CD3− CD56+ NK cells was performed in 9 of 25 patients receiving PegINFα and 3 of 7 patients treated with HU. PegINFα did not affect the proportion of NK cells in the peripheral blood. However, the proportion of dividing NK cells was significantly increased, as shown in Fig. 4a, b. Prior to PegINFα treatment, 7.8 % of NK cells were proliferating; after at least 10 weeks of treatment, the frequency increased to 17.3 %, p = 0.0078. No changes were observed in HU treated patients.
Increased CD3−/CD56+ NK cells proliferation in peripheral blood of PV and ET patients treated with PegINFα. PBMC were collected from patients prior to and after at least 10 weeks of treatment and analyzed for surface markers CD3 and CD56 and intra-cellular marker Ki-67. a shows CD56+ frequency and b shows frequency of Ki-67+ CD56+ NK cells
Correlation between peripheral blood T cells and molecular response in PegINFα treated patients
In 18 PV and 7 ET patients treated with PegINFα, molecular response was evaluated by measuring the JAK2 V617F allelic burden. One ET patient was JAK2 V617F negative and carried a calreticulin mutation and another ET patient was triple negative [35]. Patients were subdivided into two groups based on percentage reduction of JAK2 V617F allelic burden. Patients with a 20 % or greater decrease in JAK2 V617F allelic burden were categorized as responders (R), and those with a less than 20 % decrease were categorized as non-responders (NR). In this cohort, there were 10 R (9 PV and 1 ET) and 13 NR (9 PV and 4 ET).
We sought to determine whether changes in PBL correlated with changes in JAK2 V617F allelic burden. As shown in Fig. 5, we observed several trends consistent with previously published data in other malignancies. NR showed a higher increase of Treg frequency, Treg proliferation and highly suppressive CD39+ HLA-DR+ Treg frequency. When looking at changes in absolute Treg, a similar trend was observed (Additional file 3: Fig. S3). A more pronounced increase of CD38+ HLA-DR+ CD8+ T cells were observed in NR.
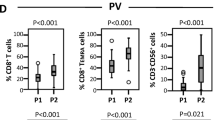
Changes in T cell subsets vary based on molecular response. Patients treated with PegINFα were separated into R and NR subsets based on a 20 % decrease in JAK2 V617 allelic burden. PBMC were analyzed as described above and fold changes compare to baseline were examined. a represents 23 patient samples (10R and 13 NR), b, c include 16 patients (6R and 10NR), and d includes 13 patients (4R and 9 NR). a–c show frequency of Treg, dividing Treg, CD39+ HLA-DR+ Treg and Helios+ Treg. d shows CD38+ HLA-DR+ T cells in R vs NR
Changes in PBL correlate with JAK2 V617F allelic burden
Using Spearman correlation analysis, we identified several Treg populations that increased in the peripheral blood and correlated with an increase in JAK2 V617F allelic burden. An increased proportion of Ki-67+ Treg, HLA-DR+ CD39+ Treg and Helios+ Treg correlated with an increase in JAK2 V617F allelic burden (Fig. 6a–c). Analysis of seven patients shows a strong positive correlation between PD-1+ Treg and JAK2 V617F allelic burden (Fig. 6d). These data need to be confirmed in a larger number of patients. We observed that an increase in the CD38+ HLA-DR+ CD8+ T cell subset also correlated with an increases in the JAK2 V617F allelic burden (Fig. 6e).
