Abstract
Background
Ginkgo biloba extract (GBE) is evidenced to be effective in the prevention and alleviation of metabolic disorders, including obesity, diabetes and fatty liver disease. However, the role of GBE in alleviating fatty liver hemorrhagic syndrome (FLHS) in laying hens and the underlying mechanisms remain to be elucidated. Here, we investigated the effects of GBE on relieving FLHS with an emphasis on the modulatory role of GBE in chicken gut microbiota.
Results
The results showed that GBE treatment ameliorated biochemical blood indicators in high-fat diet (HFD)-induced FLHS laying hen model by decreasing the levels of TG, TC, ALT and ALP. The lipid accumulation and pathological score of liver were also relieved after GBE treatment. Moreover, GBE treatment enhanced the antioxidant activity of liver and serum by increasing GSH, SOD, T-AOC, GSH-PX and reducing MDA, and downregulated the expression of genes related to lipid synthesis (FAS, LXRα, GPAT1, PPARγ and ChREBP1) and inflammatory cytokines (TNF-α, IL-6, TLR4 and NF-κB) in the liver. Microbial profiling analysis revealed that GBE treatment reshaped the HFD-perturbed gut microbiota, particularly elevated the abundance of Megasphaera in the cecum. Meanwhile, targeted metabolomic analysis of SCFAs revealed that GBE treatment significantly promoted the production of total SCFAs, acetate and propionate, which were positively correlated with the GBE-enriched gut microbiota. Finally, we confirmed that the GBE-altered gut microbiota was sufficient to alleviate FLHS by fecal microbiota transplantation (FMT).
Conclusions
We provided evidence that GBE alleviated FLHS in HFD-induced laying hens through reshaping the composition of gut microbiota. Our findings shed light on mechanism underlying the anti-FLHS efficacy of GBE and lay foundations for future use of GBE as additive to prevent and control FLHS in laying hen industry.
Similar content being viewed by others
Background
Fatty liver hemorrhagic syndrome (FLHS), a kind of nutritional metabolic disease in laying hens, is characterized by excessive accumulation of hepatic and abdominal fat, hepatic steatosis, liver rupture, inflammatory response and hepatorrhagia [1, 2]. FLHS frequently occurs in commercial caged high-production and over conditioned laying hens, accounting for 40%–70% of hen mortality [3]. Moreover, FLHS can cause sharp drop of egg production rate and a shortened egg production peak in hen flocks. Therefore, FLHS has been regarded as a “silent killer” for both laying hens and producers [1]. The pathogenesis of FLHS is poorly understood but has a great similarity to metabolic dysfunction-associated fatty liver disease (MAFLD) in human beings [4], and the common pathologic feature of FLHS and MAFLD is excess hepatic lipid deposition. It is suggested that the FLHS laying hen can be used as an ideal animal model for investigating MAFLD and hepatic steatosis in humans [4,5,6].
Studies have shown that dietary ingredients, stress, hormones, genetics, environment, inflammation, lipid metabolism disorder, oxidative stress, autophagy, gut microbiota and other pathogenic factors are related to FLHS [7]. Recently, the importance of gut microbiota in the progression of FLHS has been widely concerned [2, 4]. It is indicated that FLHS was linked with the laying hen cecal microbial dysbiosis, and the change in the abundances of Firmicutes and Bacteroidetes was closely associated with the severity of fibrosis and nonalcoholic steatohepatitis [4]. There are also studies showed that the non-hepatic steatosis group had more beneficial bacteria, while the hepatic steatosis group had more harmful bacteria [2, 8]. Additionally, treatment with Akkermansia muciniphila or the decoction of Abrus cantoniensis Hance efficiently reshaped the high-fat diet (HFD)-perturbed intestinal microbiota and thus exerted anti-FLHS effects in laying hens [2, 9]. The changes of gut microbiota were also found related to the host oxidative stress and inflammatory response, both of which are regarded as factors that contribute to the development and progression of FLHS. A recent study showed that the interaction between intestinal epithelial cells and some gut commensal bacteria induces rapid production of reactive oxygen species (ROS) in host cells [10], and the mechanism by which ROS promotes the progression of MAFLD may be related to abnormal oxidative stress markers and dysregulated redox signaling [11]. Interestingly, evidence suggest that alterations of gut microbiota regulate the metabolism of the major intracelluar antioxidant (glutathione (GSH)) in the host organism [12], as well as affect Nox2 (oxidative stress markers) activation and redox signaling [10, 13]. As for inflammation, studies have shown that the altered gut microbiota (e.g., reduction of bifidobacteria) cause an increase in plasma lipopolysaccharide levels, which lead to increases in proinflammatory cytokines [14]. However, the use of probiotic bacteria alleviates liver inflammation by inhibiting the production of pro-inflammatory cytokines and improving liver function [15, 16]. Additionally, microbiota-derived metabolites (e.g., short-chain fatty acids (SCFAs)) are evidenced to have anti-MAFLD properties. For example, Astragalus polysaccharides-enriched Desulfovibrio vulgaris attenuates MAFLD via generating acetic acid [17]. Therefore, reconstructing the micro-ecological system via prebiotics, probiotics, or microbial products is a promising method to attenuate FLHS [18].
Ginkgo biloba extract (GBE) mainly contains 24% flavonoids glycosides (quercetin, kaempferol, isorhamnetin etc.), 6% terpenoids (in which 3.1% are ginkgolides A, B, C and J and 2.9% is bilobalide), and 5%–10% organic acids [19]. GBE is a traditional Chinese medicine known for its various biological properties, such as antineoplastic, hepatoprotective, vasodilatory, antiedematogenic, antioxidant, anti-inflammatory, antiplatelet, immune modulation and metabolic regulation [20, 21]. Flavonoids and terpenoids are suggested to be the pharmacologically active components of GBE [22, 23]. Considerable evidence has shown that GBE is effective in the prevention and alleviation of atherosclerosis [24], cardiovascular diseases [25, 26], obesity [27, 28], MAFLD [29], neurological diseases [30], among others. Although the beneficial role of GBE can be partially attributed to its antioxidant activity, recent findings strongly suggested that GBE and its components can interact with gut microbiota to perform biological functions. For example, GBE has been shown to ameliorate hypercholesterolemia, systemic inflammation and atherosclerosis, and these effects are associated with rebalancing gut flora and microbial metabolism [24]. Another study showed that polysaccharide from Ginkgo biloba leaves reduced stress-induced depression and reversed depression-associated gut dysbiosis while increasing the abundance of Lactobacillus species [30]. Additionally, bilobalide is an extract of Ginkgo biloba that has been shown to attenuate the severity of ulcerative colitis by inhibiting inflammation, promoting the repair of the intestinal epithelial barrier and remodeling the intestinal microbial communities, especially by promoting the proliferation of probiotic species including Lactobacillus [31]. Similarly, supplementation with kaempferol, a flavonoid, offset the imbalance of intestinal flora associated with obesity and inflammatory [32].
Thus far, limited evidence has been provided on the role of GBE in alleviating FLHS in laying hens, and whether and how GBE modulates the chicken gut microbiota to exert the anti-FLHS effect are still needed to be answered. In the current study, we utilized HFD induced FLHS laying hens as a model to determine the effect of GBE on attenuating FLHS. We further performed FMT experiment to investigate the protective effect of the GBE-altered gut microbiota on FLHS. We demonstrated that GBE effectively alleviated FLHS in the HFD-induced laying hen model, and this effect could be largely due to the influence of GBE on the laying hen gut microbiota structure and function. Our study confirmed the therapeutic potential of GBE for FLHS in laying hens, and revealed the underlying gut microbiota-mediated mechanism.
Methods
Animals and experimental design
Ethics statement
All animal experimental procedures were conducted according to guidelines of the Institutional Studies Animal Care and Use Committee of China Agricultural University (Beijing, China). The study was approved by the Ethics Committees of Laboratory Animal Center of China Agricultural University (Beijing, China) (permit AW13501202-2–2).
