Abstract
Background
Ferulic acid esterase (FAE)-secreting Lactiplantibacillus plantarum A1 (Lp A1) is a promising silage inoculant due to the FAE’s ability to alter the plant cell wall structure during ensiling, an action that is expected to improve forage digestibility. However, little is known regarding the impacts of Lp A1 on rumen microbiota. Our research assessed the influences of Lp A1 in comparison to a widely adopted commercial inoculant Lp MTD/1 on alfalfa’s ensilage, in vitro rumen incubation and microbiota.
Results
Samples of fresh and ensiled alfalfa treated with (either Lp A1 or Lp MTD/1) or without additives (as control; CON) and ensiled for 30, 60 and 90 d were used for fermentation quality, in vitro digestibility and batch culture study. Inoculants treated silage had lower (P < 0.001) pH, acetic acid concentration and dry matter (DM) loss, but higher (P = 0.001) lactic acid concentration than the CON during ensiling. Compared to the CON and Lp MTD/1, silage treated with Lp A1 had lower (P < 0.001) aNDF, ADF, ADL, hemicellulose, and cellulose contents and higher (P < 0.001) free ferulic acid concentration. Compared silage treated with Lp MTD/1, silage treated with Lp A1 had significantly (P < 0.01) improved ruminal gas production and digestibility, which were equivalent to those of fresh alfalfa. Real-time PCR analysis indicated that Lp A1 inoculation improved the relative abundances of rumen’s total bacteria, fungi, Ruminococcus albus and Ruminococcus flavefaciens, while the relative abundance of methanogens was reduced by Lp MTD/1 compared with CON. Principal component analysis of rumen bacterial 16S rRNA gene amplicons showed a clear distinction between CON and inoculated treatments without noticeable distinction between Lp A1 and Lp MTD/1 treatments. Comparison analysis revealed differences in the relative abundance of some bacteria in different taxa between Lp A1 and Lp MTD/1 treatments. Silage treated with Lp A1 exhibited improved rumen fermentation characteristics due to the inoculant effects on the rumen microbial populations and bacterial community.
Conclusions
Our findings suggest that silage inoculation of the FAE-producing Lp A1 could be effective in improving silage quality and digestibility, and modulating the rumen fermentation to improve feed utilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Feed utilization efficiency is a highly important trait in ruminant production and significantly impacts the benefits of ruminant husbandry. In recent years, there has been an increasing demand for efficient utilization of forage in ruminant production because of increasing costs of grains and shortage of food supply for human nutrition [1]. Studies have shown that increasing ruminal digestibility of high-quality forage would be beneficial to the production of dairy cows, because it could increase the energy supply from volatile fatty acid (VFA) and protein from microbial protein synthesis [2, 3].
Ensiling is an effective way of preserving high quality forage. Lactic acid bacteria (LAB) inoculants are widely used in silage preparation due to their high efficiency in aiding fermentation and preventing spoilage [4, 5]. However, most inoculants currently used in silage had a major limitation of the absence of enzymatic capability to effectively degrade lignocellulose structure of forages [4]. Therefore, LAB inoculants are generally considered to have little or no impacts on nutrient digestibility of silage either in vitro or in vivo [4, 6].
Studies have proven that the extent of cell wall digestion in the rumen is largely dependent on ferulic acid, which is linked to lignin as well as polysaccharides by ether and/or ester bonds [7,8,9]. Therefore, silage inoculants that produce ferulic acid esterase (FAE) have been reported to have great potentials in improving the efficiency of silage digestion by secreting the FAE to breakdown the linkages between lignin and the cell wall carbohydrates of forages, and release ferulic acid from arabinoxylans during ensiling [3, 10]. Breakage of the linkage between lignin and cell wall carbohydrates facilitates further degradation of the forages in the rumen [11]. Our previous studies showed that inoculation with FAE-producing LAB during ensiling increased the permeability of plant cell wall and the accessibility of enzymes and acids to structural polysaccharides [12, 13]. The result is that FAE-producing LAB-treated silage showed lower fiber concentrations than other treatments during ensiling. Meanwhile, Li et al. [12, 14] and Usman et al. [10] showed the positive effectiveness of inoculating a FAE-producing Lactiplantibacillus plantarum A1 on silage enzymatic degradation in vitro. Kang et al. [15] and Jin et al. [16] have also reported an improved in situ DM and NDF digestibilities of silages treated with FAE-producing Lentilactobacillus buchneri. In addition, our recent research also found that inoculation of FAE-producing L. plantarum A1 in alfalfa silage improved the DM digestibility of dairy goat diet [17]. However, although the fiber-degrading characteristics of FAE-producing LAB have been investigated and there are several studies that confirmed the capability of FAE-producing LAB inoculants in improving the silage in vitro or in vivo DM digestibility [15,16,17], to date, its impacts on ruminal fermentation and microbiota are poorly understood.