Correlation between increasing JAK2 V617 allelic burden in patients treated with PegINFα and augmentation of peripheral blood Treg populations. Changes in peripheral blood Treg population were correlated with changes in JAK2 V617 allelic burden. a Ki-67+ Treg, CD39+/HLA-DR+ Treg (b), Helios+ Treg (c), PD-1+ Treg (d) and CD38+ HLA-DR+ CD8+ T cells (e)
Discussion
PegINFα is the only treatment for PV and ET patients reported to result in long-term clinical and molecular remission [34]. In some patients, we observed a marked decrease of JAK2 V617F allelic burden that persisted even after discontinuation of treatment, suggesting a PegINFα-induced immune-mediated mechanism. INFα increases tumor immunogenicity, activates antigen presenting cells, and stimulates both CD4+ and CD8+ T cells [36, 37]. Several other studies analyzed PBL, characterized the effect of PegINFα and reported similar results [28, 29]. Previously, our group also reported an increase of Treg in PV and ET patients during therapy with PegINFα [38]. However, a detailed analysis of PBL subsets and their correlation with clinical and molecular response had not previously been established.
In this study, we used multi-color flow cytometry to analyze PBL of PV and ET patients treated with PegINFα or HU. We found an increase in peripheral blood Treg in patients treated with PegINFα. In addition, the frequency of highly suppressive CD39+ HLA-DR+ Treg was also increased. In the NR subset of patients, we found a trend towards increased frequency of Treg, increased proliferation of Treg and increased CD39+ HLA-DR+ Treg compared to the R group (p = 0.55, p = 0.2530, p = 0.4078 respectively). When looking at the absolute numbers of Treg, no significant changes were observed. However, since PegINFα decreased absolute lymphocyte counts, it was interesting to observe that CD39+ HLA-DR+ Treg numbers did not decrease.
We assessed if changes in PBL subpopulations predicted molecular response. We found a positive correlation between increased proliferating Treg (Ki-67+ Treg), highly suppressive Treg (HLA-DR+/CD39+ or Helios+ Treg) and JAK2 V617F allelic burden. We also found a positive correlation between increased HLA-DR+ CD38+ CD8+ T cells and increased JAK2 V617F allelic burden. Similar results were observed in hepatitis C patients treated with INFα. Su et al. reported that patients presenting with virological response had lower circulating Treg [39]. Although peripheral blood changes may not necessarily reflect the marrow microenvironment [40], the easy accessibility of peripheral blood permits convenient monitoring of patients undergoing PegINFα therapy. Our data indicate that Treg play an important role in the pathophysiology of PV and ET and that failure of PegINFα to decrease circulating Treg predicts a poor molecular response.
Conclusion
These results suggest that activation of the immune system by PegINFα can induce either a protective immune response that contributes to elimination of the malignant clone or a suppressive immune response, suggesting that increases in Treg prevent clearance of the mutated clone by effector CD8+ T cells. Further studies analyzing changes in immune cells in bone marrow may elucidate the role of PegINFα in inducing anti-tumor responses. We found that changes in peripheral blood T cell populations correlate with achievement of a molecular response to PegINFα.
Abbreviations
- PV:
-
polycythemia vera
- ET:
-
essential thrombocytemia
- MPN:
-
myeloproliferative neoplasms
- INFα:
-
interferon alpha
- PegINFα:
-
pegylated-interferon alpha
- HU:
-
hydroxyurea
- PBL:
-
peripheral blood lymphocytes
- Treg:
-
CD4+ Foxp3+ regulatory T cells
- Teff:
-
CD4+ effector T cells
- PBMC:
-
peripheral blood mononuclear cells
References
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR, Cancer genome project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61.
James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, Garçon L, Raslova H, Berger R, Bennaceur-Griscelli A, Villeval JL. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, Adelsperger J. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97.
Liu E, Jelinek J, Pastore YD, Guan Y, Prchal JF, Prchal JT. Discrimination of polycythemias and thrombocytoses by novel, simple, accurate clonality assays and comparison with PRV-1 expression and BFU-E response to erythropoietin. Blood. 2003;101(8):3294–301.
Kiladjian JJ, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M, Bellucci S, Grandchamp B, Chomienne C, Fenaux P. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–72.