Chicken raising
HY-Line gray commercial laying hens were purchased and housed in Zhuozhou breeding base of China Agricultural University (Zhuozhou, China). All hens were housed in stainless-steel ladder cages (45 cm × 45 cm × 45 cm) and in a controlled room, in which temperature and relative humidity was maintained at 26 ± 2 °C and 50% ± 10%, respectively. The photoperiod was a 16 h light/8 h dark with a dark cycle encompassing 22:00 to 6:00. All the birds were allowed free access to water and food. All the birds were subjected to an acclimatization period of one week before formal test, during which they were fed a normal diet (ND), and meet the NRC (1994) [33] and the management manual of Hy-Line gray laying hens’ recommendations (https://www.hyline.com/literature/sonia). The ingredients and nutrient levels of ND are presented in Table 1.
Exp. 1: Effect of GBE on FLHS laying hens induced by HFD
A total of 135 24-week-old Hy-Line gray commercial laying hens of similar weight were raised in ladder cages with three bird each cage. The hens were then randomly divided into three groups, with 15 replicates per group and three hens per replicate. The control group continued receiving ND while the model group was fed high-energy low-protein diet (HFD) throughout the experiment to induce FLHS [2, 34]. Diet compositions and nutrient levels of HFD are shown in Table 1. In addition, on the basis of the HFD, GBE was supplemented at the concentration of 1,200 mg/kg diet. GBE was purchased from Xuzhou Hengkai Ginkgo Products Co., Ltd. (Xuzhou, China). GBE were first mixed with the premix and then mixed with other ingredients by the principle of step-by-step premixing and stored at 4 °C before feeding. Diets were prepared every two weeks during the experiment to ensure the quality of diets. All diets were prepared in the feed laboratory of Zhuozhou breeding base of China Agricultural University. The formal experimental period was from 25 to 40 weeks of age (total 16 weeks).
The day before the end of the experiment, all laying hens were fasted for 8 h, but water was offered ad libitum. Then ten laying hens (one hen per cage) were randomly selected and marked from 15 replicates for each treatment group, and blood was taken aseptically from the wing vein using 5-mL vacuum blood tubes. After the serum was separated naturally, it was centrifuged at 3,000 × g for 10 min to separate out the serum. Pure serum samples were aspirated by pipette, stored in 1.5-mL centrifuge tubes at −80 °C for subsequent test. At the end of the experiment, marked laying hens were euthanized via cutting the jugular vein. The liver was imaged and weighed, and approximately 1 cm of liver tissue was taken and fixed in 4% paraformaldehyde for histopathological evaluation. Three small pieces of liver tissue were taken, divided them into three centrifuge tubes, snap-frozen in liquid nitrogen and stored at −80 °C. One tube is used to measure the fat content of the liver, one tube is used to measure the antioxidant activity of the liver, and the other tube is used for mRNA analysis. The heart and abdominal fat were weighed. Cecal and ileal contents were immediately transferred to liquid nitrogen and kept in a −80 °C freezer for further analysis.
Exp. 2: Fecal microbiota transplantation
Preparation of fecal bacterial suspension for FMT
We prepared fecal bacterial suspensions as previously described [35,36,37]. Fresh feces from the hens of HFD_GBE group were immediately collected and immersed in liquid nitrogen within 2 h after defecation. Then, the frozen feces were transported on ice to a laboratory for preparation of fecal bacteria suspension. Briefly, 50 g fecal samples are diluted with 250 mL sterile saline and homogenized by hot plate magnetic stirrer (IT-09A5, Shanghai Yiheng Technology Co., Ltd., Shanghai, China). Then, the slurry was filtered one by one through stainless steel sieves of 2, 1, 0.5, 0.25 mmol/L to eliminate the undigested and small particulate matter in the fecal suspension. Next, the supernatant was removed after the fecal suspension was centrifuged with centrifuge (5804R, Eppendorf, Hamburg, Germany) at 6,000 × g for 15 min. We resuspended the precipitate in sterile saline containing 10% glycerol, and finally stored at −80 °C for subsequent FMT experiment. Before FMT, the frozen fecal suspension was thawed in a water bath at 37 °C. Upon frozen fecal suspension thawing, it was diluted with sterile saline to 1 × 109 colony forming units (CFU) per milliliter and fecal suspension was gavaged to receiver hens as soon as possible at room temperature.
Experimental design
Fourteen 24-week-old Hy-Line gray commercial laying hens were selected and fed HFD for 16 weeks to induce FLHS after an adaptation period of one week, and then were randomly divided into two groups, with 7 replicates per group and one hen per replicate. The hens were raised in ladder cages with one bird per cage and continued to receive HFD. All the hens were treated for 7 d with a antibiotics mixture of neomycin (1 g/L; N6386, Sigma-Aldrich, Saint Louis, USA), ampicillin (1 g/L; A5354, Sigma-Aldrich, Saint Louis, USA), metronidazole (1 g/L; M3761, Sigma-Aldrich, Saint Louis, USA) and vancomycin (0.5 g/L; SBR00001, Sigma-Aldrich, Saint Louis, USA) in their drinking water [38], and then a 4-d washout period ensured elimination of the residual antibiotics as previously described [39]. Then, hens of HFD_GBE-FMT group (n = 7) were administered 5 mL of fecal bacteria suspension (1 × 109 CFU/mL), and hens of CON group (n = 7) were given an equivalent volume of sterile saline by oral gavage each day at 2-week intervals until 8 weeks.
The day before the end of the experiment, all laying hens were fasted for 8 h, but water was offered ad libitum. Then all laying hens were weighed, and blood samples were collected from the wing vein. The blood was centrifuged at 3,000 × g for 10 min at 4 °C and the serum was collected and stored at −80 °C. At the end of the experiment, all laying hens were euthanized via cutting the jugular vein. The liver was imaged and weighed, and approximately 1 cm of liver tissue was taken and fixed in 4% paraformaldehyde for histopathological evaluation. Three small pieces of liver tissue were taken, divided them into three centrifuge tubes, snap-frozen in liquid nitrogen and stored at −80 °C. One tube is used to measure the fat content of the liver, one tube is used to measure the antioxidant activity of the liver, and the other tube is used for mRNA analysis. The heart and abdominal fat were weighed.
Serum biochemistry analysis
Levels of serum glucose (GLU), triglycerides (TG), total cholesterol (TC, T-CHO), alanine transaminase (ALT), aspartate transaminase (AST) and alkaline phosphatase (ALP) were measured by the automatic biochemical analyzer (KHB ZY-1280, Shanghai Kehua Bio-engineering Co., Ltd., China) as instructed by the manufacturer.
Measurement of hepatic lipid contents
Hepatic lipids were extracted according to Folch method [40] with slight modifications. Briefly, 50 mg of liver tissue was weighted and homogenized in 1 mL of chloroform:methanol (2:1) before agitation at 50 Hz for 30 s with refrigerated high-throughput tissue grinder (SCIENTZ-48L, Ningbo SCIENTZ Biotechnology Co., Ltd., China). 200 µL of distilled water was added and vortex to mix, following by centrifugation at 4,000 × g for 10 min and the lower solvent layer was extracted and then dried with a centrifugal vacuum concentrator (CV200, Beijing JM Technology Co., Ltd., China). The residue was resuspended with an equivalent volume of PBS supplemented with 5% Triton X-100 as a total lipid extract sample. The contents of TG and TC in liver were assayed by using commercial kits based on manufacturer’s guidelines (Biosino Biotechnolgy and Science Inc., Beijing, China).
Histopathological evaluation
Fresh samples of liver were resected and fixed with 4% paraformaldehyde at room temperature. The samples were dehydrated and embedded in paraffin, and then sectioned and further stained with hematoxylin–eosin staining (HE). Images were obtained using a LIOO 3.7 for digital camera software (Leica DM750, Leica Biosystems, Nussloch, Germany). The non-alcoholic fatty liver disease (NAFLD) activity score (NAS) of pathological sections of liver tissues, a semi-quantitative evaluation, was conducted based on the levels of hepatocyte steatosis, inflammation within the lobules and ballooning degeneration of hepatocyte [41].