Thus, the objectives of the present study were to investigate the effect of FAE-producing LAB inoculation on fermentation characteristics of alfalfa silage, in vitro feed digestion, and the major microbial groups and bacterial community involved in feed digestion and rumen fermentation, with comparison to a same type of inoculant that is widely used in silage.
Materials and methods
The whole animal-handing protocols were reviewed and approved by Animal Ethics Committee of Lanzhou University (file No. 2010–1 and 2010–2), following the Chinese Standards for the Use and Care of Research Animals, and confirming that all experiments were performed in accordance with relevant guidelines and regulations.
Inoculants used
In the current study, L. plantarum MTD/1 is a widely adopted commercial inoculant (Ecosyl Products Ltd., Stokesley, UK), generally used to improve the fermentation quality of silages, but it does not exhibit FAE activity [13, 18]. L. plantarum A1 is a strain that previously isolated and screened from the ensiled grass of Elymus nutans harvested from the Qinghai-Tibet plateau, which has been proved possessing FAE activity in addition to improving silage fermentation quality in our previous study [19]. In addition, the results showed that inoculation with FAE-producing L. plantarum A1 during ensiling decreased the forage (including corn stalk, alfalfa, Pennisetum sinese and sorghum) lignocellulose concentration with a concomitant increase in free ferulic acid concentration in silage [10, 12,13,14]. During the silage preparation, both strains were provided in the form of freeze-dried powder.
Alfalfa silage preparation
Four plots of first cut alfalfa (Medicago sativa L.) at the late bud to early bloom stage were randomly selected and harvested from an established alfalfa field in Dingxi, Gansu Province, China. The fresh alfalfa was immediately transported to the laboratory, then the harvested alfalfa was evenly spread on the laboratory floor for naturally wilting. During the wilting period, the DM content was frequently detected using a microwave oven according to the method described by Zhang et al. [20] until the DM content reached to around 30%. Thereafter, the wilted alfalfa was chopped into approximately 2-cm fragments using a paper cutter (deli8015, Deli group Co. Ltd., Ningbo, China). The chopped forages were mixed thoroughly by plot, and about 500 g from each plot was subsampled and frozen for further chemical analysis (Table 1); the rest of the chopped forages of each plot were apportioned into nine piles (about 500 g each), randomly assigned into three inoculant treatments. The treatments were: no additives (control, CON), L. plantarum A1 (Lp A1), and L. plantarum MTD/1 (Lp MTD/1), with four replicates (plots in the field) for each treatment and three ensiling periods. During ensiling, the inoculants were dissolved in 5 mL distilled water and sprayed on the chopped alfalfa to achieve a rate of 1 \(\times\)106 colony forming units (CFU)/g fresh alfalfa (FM); meanwhile, the CON was treated with equal volume of distilled water. All the treated piles were packed into 25 cm \(\times\) 35 cm vacuum-packed polyethylene plastic bags (Shanghai Yhpak Co., Ltd., Shanghai, China) that were used as laboratory silos and vacuum-sealed to a density of approximately 0.534 g/cm3. The silos then were stored and ensiled at 25 ± 0.2 °C for 30, 60 and 90 d.