Quintás-Cardama A, Kantarjian H, Manshouri T, Luthra R, Estrov Z, Pierce S, Richie MA, Borthakur G, Konopleva M, Cortes J, Verstovsek S. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27(32):5418–24.
Kiladjian JJ, Mesa RA, Hoffman R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood. 2011;117(18):4706–15.
King KY, Matatall KA, Shen CC, Goodell MA, Swierczek SI, Prchal JT. Comparative long-term effects of interferon α and hydroxyurea on human hematopoietic progenitor cells. Exp Hematol. 2015;43(10):912–8.
Drake CG. Combined immune checkpoint blockade. Semin Oncol. 2015;42(4):656–62.
Atkins MB, Sznol M. Cancer immunotherapy: past progress and future directions. Semin Oncol. 2015;42(4):518–22.
Fattovich G, Betterle C, Brollo L, Pedini B, Giustina G, Realdi G, Alberti A, Ruol A. Autoantibodies during α-interferon therapy for chronic hepatitis B. J Med Virol. 1991;34(2):132–5.
Dumoulin FL, Leifeld L, Sauerbruch T, Spengler U. Autoimmunity induced by interferon-α therapy for chronic viral hepatitis. Biomed Pharmacother. 1999;53(5):242–54.
Huber JP, Farrar JD. Regulation of effector and memory T-cell functions by type I interferon. Immunology. 2011;132(4):466–74.
Golding A, Rosen A, Petri M, Akhter E, Andrade F. Interferon-alpha regulates the dynamic balance between human activated regulatory and effector T cells: implications for antiviral and autoimmune responses. Immunology. 2010;131(1):107–17.
Sprengers D, Stoop JN, Binda RS, Kusters JG, Haagmans BL, Carotenuto P, Artsen A, van der Molen RG, Janssen HL. Induction of regulatory T-cells and interleukin-10-producing cells in non-responders to pegylated interferon-alpha therapy for chronic hepatitis B. Antiviral therapy. 2007;12(7):1087.
Flaherty LE, Othus M, Atkins MB, Tuthill RJ, Thompson JA, Vetto JT, Haluska FG, Pappo AS, Sosman JA, Redman BG, Moon J. Southwest Oncology Group S0008: a phase III trial of high-dose interferon Alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma—an intergroup study of cancer and leukemia Group B, Children’s Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. J Clin Oncol. 2014;32(33):3771–8.
Chevolet I, Schreuer M, Speeckaert R, Neyns B, Hoorens I, van Geel N, Krüse V, Hennart B, Allorge D, Van Gele M, Brochez L. Systemic immune changes associated with adjuvant interferon-α2b-therapy in stage III melanoma patients: failure at the effector phase? Melanoma Res. 2015;25(4):357–61.
Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM. Expression of ectonucleotidase CD39 by Foxp3 + Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–32.
Getnet D, Grosso JF, Goldberg MV, Harris TJ, Yen HR, Bruno TC, Durham NM, Hipkiss EL, Pyle KJ, Wada S, Pan F. A role for the transcription factor Helios in human CD4+, CD25+ regulatory T cells. Mol Immunol. 2010;47(7):1595–600.
Kisielewicz A, Schaier M, Schmitt E, Hug F, Haensch GM, Meuer S, Zeier M, Sohn C, Steinborn A. A distinct subset of HLA-DR+-regulatory T cells is involved in the induction of preterm labor during pregnancy and in the induction of organ rejection after transplantation. Clin Immunol. 2010;137(2):209–20.
Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90(10):935–44.
Zabransky DJ, Nirschl CJ, Durham NM, Park BV, Ceccato CM, Bruno TC, Tam AJ, Getnet D, Drake CG. Phenotypic and functional properties of Helios + regulatory T cells. PLoS ONE. 2012;7(3):e34547.
Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–20.
Redmond WL, Ruby CE, Weinberg AD. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit Rev Immunol. 2009;29(3):187–201.