Measurement of serum and liver antioxidant indexes
0.2 g of liver tissue was weighted and homogenized in 2 mL of physiological saline, following by centrifugation at 3,500 × g for 15 min and the lower solvent layer was obtained as liver homogenate. Then, the BCA protein concentration assay kit (P0012 BCA, Beyotime Biotechnology, Nantong, Jiangsu, China) was used to detect the protein content in liver homogenate. The contents of malondialdehyde (MDA), superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), GSH and glutathione peroxidase (GSH-Px) in serum and liver homogenate were determined with microplate reader (Epoch 2, BioTek Instruments, Inc., Winooski, Vermont, USA) by using commercial kits based on manufacturer’s guidelines (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Total RNA isolation and real-time PCR
Total RNA from tissues or cells were isolated using the TRIzol method, and then used to synthesize the first-strand cDNA with the BeyoRT™ II cDNA Synthesis Kit with gDNA Eraser (D7170M, Beyotime Biotechnology, Nantong, Jiangsu, China). Real-time quantitative polymerase chain reaction (RT-qPCR) was carried out with BeyoFast™ SYBR Green qPCR Mix (2X, Low ROX) (D7262, Beyotime Biotechnology, Nantong, Jiangsu, China) on an Applied Biosystems® QuantStudio™ 7 Flex Real-Time PCR System (QuantStudio7 Flex, Thermo Fisher Scientific, Waltham, USA). The results were analyzed using the 2−ΔΔCt method with β-actin serving as the reference. The primers used were synthesized by Sangon Biotech (Shanghai, China), and listed in Table 2.
16S rRNA gene sequencing and analysis
Bacterial genomic DNA was extracted from cecal content or ileal content of animals with a QIAamp Fast DNA stool Mini Kit (51604, Qiagen, Dusseldorf, North Rhine-Westphalia, Germany) for subsequent 16S rRNA gene sequencing. Qualified DNA samples were purified and amplified using specific bacterial primers targeting the variable region V3–V4 of the 16S rRNA gene, and amplification primers were 341F (5′-CCTACGGGNBGCASCAG-3′) and 805R (5′-GACTACNVGGGTATCTAATCC-3′) [42]. Purified amplicons were pooled in equimolar and then paired-end sequencing was performed on an Illumina Hiseq 2500 sequencing platform at the Institute of Microbiology, Chinese Academy of Sciences (Beijing, China) according to standard protocols. The generated raw fastq files were quality-filtered and taxonomically analyzed using QIIME2 (V2019.7) [43]. In brief, primers of imported sequences were removed via Cutadapt (V2.4) [44], and then DADA2 was used to filter and denoise sequences, remove chimeras, infer amplicon sequence variants (ASVs) and generate the abundance table [45]. The ASVs were clustered with 100% similarity, and species annotation was performed for each ASV using the Naive Bayes classifier method based on the SILVA database [46]. Alpha diversity of each sample was assessed using the vegan package for R to assess bacterial richness and evenness. The weighted Unifrac based principal co-ordinates (PCoA) analysis was conducted to reflect differences in community structure of gut microbiota in each group, ANOSIM analysis was used to evaluate the statistical differences among groups.
GC–MS analysis of SCFAs
Targeted metabolomics was performed with GC–MS to quantify the concentrations of SCFAs in cecal content. Briefly, for cecal content samples, the extraction procedures have been carried out 4 °C to protect the volatile SCFAs. 50 mg cecal content were diluted with distilled water (4 × the volume), tubes were homogenized and centrifuged at 12,000 × g for 15 min at 4 °C. Following centrifugation supernatants were transferred into centrifuge tubes for the derivatization reaction. 200 µL of 25% metaphosphoric acid solution was added to the supernatants, the solution was kept standing for 0.5 h with an environmental temperature of 4 °C, and then centrifuged at 12,000 × g for 10 min at 4 °C. Next, transfered the obtained supernatant to glass bottles and sent to the Institute of Animal Sciences of Chinese Academy of Agricultural Sciences at a low temperature for SCFAs determination using GC-MS (Agilent 5975C, Santa Clara, CA, USA). The concentration gradient of standard curve was 0 to 100 μg/mL for low concentration and 0 to 600 μg/mL for high concentration. SCFAs levels in the cecal content were normalized to the weight.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA) and data are shown as means ± SEM. Significant differences between the two groups were assessed by unpaired two-tailed Student’s t-test or Mann–Whitney U test for samples that were not normally distributed. Data sets involving more than two groups were evaluated by one-way analysis of variance (ANOVA) followed by the Least Significant Difference (LSD) or by the non-parametric Kruskal–Wallis test with IBM SPSS Statistics 24.0 (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
GBE supplementation ameliorates the HFD-induced FLHS in laying hens
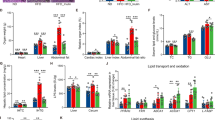
To evaluate the therapeutic effects of GBE on FLHS, we treated HFD-induced laying hens with 0.12% GBE for a period of 16 weeks. The body weight, heart weight, liver weight, abdominal fat weight and abdominal fat ratio of the laying hens in the HFD group were significantly elevated compared with the ND group, but GBE treatment effectively decreased these indexes compared with the HFD group (Fig. 1A–C). According to liver tissue H&E staining and phenotype pictures, FLHS laying hens exhibited severe hepatic steatosis with yellowish and larger liver, a great number of fat vacuoles and higher liver NAS score, which was alleviated after GBE treatment (Fig. 1D–E). Additionally, FLHS laying hens showed a significant increase in the concentrations of TC and GLU in the serum and intrahepatic lipid contents (IHTG and IHTC). In contrast, GBE intervention obviously masked off the effects of the HFD on these disturbed metabolic parameters in FLHS laying hens, implying its regulative effects on hepatic lipid metabolism (Fig. 1F–G). ALT, AST and ALP activities in serum are often used as reliable indicators for assessing liver damage. When the hepatocytes are damaged, these enzymes enter the circulatory system from the hepatocytes, thereby increasing the enzyme activities in the serum [47]. Notably, these enzymes were significantly increased in HFD group compared with the ND group, while the HFD_GBE group showed significant improvement after GBE intervention (Fig. 1H). Overall, the above results indicate the therapeutical effects of GBE on the pathological progression of FLHS.
GBE relieves HFD-induced FLHS in laying hens. A Body weight. B Organ weight. C Organ index (%, organ weight (g)/body weight (g) × 100%). D Representative photomicrographs of liver tissues with H&E staining and phenotype pictures. E Assessment of non-alcoholic fatty liver disease (NAFLD) activity score (NAS) of liver tissues based on histological sections. F Serum lipid and glucose levels. G Hepatic lipid levels. H Serum liver function index. Data are presented as the mean ± SEM; n = 10 hens per group. Statistical analysis was performed using one-way ANOVA followed by the LSD. *P < 0.05, **P < 0.01, ***P < 0.001
Effects of GBE on antioxidant activity of FLHS laying hens
Studies have pointed out that GBE exerts protective and antioxidative effects by up-regulating the expression of SOD and GSH which in turn inhibits the synthesis of ROS [48, 49], we thereby measured the antioxidant activity in liver and serum of laying hens. Compared with the ND group, the hepatic GSH, SOD, T-AOC and GSH-PX activities of laying hens in HFD group were decreased, and the hepatic MDA contents were significantly increased. Whereas GBE treatment significantly enhanced the activities of GSH, SOD, T-AOC and GSH-PX, and decreased the activity of MDA. Interestingly, similar results were also found in serum (Fig. 2A–E). GBE caused increase in the level of SOD and T-AOC, and reduction of 74.2% in the level of MDA (Fig. 2F–I). Thus, GBE improves the antioxidant capacity of HFD-induced FLHS laying hens.