Chemical analysis of fresh alfalfa and silage
Samples of fresh and ensiled alfalfa (20 g) that collected at d 30, 60 and 90 were homogenized with 180 mL distilled water in a juice extractor for 30 s, filtered through 4 layers of medical gauze, and with pH of filtrates measured immediately using a glass electrode pH meter (Orion StarTM A111, Thermo Fisher Scientific, Waltham, MA, USA). Then, the filtrates were acidified with 7.14 mol/L H2SO4 to pH = 2 and filtered using 0.22-μm dialyzer. The filtrates were used to quantify the concentrations of lactic acid, acetic acid, propionic acid, and butyric acid referring to our previously published method [14]. DM content (AOAC method 943.01) of fresh and silage samples were determined according to AOAC [21]. The dried samples were ground through a 1-mm sieve and sealed in plastic sample bags pending the subsequent analysis of the fiber fractions [amylase-treated neutral detergent fiber (aNDF), acid detergent fiber (ADF), acid detergent lignin (ADL)], crude protein (CP), and water-soluble carbohydrates (WSC). Concentrations of aNDF and ADF were determined by digestion in neutral detergent and acid detergent, respectively, using the batch procedures outlined for an ANKOM 2000 Fiber Analyzer (ANKOM Technology Corporation, Fairport, NY, USA). During the aNDF analysis, heat stable alpha-amylase and sodium sulfite were added to eliminate starch and protein effects, respectively, while the aNDF content is inclusive of the residual ash. The concentration of ADL was determined according to the procedures as reported earlier [22]. Hemicellulose and cellulose were calculated using aNDF−ADF and ADF−ADL, respectively. The nitrogen was measured using Kjeldahl automated apparatus (K9805, Shanghai Analytical Instrument Co., Ltd, Shanghai, China) and CP was computed by multiplying the Kjeldahl nitrogen with 6.25 [13]. The colorimetric method was used to quantify the WSC concentration of samples after fully reacting with anthrone reagents [23]. The method of Zhao et al. [24] with modification was used to extract and measure ferulic acid concentration in both fresh and silage samples.
In vitro batch culture
The in vitro batch culture was conducted by a twostep approach using sheep as ruminal fluid donor:
Step 1 (preliminary experiment)
In order to achieve an obvious difference among treatments, an in vitro digestibility experiment of all silage samples was conducted using a DaisyII Incubator (ANKOM Technology Corporation, Fairport, NY, USA) before in vitro batch culture. Four parallel subsamples of each silo (500 ± 5 mg) and glass beads (about 8 g) were heat-sealed within fiber bags (ANKOM F57, 50 mm \(\times\) 55 mm, 25 μm porosity, ANKOM Technology Corporation, Fairport, NY, USA) using an impulse sealer (SF-400, Qingdao Ausense Packing Equipment Co., Ltd., Qingdao, China). Before sample placement, empty F57 filter bags were pre-rinsed in acetone, air-dried, and then dried thoroughly in a forced-air oven at 105 °C for 5 h. Similarly, four empty fiber bags with only glass beads were used as blanks. A total of 148 fiber bags (36 silos \(\times\) 4 parallel samples + 4 blanks) were prepared for in vitro digestibility determination.
Five healthy rumen-fistulated dorper sheep (18–24 months old) were used as rumen fluid donors. The sheep were fed ad libitum at 0730 and 1730 h, and the diet of sheep comprising 40% corn silage, 20% alfalfa hay, and 40% concentrate mixture (DM basis), which was formulated to include 46.7% NDF, 35.2% ADF, 16.7% CP, and 12.6 MJ/kg of digestible energy. About 620 mL of filtered mixed rumen fluid from donors was added to each incubation jar containing pre-warmed (39 °C) buffers (rumen fluid:buffers = 1:2, v/v) and samples (each containing 37 bags) under continuous flushing with CO2 until final lid placement [25]. About 50 mL of ruminal mixture was prepared for each fiber bag. Four prepared incubation jars were then placed into DaisyII Incubator and writhed continuously for 48 h at 39 °C. Following incubation, fiber bags were collected and rinsed with cold distilled water until the water became clear. Then, the fiber bags were squeezed gently to remove excess water, dried in an oven at 65 °C for 48 h, and finally weighed to calculate DM digestibility by the difference of residues before and after digestion.