Bastidas S, Graw F, Smith MZ, Kuster H, Gunthard HF, Oxenius A. CD8+ T cells are activated in an antigen-independent manner in HIV-infected individuals. J Immunol. 2014;192(4):1732–44.
Kestens L, Vanham G, Gigase P, Young G, Hannet I, Vanlangendonck F, Hulstaert F, Bach BA. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6(8):793–7.
Riley CH, Brimnes MK, Hansen M, Jensen MK, Hasselbalch HC, Kjaer L, Svane IM. Interferon-alpha induces marked alterations in circulating regulatory T cells, NK cell subsets and dendritic cells in patients with JAK2 -positive essential thrombocythemia and polycythemia vera. Eur J Haematol. 2015.
Riley CH, Hansen M, Brimnes MK, Hasselbalch HC, Bjerrum OW, Svane IM, Jensen MK. Expansion of circulating CD56bright natural killer cells in patients with JAK2-positive chronic myeloproliferative neoplasms during treatment with interferon-α. Eur J Haematol. 2015;94(3):227–34.
Riley CH, Jensen MK, Brimnes MK, Hasselbalch HC, Bjerrum OW, Straten PT, Svane IM. Increase in circulating CD4(+) CD25(+) Foxp3(+) T cells in patients with Philadelphia-negative chronic myeloproliferative neoplasms during treatment with IFN-alpha. Blood. 2011;118(8):2170–3.
Prchal JT, Throckmorton DW, Carroll AJ, Fuson EW, Gams RA, Prchal JF. A common progenitor for human myeloid and lymphoid cells. Nature. 1978;274(5671):590–1.
Jelinek J, Prchal JT. Oxygen-dependent regulation of erythropoiesis. Methods Enzymol. 2004;381:201–10.
Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45(3):194–205.
Nussenzveig RH, Swierczek SI, Jelinek J, Gaikwad A, Liu E, Verstovsek S, Prchal JF, Prchal JT. Polycythemia vera is not initiated by JAK2V617F mutation. Exp Hematol. 2007;35(1):32–8.
Tefferi A, Thiele J, Vannucchi AM, Barbui T. An overview on CALR and CSF3R mutations and a proposal for revision of WHO diagnostic criteria for myeloproliferative neoplasms. Leukemia. 2014;28(7):1407–13.
Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17(9):2619–27.
Tough DF. Modulation of T-cell function by type I interferon. Immunol Cell Biol. 2012;90(5):492–7.
Kelley TW, Swierczek S, Efimova O, Wilson AR, Kim SJ, Prchal J. The number of peripheral blood CD4+, CD25+, FOXP3+ regulatory T-cells is a marker of response in patients with JAK2 V617F positive myeloproliferative neoplasms treated with pegylated-interferon-α. ASH abstract, 2013.
Suuu SS, He H, Kong LB, Zhang YG, Zhao SX, Wang RQ, Zheng HW, Sun DX, Nan YM, Yu J. Regulatory phenotype, PD-1 and TLR3 expression in T cells and monocytes from HCV patients undergoing antiviral therapy: a randomized clinical trial. PLoS ONE. 2014;9(4):e93620.
Kovacsovics-Bankowski M, Chisholm L, Vercellini J, Tucker CG, Montler R, Haley D, Newell P, Ma J, Tseng P, Wolf R, Vetto JT. Detailed characterization of tumor infiltrating lymphocytes in two distinct human solid malignancies show phenotypic similarities. J Immunother Cancer. 2014;2(1):1.
Authors’ contributions
MKB designed the study, performed, analyzed and interpreted the flow cytometry experiments and wrote the manuscript. OE performed the flow cytometry experiments. SJK carried out the detection of the JAK2 V617F allelic burden. AW performed the statistical analysis. TWK designed the study and revised the manuscript. SS carried out detection of the JAK2 V617F allelic burden and revised the manuscript. JP designed the study and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors want to thank Mrs. S. Madden for her editorial help and Mrs. C. Warby for helping preparing patients samples.