Effect of GBE on antioxidant indices in liver and serum. A The reduced GSH content of liver tissues. B The SOD activity in liver tissues. C The T-AOC of liver tissues. D The MDA content of liver tissues. E The GSH-PX activity in liver tissues. F The reduced GSH content of serum. G The SOD activity in serum. H The T-AOC of serum. I The MDA content of serum. Data are presented as the mean ± SEM; n = 10 hens per group. Statistical analysis was performed using one-way ANOVA followed by the LSD. *P < 0.05, **P < 0.01, ***P < 0.001
GBE decreased gene expression of lipid synthesis and inflammatory cytokines in liver of FLHS laying hens
To further reveal the mechanism of GBE in alleviating FLHS, we measured the related genes expression of de novo lipogenesis, the lipid beta-oxidation, and the inflammatory cytokine from the liver in the FLHS laying hens with or without GBE treatment. Compared with the ND group, the HFD group significantly increased the expression levels of genes related to lipid synthesis, such as fatty acid synthase (FAS), liver X receptor alpha (LXRα), peroxisome proliferator-activated receptor gamma (PPARγ) and carbohydrate response element-binding protein 1 (ChREBP1) (Fig. 3A). The intake of HFD also significantly up-regulated the expression level of genes related to lipid transport and oxidation, such as acyl-CoA oxidase 1 (ACOX1), peroxisome proliferator-activated receptor alpha (PPARα), liver fatty acid binding protein (L-FABP) and farnesoid X receptor (FXR) (Fig. 3B). In addition to the excessive accumulation of lipids in livers, steatohepatitis is also an important pathological feature of FLHS. HFD increased the expression levels of genes related to inflammatory cytokine, such as tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), toll-like receptors 4 (TLR4) and nuclear factor kappa-B (NF-κB) (Fig. 3B). After GBE intervention, the treated group displayed markedly reduced expression levels of genes related to lipid synthesis, such as FAS, LXRα, glycerol-3-phosphate acyltransferase1 (GPAT1), PPARγ and ChREBP1 (Fig. 3A), and also significantly downregulated expression levels of several inflammatory cytokine markers (TNF-α, IL-6, TLR4 and NF-κB) (Fig. 3C), despite no effect on the expression of lipid transport and oxidation related genes compared with the HFD group (Fig. 3B). These data support the effectiveness of GBE in attenuating FLHS by reducing the expression of genes related to lipid synthesis and inflammatory cytokines in the liver.
Effects of GBE supplementation on the expression of genes related to lipid metabolism and inflammatory factors in the liver of laying hens. The relative mRNA expression of lipid synthesis (A), lipid transport and oxidation (B) and inflammatory cytokines (C) related genes in liver tissues. Data are presented as the mean ± SEM; n = 10 hens per group. Statistical analysis was performed using one-way ANOVA followed by the LSD or non-parametric Kruskal–Wallis test. *P < 0 .05, **P < 0.01, ***P < 0.001
The anti-FLHS effect of GBE is associated with gut microbiota changes
To assess the effect of GBE on the gut microbiota of FLHS laying hens, we performed 16S rRNA gene sequencing on the cecal and ileal contents of laying hens. We found that GBE had no significant influence on α-diversity (as analysed by the Shannon index) and β-diversity (as determined by PCoA analysis) of ileal microbiota (Fig. 4A and B), and no differentially abundant taxa were observed at the genus levels (Fig. 4C). In contrast, both the Shannon analysis and principal coordinate analysis (PCoA) showed significant differences of the cecal microbiota among the three groups (Fig. 5A and B). The co-occurrence network analysis based on the relative abundance of genus showed that there were 65 nodes and 231 edges in the HFD group, while ND group had 76 nodes and 190 edges, indicating that bacterial network of the HFD group is more complex than that of the ND group. Moreover. The core genera of the ND group and the HFD group are contained in 7 phyla and 8 phyla, respectively. Notably, HFD_GBE group contains only 58 nodes, 110 edges and 5 phyla, indicating GBE intervention reversed the complex bacterial network caused by HFD (Fig. 5C–E). Subsequent linear discriminant analysis (LDA) effect size (LEfSe) results revealed obvious differences in the cecal bacterial communities between different groups. At the genus level, compared with the ND group, HFD reduced several beneficial bacteria, such as Butyricicoccus, Enterococcus, Flavonifractor, Faecalicoccus, Parabacteroides and Megasphaera, but enhanced Intestinimonas, Ruminococcaceae UCG-005, CHKCI001, unclassified Lachnospiraceae and Lactobacillus (Fig. 5F). After GBE intervention, the abundances of uncultured rumen bacterium, unclassified Tannerellaceae, Megasphaera, and uncultured Muribaculaceae were significantly enriched, but the abundances of Ruminococcaceae UCG-005, Anaerostipes, Fusobacterium and Christensenellaceae R-7 group, etc. were significantly reduced (Fig. 5G). Further correlation analysis found that GBE-enriched bacteria had a significant negative correlation with the markers of FLHS and a significant positive correlation with antioxidant capacity, while HFD-enriched bacteria showed the opposite results (Fig. 5H). Notably, Megasphaera was the only genus that was significantly reduced by HFD but rescued by GBE (Fig. 5F and G), which was significantly negatively correlated with different FLHS markers including abdominal fat weight and ratio, ALT, IHTG, IHTC and serum TG (Fig. 5H). Collectively, these data suggested that GBE may be involved in the alleviation of FLHS through modulating cecal microbiota rather than ileal microbiota, especially the abundance of Megasphaera in the cecum.
GBE does not alter ileal microbial diversity and composition of FHLS laying hens. A Alpha diversity of ileal microbiota in different groups. Data were analyzed by nonparametric Kruskal–Wallis test. B Principal coordinate analysis (PCoA) of ileal microbiota in different groups. PCoA plots were generated using OUT abundance data according to the Bray–Curtis distance, and statistical significance was measured using adonis analysis. C Relative abundance of top 20 genera in each sample. n = 10 hens per group
The impacts of GBE on cecal microbiota of HFD-induced FLHS laying hens. A The alpha diversity of cecal microbiota in different groups. Data were analyzed by nonparametric Kruskal–Wallis test. B Principal coordinate analysis (PCoA) of cecal microbiota in different groups. PCoA plots were generated using OUT abundance data according to the Bray–Curtis distance, and statistical significance was measured using adonis analysis. C–E Gut microbial co-occurrence network analysis based on core genus (average relative abundance > 0.1% in the ND, HFD and HFD_GBE groups). Different colors of nodes indicate different phylum levels. Edges represent significant correlations (P < 0.05). F–G Differences in the bacterial communities between different groups at the genus level. Significant differences in Linear discriminant analysis (LDA) scores (P < 0.05) were produced between groups (Wilcoxon’s test). The threshold of the logarithmic LDA score was 2.0. H Spearman’s correlation analysis between related indicators of metabolic disorders and cecal microbes at the genus level: *P < 0.05, **P < 0.01, ***P < 0.001. Red represents positive correlations and blue represents negative correlations. n = 10 hens per group
GBE increases the cecal SCFAs concentration
The microbiota-derived SCFAs play an important role in alleviating MAFLD [17]. We observed that the HFD feeding led to a total reduction in SCFAs levels of cecal contents, especially decreased the concentrations of acetate and propionate (Fig. 6A). However, GBE treatment remarkably elevated the total SCFAs, acetate and propionate levels compared with the HFD group. Correlation analysis between the SCFAs concentration and the differentially abundant microbes revealed that uncultured rumen bacterium, Megasphaera and uncultured Muribaculaceae enriched in the HFD_GBE group and Faecalicoccus, Ruminococcaceae UCG-013, unclassified Bacteroidales and uncultured Ruminococcaceae enriched in the ND group were significantly positively correlated with the SCFAs concentration (Fig. 6B). In contrast, Lactobacillus, CHKCI001, uncultured Barnesiellaceae, Christensenellaceae R-7 group and uncultured Lachnospiraceae enriched in the HFD group were significantly negatively correlated with the SCFAs concentration (Fig. 6B). These results demonstrated the ability of GBE to promote the growth of SCFAs-producing bacteria.