Step 2 (in vitro batch culture)
According to the results of step 1, three silage samples of each treatment from a suitable ensiling period (9 silage samples = 3 treatments \(\times\) 3 replicates) and 3 fresh alfalfa samples were subjected to in vitro batch culture. The in vitro batch culture was carried out in 100 mL calibrated glass syringes gas production system and repeated in two separate incubations. The ground fresh alfalfa and silage samples (500 ± 5 mg) were weighed and heat-sealed within fiber bags like step 1 but with 3 replicates for each sample. Before morning feeding, fresh rumen fluid was obtained through rumen cannula from 5 dorper donor sheep. Subsequently, rumen fluid was mixed equally and squeezed through 4 layers of cheesecloth into a sterilized flask (2500 mL) that was pre-warmed in a water bath of 39 °C. The anaerobic buffer medium was prepared according to Theodorou et al. [25] and combined with the rumen fluid in a ratio of 2:1 (v/v) under anaerobic conditions. A 50 mL mixture was immediately dispensed into each incubation glass syringe containing substrates. Four syringes containing only incubation media and empty bags were used as blanks for correcting gas production. A total of 40 glass syringe gas production systems [(9 silage samples + 3 fresh alfalfa) \(\times\) 3 replicates + 4 blanks] were prepared and incubated in a 39 °C-water bath for 48 h. All of syringes were affixed to a rotary shaker to ensure that the fermenters were shaken gently during fermenting. The accumulated gas production was recorded and gas samples (5 mL each) were collected using a gastight syringe at 6, 12, 24, and 48 h of incubation, respectively. Methane concentration of mixed gas was measured using a gas chromatography (SP-3420A, Shimadzu, Japan) according to Wang et al. [26]. After 48 h of incubation, fiber bags were removed from glass syringes and rinsed 4 to 6 times using cold distilled water, squeezed gently to remove excess water, placed in an oven at 65 °C for 48 h to calculate DM digestibility. Fermentation fluid of each in vitro culture was collected for measurements of VFA, ammonia nitrogen (NH3-N) and used for DNA extraction and subsequent microbial analysis. The rumen fluid was immediately treated by adding 250 g/L (w/v) HPO3 at a ratio of 1:5 (v/v, HPO3:rumen fluid) for further VFA analysis using a gas chromatography (SP-3420A, Shimadzu, Japan) according to the method described by Wang et al. [26]. The concentration of NH3-N in rumen fluid was quantified using a colorimetric method following the procedure of Broderick and Kang [27]. The remaining part of rumen fluid was stored at −80 °C for subsequent DNA analysis.
DNA extraction, PCR amplification, and sequencing
DNA of frozen rumen liquid (about 10 mL) was extracted with the EasyPure Stool Genomic DNA Kit (TransGen Biotech, Beijing, China) following the manufacturer’s instructions. The DNA concentration and purity was checked using a Nanodrop 2000 (Thermo Fisher Scientific, Inc., Madison, WI, USA). The polymerase chain reaction (PCR) amplification of the V3-V4 region of the bacterial 16S rRNA gene was carried out applying universal primer pairs 338F (5'-ACTCCTACGGGAGGCAGCAG-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3') [28]. Amplicon was examined on a 2% (w/v) agarose gel to verify their expected bands and size. The PCR products were extracted and purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), and the purified DNA amplicons were quantified using a Quantus™ Fluorometer (Promega, Madison, WI, USA). DNA-Seq was done on an Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA) provided by Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The sequence information reported in this study had been deposited in the NCBI database (https://www.ncbi.nlm.nih.gov/sra/PRJNA793346).
Sequences were processed, clustered and taxonomically classified with the QIIME2 software package (Quantitative Insights Into Microbial Ecology) [29]. Clustering of operational taxonomic units (OTU) with 97% similarity using UPARSE version 7.1 [30], and at the same time, the data were identified and some chimeric DNA sequences were removed. Representative sequences for each OTU were classified and analyzed by RDP Classifier version 2.2 [31] and the 16S rRNA database (V201305, Greengenes database) with a confidence threshold of 0.8. The community diversity was estimated using the four commonly used indices in the alpha diversity index (ACE, Chao1, Shannon, and Simpson). Then, principal coordinate analysis (PCoA) was used to obtain the interrelationships of bacterial community composition from four different treatment’s samples. Venn diagram was used to detected the similarities and differences of ruminal microorganisms’ OUT among treatments.
Quantitative real-time PCR (qPCR) analysis
The qPCR was used for absolute quantification of total bacteria, protozoa, fungi, methanogens, Ruminococcus albus, Ruminococcus flavefaciens and Fibrobacter succinogenes. The qPCR assay for total bacteria, methanogens and the three cellulolytic bacteria [29, 32] targeted partial sequences of the 16S rRNA gene, whereas that for protozoa and fungi targeted partial sequences of the 18S rRNA gene. A full description of the primers and thermal profiles used for total bacteria, protozoa, fungi, methanogens, R. albus, R. flavefaciens and F. succinogenes could be seen in Table S1. The specific operation refers to the method of Shen et al. [29].