Competing interests
The authors declare that they do not have competing interests.
Availability of supporting data
All raw flow cytometry data from which the conclusions have been drawn are available are available upon request.
Ethical approval and consent to participate
All patients were consented under the following protocols: IRB_00017793, Grant # 1P01CA108671-O1A2, Sponsor: MPDRC/NIH, University of Utah IRB approval date: 7/17/2006; IRB_45342 University of Utah IRB approval date: 1/12/2011,NCI Trial ID:NCI-2012-00268; IRB_36791 University of Utah IRB approval date: 1/12/2011,NCI Trial ID, NCI-2012-00269; NCT01259856, December 7. 2010; NCT01259817, December 6. 2010; This study was approved by the University of Utah Institutional Review Board; Research Administration Building 75 South 2000 East Salt Lake City, UT 84112; 801-581-3655.
Funding
NIH P01 CA108671; LLS Leukemia and Lymphoma SocietyR6404-13.
Author information
Authors and Affiliations
Corresponding author
Additional files
40164_2016_57_MOESM1_ESM.pdf
Additional file 1: FigS1. Flow cytometry gating strategy. PBMC were processed as described in Material and Methods. Single cell suspensions were analyzed by flow cytometry. FSC and SSC were used to determine lymphocyte population. CD4+ T cells were analyzed for expression of CD25 and Foxp3 (Treg) (Panel B and F). In Panel C-D and G-H, CD3+ CD4+ CD25+ Foxp3+ Treg were analyzed using CD39 and HLA-DR (Panel C and G) and Helios and CD45RO (panel D and H). CD39, HLA-DR and Helios were used to characterize highly suppressive Treg. Treg were also assessed for proliferation by using Ki-67. Panels A-D represent analysis prior to PegINFα, and panels E-H after at least 10 weeks of PegINFα treatment.
40164_2016_57_MOESM2_ESM.pdf
Additional file 2: FigS2. Absolute numbers of Treg and highly suppressive Treg in peripheral blood of PV and ET patients are not significantly affected by Peginfα. PBMC were collected from patients with PV or ET treated with PegINFα or HU for at least 70 days (range 70-616 days, median 112 days, for PegINFα and range 113-2422 days, median 175 days, for HU treated patients). Lymphocytes were analyzed by flow cytometry using surface markers CD3, CD4, CD25, CD39, HLA-DR and intracellular markers Foxp3, Ki-67 and Helios. Panel A represents absolute numbers of CD4+ CD25+ Foxp3+ Treg cells. In panel B, the absolute number of Treg was analyzed at different time points after initiation of PegINFα treatment. Panels C-D-E show the absolute number of Ki-67+ Treg, Helios+ Treg, and CD39+/HLA-DR+ Treg.
40164_2016_57_MOESM3_ESM.pdf
Additional file 3: FigS3. Changes in numbers of Treg subsets in Peginfa treated patients based on molecular response. Patients treated with PegINFα were separated into R and NR subsets based on a 20% decrease in JAK2 V617 allelic burden. PBMC were analyzed as described above and fold changes compare to baseline were examined. Panel A represents 23 patient samples (10R and 13NR), panels B-C include 16 patients (6 R and 10 NR), and panel D includes 13 patients (4R and 9NR). A-C show the number of Treg, of dividing Treg, of CD39+ HLA-DR+ Treg and Helios+ Treg. Panel D shows CD38+ HLA-DR+ T cells in R vs NR.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kovacsovics-Bankowski, M., Kelley, T.W., Efimova, O. et al. Changes in peripheral blood lymphocytes in polycythemia vera and essential thrombocythemia patients treated with pegylated-interferon alpha and correlation with JAK2 V617F allelic burden. Exp Hematol Oncol 5, 28 (2015). https://doi.org/10.1186/s40164-016-0057-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-016-0057-y