Targeted metabolomic analyses of SCFAs levels using GC–MS in laying hens. A SCFAs concentration in cecal contents of laying hens. B Correlation analysis of SCFAs concentration and the differentially abundant bacteria. Red represents positive correlations and blue represents negative correlations. *P < 0.05, **P < 0.01, ***P < 0.001. n = 10 hens per group
FMT attenuates HFD-induced FLHS in laying hens
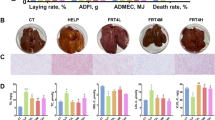
To investigate whether GBE-altered gut microbiota had therapeutic effects on FLHS, we performed a FMT experiment. The results showed that HFD-induced FLHS laying hens orally treated with fecal bacterial suspension from GBE-treated donors significantly decreased the body weight, heart weight, abdominal fat weight and abdominal fat ratio (Fig. 7A–C). Notably, FMT showed apparent decrease in size and conspicuous color change toward to fuchsia from yellowish relative to the model group, and effectively reduced the fat vacuoles number in liver of FLHS laying hens (Fig. 7D). The liver NAS score in the FMT group was significantly lower than the model group (Fig. 7E). Additionally, FMT exhibited little improvements in serum TC and GLU, but significantly decreased lipid deposition (as indicated by serum TG, IHTC and IHTG) and liver injury (Fig. 7F–H). These findings further support the notion that GBE alleviates FLHS in laying hens is mediated by gut microbes.
FMT attenuates HFD-induced FLHS in laying hens. A Body weight. B Organ weight. C Organ index. D Representative photomicrographs of liver tissues with H&E staining and phenotype pictures. E Assessment of non-alcoholic fatty liver disease (NAFLD) activity score (NAS) of liver tissues based on histological sections. F Serum lipid and glucose levels. G Hepatic lipid levels. H Serum liver function index. Data are presented as the mean ± SEM; n = 7 hens per group. Statistical analysis was performed using independent-samples t test. *P < 0 .05, **P < 0.01, ***P < 0.001
Effect of FMT on antioxidant activity in liver and serum of FLHS laying hens
FMT significantly decreased the activities of MDA in liver of FLHS laying hens, and the hepatic GSH, SOD and T-AOC activities were significantly increased by 44.83%, 20.95%, and 172.55% compared with those in the model group, respectively (Fig. 8A–E). Likewise, FMT significantly reversed the effects of HFD on serum SOD, T-AOC and MDA activities (Fig. 8F–I). Overall, FMT enhances the antioxidant capacity of FLHS laying hens.
Effect of FMT on antioxidant activity in liver and serum. A The reduced GSH content of liver tissues. B The SOD activity in liver tissues. C The T-AOC of liver tissues. D The MDA content of liver tissues. E The GSH-PX activity in liver tissues. F The reduced GSH content of serum. G The SOD activity in serum. H The T-AOC of serum. I The MDA content of serum. Data are presented as the mean ± SEM; n = 7 hens per group. Statistical analysis was performed using independent-samples t test. *P < 0 .05, **P < 0.01, ***P < 0.001
Effect of FMT on gene expression of lipid metabolism and inflammatory factors in liver of FLHS laying hens
qPCR analysis of the liver of FLHS laying hens revealed that FMT significantly decreased the expression levels of genes related to lipid synthesis, such as FAS, LXRα, GPAT1, PPARγ, SREBP-1c, SCD1 and ChREBP1 (Fig. 9A), and increased the expression levels of genes related to inflammatory cytokine, such as TNF-α, IL-6, TLR4 and NF-κB (Fig. 9C); however, no effect was observed on the expression levels of genes related to lipid transport and oxidation (Fig. 9B). Altogether, FMT inhibited lipid synthesis and inflammatory responses of FLHS laying hens.
Effects of FMT on the expression of genes related to lipid metabolism and inflammatory factors in the liver of FLHS laying hens. The relative mRNA expression of lipid synthesis (A), lipid transport and oxidation (B) and inflammatory cytokines (C) related genes in liver tissues. Data are presented as the mean ± SEM; n = 7 hens per group. Statistical analysis was performed using independent-samples t test or non-parametric Mann-Whitney U test. *P < 0 .05, **P < 0.01, ***P < 0.001
Discussion
As a natural antioxidant, GBE has been reported to be beneficial to health by helping the body resist harmful oxidative stress and reducing the infiltration of inflammatory cells and inflammatory cytokines [20, 49]. Currently, GBE is widely used in the alleviation of metabolic diseases such as obesity and MAFLD due to its excellent lipid-lowering effect [27,28,29], while the pharmacological mechanism of GBE on anti-FLHS remains unelucidated. In the current study, we found that the anti-FLHS effect of GBE was associated with the reshaping of the chicken gut microbiota.
Our study showed that FLHS laying hens with GBE intervention eliminated hepatic steatosis, inflammation and liver injury, and increased antioxidant activity. These therapeutic effects largely meet the recommended criteria for the treatment of MAFLD [50]. Interestingly, the anti-FLHS function of GBE in FLHS laying hens was transferred through FMT to new receivers, which further demonstrated that the anti-FLHS effects of GBE was associated with its modulation on gut microbiota. Our study provided new clues in explaining the metabolic benefits of GBE.
In this study, the disorder of lipid metabolism in FLHS model was effectively reversed by supplementing GBE, which was consistent with the fact that GBE could inhibit lipid accumulation in the liver of obese rats reported previously [28, 51]. FAS, SREBP-1c, GPAT, PPARγ, LXRα, SCD1 and ChREBP1 are key genes involved in de novo lipid synthesis. FAS is a key enzyme that catalyzes fatty acid synthesis [52]. SREBPs is an important transcription factor that regulates the biosynthesis and uptake of cholesterol and fatty acids [53]. GPAT1 is the rate-limiting enzyme for the synthesis of glycerophospholipids and triglycerides [54]. PPARγ mainly promotes lipogenesis and preadipocyte differentiation [55] and its upregulation contributes to hepatic steatosis [56]. LXRα, as a ligand-dependent nuclear receptor, which can form a positive feedback loop with SREBP-1c and enhance lipogenesis in the liver and directly activate target genes ACC, FAS and SCD1 by the binding of LXREs to their promoter regions [2, 57]. In the present study, GBE inhibited lipid synthesis by significantly downregulated the gene expression of hepatic FAS, LXRα, GPAT1, PPARγ and ChREBP1, thus alleviating the fat accumulation in FLHS laying hens.
Fatty acid accumulation is not only a characteristic of FLHS, but also causes insulin resistance, lipid peroxidation, hepatic damage, inflammatory response, and energy metabolism disorders [58]. A study found that excessive deposition of palmitic acid in the liver can activate NF-κB and cause an inflammatory response [59]. TLR4 is expressed in hepatic stellate cells (HSCs), where it triggers an inflammation-like response with NF-κB activation and further NF-κB-dependent gene expression [60]. TNF-α is the most prominent “first-line” cytokines [61], which stimulates and induces ROS production and lipid peroxidation, and it also activates oxidative stress response genes which amplify and prolong inflammation [62]. Meanwhile, Oxygen free radicals and TNF-α released during inflammation could also activate and induce NF-κB, and then upregulate the expression of TNF-α, involved in immune and inflammatory responses [57, 63]. Of note, TNF-α can induce the release of IL-6 from Kupffer cells in the liver, which hinders the transport and secretion of TG [64]. On the other hand, elevated TNF-α can lead to hepatic TG accumulation and steatosis, which in turn activates NF-κB, thus form a vicious cycle of aggravating liver damage [65]. Our results showed that GBE supplementation significantly reduced the activity of serum ALT, AST and ALP in the FLHS laying hens, reflecting alleviation of liver damage. Likewise, the expression levels of TLR4, NF-κB, TNF-α and IL-6 were downregulated, which is consistent with previous studies that GBE ameliorates inflammation by decreasing the expression of NF-κBp65, TNF-α and IL-6 [63, 66]. Our results together with previous findings suggested that GBE could inhibit the activation of TNF-α, IL-6 and TLR4-NF-κB pathway, thereby attenuating inflammation cascade effects and tissue damage.
Oxidative stress induces hepatic lipid metabolism disturbance, which is also an important mechanism for liver-related diseases [67]. It has been shown that oxidative stress and its consequent lipid peroxidation aggravate the free radicals chain reaction and activate inflammatory mediators [63]. MDA activities are often used as a marker of free radicals-induced lipid peroxidation and as an indicator of oxidative damage [63], which can interact with NF-κB to increase the release of TNF-α [68]. As a primary defense, SOD reduces the activation of inflammatory mediators and oxidative stress [63]. It has been reported that SOD treatment significantly reduces lipid oxidation and improves colonic inflammation in ulcerative colitis [69]. Of note, GBE acts as a free radical scavenger with SOD-like activity [63], which reduces lipid peroxidation in CCl4-induced liver fibrosis by enhancing SOD activities [70]. GSH, T-AOC and GSH-PX are other commonly used indicators to evaluate the degree of oxidative stress. In the present study, GBE treatment significantly increased the activities of SOD and T-AOC, and reduced MDA contents in the liver and serum, indicating that GBE increased the body’s total antioxidant capacity and effectively inhibited lipid peroxidation induced by HFD.
Gut microbiota and its metabolites play an important role in host metabolism. Our results showed that GBE supplementation dramatically reversed the HFD-induced flora imbalance of FLHS laying hens, and transplanting GBE-altered intestinal flora to FLHS laying hens improved hepatic steatosis, inflammation and anti-oxidation. This result strongly suggested that the anti-FLHS effect of GBE was related to its modulation of gut microbiota. Interestingly, Megasphaera is the only genus that was significantly decreased by HFD but enriched by GBE, and was significantly negatively correlated with FLHS traits. Megasphaera is obligately anaerobic, Gram-negative, non-motile, coccoid bacteria, which belongs to the family Veillonellaceae under the order Veillonellales, class Negativicutes, and phylum Firmicutes [71]. Studies have found that Megasphaera is a dominant genus of gut microbiota in different life stages of primates, and is also a key driving factor for the long-term development of gut microbiota in adults [72]. Megasphaera isolated from the human gut have been found to contain a broad range of carbohydrate active enzymes, and oligopeptide transport systems that enables them to use carbohydrates, as well as a broad range of amino acids as carbon sources [73]. These capabilities endow Megasphaera with a competitive advantage in the gut microenvironment especially when the microenvironment changes. Previous studies have demonstrated that Megasphaera is able to produce SCFAs, including acetate, propionate, butyrate, valerate, caproate, isobutyrate, isovalerate and isocaproate [71, 74]. In particular, Megasphaera can metabolize lactate and buffer fat to produce acetate, propionate and butyrate [75, 76]. These SCFAs may regulate lipid levels by downregulating cholesterol biosynthesis and increasing bile acid excretion [77,78,79]. A recent study showed that dietary Lactobacillus delbrueckii lower serum TG levels of growing-finishing pigs via enhancing the abundance of bacteria such as Megasphaera and butyrate content in the colon [80]. Another study showed that Megasphaera exerts an important role in lowering blood lipids in hyperlipidemia animals [76]. Compared with HFD-induced monkeys, the abundance of Megasphaera was found dramatically increased in high-fat-diet-tolerant cynomolgus monkeys (HFD-T). Notably, the abundance of Megasphaera was also dramatically increased in HFD-induced rats after transplantation of fecal microbiota from HFD-T monkeys and this increasing trend was consistent with that in monkeys. All these facts indicate that Megasphaera may have beneficial effects on lipid metabolism-related diseases. Therefore, we speculate that the Megasphaera might play a critical role in the treatment of FLHS, which deserved to be further investigated.
Conclusions
In conclusion, we demonstrated that GBE is effective in improving steatosis, antioxidant activity, inflammation and FLHS in laying hen model. We also evidenced that these effects are probably mediated by GBE-altered gut microbiota. Our work provides fundamental evidence for the promising therapeutic potential of GBE in the treatment of FLHS and related metabolic diseases, and also shed lights on manipulating the chicken gut microbiota to design and development of new therapeutic strategies for prevention and control of FLHS in laying hens.
Availability of data and materials
The data analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACOX1:
-
Acyl-CoA oxidase 1
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- ChREBP1:
-
Carbohydrate response element-binding protein 1
- CPT1:
-
Carnitine palmitoyl transferase 1
- FAS:
-
Fatty acid synthase
- FLHS:
-
Fatty liver hemorrhagic syndrome
- FMT:
-
Fecal microbiota transplantation
- FXR:
-
Farnesoid X receptor
- GBE:
-
Ginkgo biloba extract
- GLU:
-
Glucose
- GPAT1:
-
Glycerol-3-phosphate acyltransferase 1
- GSH:
-
Glutathione
- GSH-Px:
-
Glutathione peroxidase
- HE:
-
Hematoxylin–eosin staining
- HFD:
-
High-fat diet
- HMGCR:
-
3-Hydroxy-3-methylglutaryl-CoA reductase
- IL-6:
-
Interleukin 6
- LXRα:
-
Liver X receptor alpha
- L-FABP:
-
Liver fatty acid binding protein
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- MDA:
-
Malondialdehyde
- NAFLD:
-
Non-alcoholic fatty liver disease
- NAS:
-
NAFLD activity score
- NF-κB :
-
Nuclear factor kappa-B
- PPARα:
-
Peroxisome proliferator-activated receptor alpha
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- ROS:
-
Reactive oxygen species
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- SCD1:
-
Stearoyl-CoA desaturase 1
- SCFAs:
-
Short-chain fatty acids
- SREBP-1c:
-
Sterol regulatory element-binding protein 1c
- T-AOC:
-
Total antioxidant capacity
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- TLR4:
-
Toll-like receptors 4
- TNF-α:
-
Tumor necrosis factor alpha
- T-SOD:
-
Superoxide dismutase
References
Miao YF, Gao XN, Xu DN, Li MC, Gao ZS, Tang ZH, et al. Protective effect of the new prepared Atractylodes macrocephala Koidz polysaccharide on fatty liver hemorrhagic syndrome in laying hens. Poult Sci. 2021;100(2):938–48. https://doi.org/10.1016/j.psj.2020.11.036.
Wei F, Yang X, Zhang M, Xu C, Hu Y, Liu D. Akkermansia muciniphila enhances egg quality and the lipid profile of egg yolk by improving lipid metabolism Front. Microbiol. 2022;13:927245. https://doi.org/10.3389/fmicb.2022.927245.
Shini A, Shini S, Bryden WL. Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system. Avian Pathol. 2019;48(1):25–34. https://doi.org/10.1080/03079457.2018.1538550.
Hamid H, Zhang JY, Li WX, Liu C, Li ML, Zhao LH, et al. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult Sci. 2019;98(6):2509–21. https://doi.org/10.3382/ps/pey596.
Zhuang Y, Xing C, Cao H, Zhang C, Luo J, Guo X, et al. Insulin resistance and metabonomics analysis of fatty liver haemorrhagic syndrome in laying hens induced by a high-energy low-protein diet. Sci Rep. 2019;9(1):10141. https://doi.org/10.1038/s41598-019-46183-y.
Wu Q, Tang H, Wang H. The anti-oxidation and mechanism of essential oil of paederia scandens in the NAFLD Model of chicken. Animals (Basel). 2019;9(10):850. https://doi.org/10.3390/ani9100850.
Meng J, Ma N, Liu H, Liu J, Liu J, Wang J, et al. Untargeted and targeted metabolomics profiling reveals the underlying pathogenesis and abnormal arachidonic acid metabolism in laying hens with fatty liver hemorrhagic syndrome. Poult Sci. 2021;100(9):101320. https://doi.org/10.1016/j.psj.2021.101320.
Li S, Yan C, Liu T, Xu C, Wen K, Liu L, et al. Research note: increase of bad bacteria and decrease of good bacteria in the gut of layers with vs. without hepatic steatosis. Poult Sci. 2020;99(10):5074–8. https://doi.org/10.1016/j.psj.2020.07.007.
Liu X, Pan Y, Shen Y, Liu H, Zhao X, Li J, et al. Protective effects of Abrus cantoniensis Hance on the fatty liver hemorrhagic syndrome in laying hens based on liver metabolomics and gut microbiota. Front Vet Sci. 2022;9:862006. https://doi.org/10.3389/fvets.2022.862006.
Jones RM, Neish AS. Redox signaling mediated by the gut microbiota. Free Radic Biol Med. 2017;105:41–7. https://doi.org/10.1016/j.freeradbiomed.2016.10.495.
Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155(3):629–47. https://doi.org/10.1053/j.gastro.2018.06.083.
Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E, et al. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol. 2015;11(10):834. https://doi.org/10.15252/msb.20156487.
Ferro D, Baratta F, Pastori D, Cocomello N, Colantoni A, Angelico F, et al. New insights into the pathogenesis of non-alcoholic fatty liver disease: gut-derived lipopolysaccharides and oxidative stress. Nutrients. 2020;12(9):2762. https://doi.org/10.3390/nu12092762.
Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15(13):1546–58. https://doi.org/10.2174/138161209788168164.
Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37(2):343–50. https://doi.org/10.1053/jhep.2003.50048.
Xue L, He J, Gao N, Lu X, Li M, Wu X, et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. 2017;7:45176. https://doi.org/10.1038/srep45176.
Hong Y, Sheng L, Zhong J, Tao X, Zhu W, Ma J, et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes. 2021;13(1):1930874. https://doi.org/10.1080/19490976.2021.1930874.
Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Nonalcoholic fatty liver disease: modulating gut microbiota to improve severity? Gastroenterology. 2020;158(7):1881–98. https://doi.org/10.1053/j.gastro.2020.01.049.
Singh SK, Srivastav S, Castellani RJ, Plascencia-Villa G, Perry G. Neuroprotective and antioxidant effect of Ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics. 2019;16(3):666–74. https://doi.org/10.1007/s13311-019-00767-8.
Mahadevan S, Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008;73(1):R14-9. https://doi.org/10.1111/j.1750-3841.2007.00597.x.
Tan X, Sun Z, Liu Q, Ye H, Zou C, Ye C, et al. Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol. 2018;72:399–409. https://doi.org/10.1016/j.fsi.2017.10.022.
Smith PF, Maclennan K, Darlington CL. The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). J Ethnopharmacol. 1996;50(3):131–9. https://doi.org/10.1016/0378-8741(96)01379-7.
Shi C, Zhao L, Zhu B, Li Q, Yew DT, Yao Z, et al. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against beta-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem Biol Interact. 2009;181(1):115–23. https://doi.org/10.1016/j.cbi.2009.05.010.
Wang Y, Xu Y, Xu X, Wang H, Wang D, Yan W, et al. Ginkgo biloba extract ameliorates atherosclerosis via rebalancing gut flora and microbial metabolism. Phytother Res. 2022;36(6):2463–80. https://doi.org/10.1002/ptr.7439.
Rodríguez M, Ringstad L, Schäfer P, Just S, Hofer HW, Malmsten M, et al. Reduction of atherosclerotic nanoplaque formation and size by Ginkgo biloba (EGb 761) in cardiovascular high-risk patients. Atherosclerosis. 2007;192(2):438–44. https://doi.org/10.1016/j.atherosclerosis.2007.02.021.
Sun M, Chai L, Lu F, Zhao Y, Li Q, Cui B, et al. Efficacy and safety of Ginkgo biloba pills for coronary heart disease with impaired glucose regulation: study protocol for a series of N-of-1 randomized, double-blind, placebo-controlled trials. Evid Based Complement Alternat Med. 2018;2018:7571629. https://doi.org/10.1155/2018/7571629.
Hirata BKS, Pedroso AP, Machado MMF, Neto NIP, Perestrelo BO, de Sá R, et al. Ginkgo biloba extract modulates the retroperitoneal fat depot proteome and reduces oxidative stress in diet-induced obese rats. Front Pharmacol. 2019;10:686. https://doi.org/10.3389/fphar.2019.00686.
Hirata BKS, Cruz MM, de Sá R, Farias TSM, Machado MMF, Bueno AA, et al. Potential anti-obesogenic effects of Ginkgo biloba observed in epididymal white adipose tissue of obese rats. Front Endocrinol (Lausanne). 2019;10:284. https://doi.org/10.3389/fendo.2019.00284.
Wang SD, Xie ZQ, Chen J, Wang K, Wei T, Zhao AH, et al. Inhibitory effect of Ginkgo biloba extract on fatty liver: regulation of carnitine palmitoyltransferase 1a and fatty acid metabolism. J Dig Dis. 2012;13(10):525–35. https://doi.org/10.1111/j.1751-2980.2012.00627.x.
Chen P, Hei M, Kong L, Liu Y, Yang Y, Mu H, et al. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 2019;10(12):8161–71. https://doi.org/10.1039/c9fo01178a.
Zhang H, Wang Y, Su Y, Fang X, Guo W. The alleviating effect and mechanism of Bilobalide on ulcerative colitis. Food Funct. 2021;12(14):6226–39. https://doi.org/10.1039/d1fo01266e.
Bian Y, Lei J, Zhong J, Wang B, Wan Y, Li J, et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J Nutr Biochem. 2022;99:108840. https://doi.org/10.1016/j.jnutbio.2021.108840.
National Research Council. Nutrient requirements of poultry. 9th Rev. Ed. Washington: National Academy Press; 1994.
Qiu K, Zhao Q, Wang J, Qi GH, Wu SG, Zhang HJ. Effects of Pyrroloquinoline quinone on lipid metabolism and anti-oxidative capacity in a high-fat-diet metabolic dysfunction-associated fatty liver disease chick model. Int J Mol Sci. 2021;22(3):1458. https://doi.org/10.3390/ijms22031458.
Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent clostridium difficile infection: a randomized clinical trial. JAMA. 2016;315(2):142–9.
Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(5):761.
Satokari R, Mattila E, Kainulainen V, Arkkila PET. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection–an observational cohort study. Aliment Pharmacol Ther. 2015;41(1):46.
Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, et al. Gut microbial metabolism drives transformation of Msh2-deficient colon epithelial cells. Cell. 2014;158(2):288–99.
Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med. 2018;24(7):1070–80.
Folch J. A simple method for the isolation and purification of total lipides from animal tissues. J Bid Chem. 1957;226:497–509.
Liang W, Menke AL, Driessen A, Koek GH, Lindeman JH, Stoop R, et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One. 2014;9(12):e115922. https://doi.org/10.1371/journal.pone.0115922.
Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and Archaea using next-generation sequencing. PLoS One. 2014;9(8):e105592. https://doi.org/10.1371/journal.pone.0105592.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. https://doi.org/10.1038/nmeth.f.303.
Kechin A, Boyarskikh U, Kel A, Filipenko M. cutPrimers: a new tool for accurate cutting of primers from reads of targeted next generation sequencing. J Comput Biol. 2017;24(11):1138–43. https://doi.org/10.1089/cmb.2017.0096.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. https://doi.org/10.1038/nmeth.3869.
Quast C, Pruesse E, Yilmaz P, Gerken J, Glckner FO. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2021;41(D1):D590–6.
Dong Y, Zhang J, Gao Z, Zhao H, Sun G, Wang X, et al. Characterization and anti-hyperlipidemia effects of enzymatic residue polysaccharides from Pleurotus ostreatus. Int J Biol Macromol. 2019;129:316–25. https://doi.org/10.1016/j.ijbiomac.2019.01.164.
Bridi R, Crossetti FP, Steffen VM, Henriques AT. The antioxidant activity of standardized extract of Ginkgo biloba (EGb 761) in rats. Phytother Res. 2001;15(5):449–51. https://doi.org/10.1002/ptr.814.
Marcocci L, Packer L, Droy-Lefaix MT, Sekaki A, Gardès-Albert M. Antioxidant action of Ginkgo biloba extract EGb 761. Methods Enzymol. 1994;234:462–75. https://doi.org/10.1016/0076-6879(94)34117-6.
Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063–72. https://doi.org/10.1056/NEJMra1503519.
Gu X, Xie Z, Wang Q, Liu G, Qu Y, Zhang L, et al. Transcriptome profiling analysis reveals multiple modulatory effects of Ginkgo biloba extract in the liver of rats on a high-fat diet. Febs j. 2009;276(5):1450–8. https://doi.org/10.1111/j.1742-4658.2009.06886.x.
Yan H, Zheng P, Yu B, Yu J, Mao X, He J, et al. Postnatal high-fat diet enhances ectopic fat deposition in pigs with intrauterine growth retardation. Eur J Nutr. 2015;56(2):1–8.
Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. PNAS. 1999;96(24):13656–61. https://doi.org/10.1073/pnas.96.24.13656.
Karasawa K, Tanigawa K, Harada A, Yamashita A. Transcriptional regulation of Acyl-CoA:Glycerol-sn-3-phosphate acyltransferases. Int J Mol Sci. 2019;20(4):964. https://doi.org/10.3390/ijms20040964.
Vosper H, Patel L, Graham TL, Khoudoli GA, Hill A, Macphee CH, et al. The peroxisome proliferator-activated receptor delta promotes lipid accumulation in human macrophages. J Biol Chem. 2001;276(47):44258–65. https://doi.org/10.1074/jbc.M108482200.
Hoekstra M, Lammers B, Out R, Li Z, Van Eck M, Van Berkel TJ. Activation of the nuclear receptor PXR decreases plasma LDL-cholesterol levels and induces hepatic steatosis in LDL receptor knockout mice. Mol Pharm. 2009;6(1):182–9. https://doi.org/10.1021/mp800131d.
Lv Z, Xing K, Li G, Liu D, Guo Y. Dietary Genistein alleviates lipid metabolism disorder and inflammatory response in laying hens with fatty liver syndrome. Front Physiol. 2018;9:1493. https://doi.org/10.3389/fphys.2018.01493.
Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276(20):5738–46. https://doi.org/10.1111/j.1742-4658.2009.07303.x.
Joshi-Barve S, Barve SS, Amancherla K, Gobejishvili L, Hill D, Cave M, et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46(3):823–30. https://doi.org/10.1002/hep.21752.
Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2019;76(1):99–128. https://doi.org/10.1007/s00018-018-2947-0.
Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94(1):174–81. https://doi.org/10.1111/j.1365-2249.1993.tb05997.x.
Larrick JW, Wright SC. Cytotoxic mechanism of tumor necrosis factor-alpha. FASEB J. 1990;4(14):3215–23. https://doi.org/10.1096/fasebj.4.14.2172061.
Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M, Lv P, et al. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm. 2006;2006(5):92642. https://doi.org/10.1155/mi/2006/92642.
Navasa M, Gordon DA, Hariharan N, Jamil H, Shigenaga JK, Moser A, et al. Regulation of microsomal triglyceride transfer protein mRNA expression by endotoxin and cytokines. J Lipid Res. 1998;39(6):1220–30.
Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40(1):185–94. https://doi.org/10.1002/hep.20283.
Rhee KJ, Lee CG, Kim SW, Gim DH, Kim HC, Jung BD. Extract of Ginkgo biloba ameliorates streptozotocin-induced type 1 diabetes mellitus and high-fat diet-induced type 2 diabetes mellitus in mice. Int J Med Sci. 2015;12(12):987–94. https://doi.org/10.7150/ijms.13339.
Hu L, Tian K, Zhang T, Fan CH, Zhou P, Zeng D, et al. Cyanate induces oxidative stress injury and abnormal lipid metabolism in liver through Nrf2/HO-1. Molecules. 2019;24(18):3231. https://doi.org/10.3390/molecules24183231.
Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282(1):G6-15. https://doi.org/10.1152/ajpgi.00328.2001.
Seguí J, Gil F, Gironella M, Alvarez M, Gimeno M, Coronel P, et al. Down-regulation of endothelial adhesion molecules and leukocyte adhesion by treatment with superoxide dismutase is beneficial in chronic immune experimental colitis. Inflamm Bowel Dis. 2005;11(10):872–82. https://doi.org/10.1097/01.mib.0000183420.25186.7a.
Liu SQ, Yu JP, Chen HL, Luo HS, Chen SM, Yu HG. Therapeutic effects and molecular mechanisms of Ginkgo biloba extract on liver fibrosis in rats. Am J Chin Med. 2006;34(1):99–114. https://doi.org/10.1142/s0192415x06003679.
Maki JJ, Looft T. Megasphaera stantonii sp. nov., a butyrate-producing bacterium isolated from the cecum of a healthy chicken. Int J Syst Evol Microbiol. 2018;68(11):3409–15. https://doi.org/10.1099/ijsem.0.002991.
Wei ZY, Rao JH, Tang MT, Zhao GA, Li QC, Wu LM, et al. Characterization of changes and driver microbes in gut microbiota during healthy aging using a captive monkey model. Genom Proteom Bioinf. 2022;20(2):350–65. https://doi.org/10.1016/j.gpb.2021.09.009.
Shetty SA, Marathe NP, Lanjekar V, Ranade D, Shouche YS. Comparative genome analysis of Megasphaera sp. reveals niche specialization and its potential role in the human gut. PLoS One. 2013;8(11):e79353. https://doi.org/10.1371/journal.pone.0079353.
Burakova I, Smirnova Y, Gryaznova M, Syromyatnikov M, Chizhkov P, Popov E, et al. The effect of short-term consumption of lactic acid bacteria on the gut microbiota in obese people. Nutrients. 2022;14(16):3384. https://doi.org/10.3390/nu14163384.
Chen L, Shen Y, Wang C, Ding L, Zhao F, Wang M, et al. Megasphaera elsdenii lactate degradation pattern shifts in rumen acidosis models. Front Microbiol. 2019;10:162. https://doi.org/10.3389/fmicb.2019.00162.
Gao JM, Rao JH, Wei ZY, Xia SY, Huang L, Tang MT, et al. Transplantation of gut microbiota from high-fat-diet-tolerant cynomolgus monkeys alleviates hyperlipidemia and hepatic steatosis in rats. Front Microbiol. 2022;13:876043. https://doi.org/10.3389/fmicb.2022.876043.
Illman RJ, Topping DL, McIntosh GH, Trimble RP, Storer GB, Taylor MN, et al. Hypocholesterolaemic effects of dietary propionate: studies in whole animals and perfused rat liver. Ann Nutr Metab. 1988;32(2):95–107.
Marcil V, Delvin E, Garofalo C, Levy E. Butyrate impairs lipid transport by inhibiting microsomal triglyceride transfer protein in Caco-2 cells. J Nutr. 2003;133(7):2180–3. https://doi.org/10.1093/jn/133.7.2180.
Falcinelli S, Picchietti S, Rodiles A, Cossignani L, Merrifield DL, Taddei AR, et al. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci Rep. 2015;5:9336. https://doi.org/10.1038/srep09336.
Hou G, Yin J, Wei L, Li R, Peng W, Yuan Y, et al. Lactobacillus delbrueckii might lower serum triglyceride levels via colonic microbiota modulation and SCFA-mediated fat metabolism in parenteral tissues of growing-finishing pigs. Front Vet Sci. 2022;9:982349. https://doi.org/10.3389/fvets.2022.982349.
Acknowledgements
We would like to express our gratitude to researchers in our laboratories for their dedication and the staff of the Zhuozhou Teaching Experimental Base of China Agricultural University for their selfless support.
Funding
This study was funded by the National Key Research and Development Program of China (2022YFA1304201), the Beijing Natural Science Foundation (6222032), the Starting Grants Program for Young Talents at China Agricultural University, the 2115 Talent Development Program of China Agricultural University, and Chinese Universities Scientific Fund.
Author information
Authors and Affiliations
Contributions
YFH and XYY conceived the project, contributed to experimental design, interpreted the results, prepared the figures, analyzed the data and wrote the manuscript; XYY and DPL performed experiments and collected samples; MHZ and YQF analyzed the data; XLJ, DL and YMG participated in the experimental design and interpreted the results. All authors discussed the results and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experimental procedures were conducted according to guidelines of the Institutional Studies Animal Care and Use Committee of China Agricultural University (Beijing, China). The study was approved by the Ethics Committees of Laboratory Animal Center of China Agricultural University (Beijing, China) (permit AW13501202-2–2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, X., Li, D., Zhang, M. et al. Ginkgo biloba extract alleviates fatty liver hemorrhagic syndrome in laying hens via reshaping gut microbiota. J Animal Sci Biotechnol 14, 97 (2023). https://doi.org/10.1186/s40104-023-00900-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-023-00900-w