Statistical analysis
Chemical composition of wilted alfalfa before ensiling was presented as mean ± standard deviation (SD). The dynamical results of silage fermentation parameters, concentrations of fiber components and ferulic acid during ensiling, and digestibility in step 1 of in vitro batch culture were analyzed using general linear model of Statistical Package for Social Science (SPSS 21.0, SPSS, Inc., Chicago, IL, USA) according to a 3 \(\times\) 3 factorial treatment design (three treatments and three ensiling days):
where Yij represents observation; μ is the overall mean; Ti represents the effect of three treatments (i = 1, 2, 3); Dj represents the effect of three ensiling days (j = 1, 2, 3); (T \(\times\)D)ij represents the interaction of treatments and ensiling days, and eij represents the random residual error. Significance was considered at P ≤ 0.05.
Data on in vitro ruminal fermentation characteristics, qPCR of the microbial population, alpha diversity (ACE, Chao1, Shannon, and Simpson) of the bacteria, and the microbial communities’ relative abundances at phylum, and genus levels were analyzed using one-way ANOVA of SPSS 21.0. The absolute quantification data of the total bacteria, protozoa, fungi, methanogens, R. albus, R. flavefaciens and F. succinogenes generated by qPCR data were log transformed before conducting the one-way ANOVA to improve normality. Significant means were separated using Tukey’s multiple comparison tests at P ≤ 0.05. RStudio (Version 1.0.136) was used to calculate Pearson correlation coefficients and generate a heatmaps to examine the correlation between the relative abundances of bacterial genera and each of the fermentation and chemical parameters. Significant correlation was considered at P ≤ 0.05.
Results
Fermentation characteristics, fiber component and ferulic acid of alfalfa silage during ensiling
The interaction of T \(\times\) D influenced both pH and lactic acid (Table 2; P < 0.01). As expected, both inoculants decreased silage pH and improved lactic acid concentration during ensiling, but no differences were observed in pH and lactic acid concentration between the Lp MTD/1-treated silages ensiled for 30, 60 and 90 d, while for Lp A1-treated silages, a lower pH and a higher lactic acid concentration were found in 90 d silage compared with 30 and 60 d silages. Similarly, both Lp A1 and Lp MTD/1 reduced silage DM loss during ensiling (P < 0.001), but no difference was observed in DM loss between Lp A1-treated silages ensiled for 30, 60 and 90 d, while the DM loss increased with the extension of ensiling time in silages inoculated with Lp MTD/1.
As presented in Table 3, unlike silages treated with Lp MTD/1, the silages inoculated with Lp A1 had lower aNDF and ADF concentrations compared with CON silages regardless of the ensiling times (P < 0.05). There was an T \(\times\) D interaction for ferulic acid (P < 0.001). Ferulic acid concentration was highest in Lp A1-treated silages. Although ferulic acid concentrations were higher in Lp MTD/1-treated silages than CON ensiled for 30 and 60 d (P < 0.05), no difference was observed between the two treatments for 90 d silages.
Effects of Lp A1 inoculation in silage on rumen fermentation characteristics and the correlations of rumen fermentation indicators with silage fiber components
In order to investigate the effect of ensiling period on in vitro DM digestibility of silage, we measured the in vitro DM digestibility of silage samples at each ensiling stage using DaisyII Incubator before the experiment. The results showed that alfalfa silages inoculated with Lp A1 and Lp MTD/1 had higher in vitro DM digestibility at 30 d of ensiling than CON, but by d 60 and 90, the highest DM digestibility was observed in Lp A1-treated silages, and a greater difference was found between Lp A1 and other treatments at d 60 (P < 0.05, Fig. 1). Thus, the 60th d silage samples were used for further in vitro batch culture.
In vitro dry matter (DM) digestibility of alfalfa silages ensiled for 0 (fresh material), 30, 60 and 90 d. Treatments: CON = control (no additives); Lp A1 = alfalfa silage inoculated with L. plantarum A1; Lp MTD/1 = alfalfa silage inoculated with L. plantarum MTD/1. Means with different lowercase letters shows difference among treatments in the same ensiling day at P < 0.05 (n = 4, the number of replicates in each mean). T, effect of treatment; D, effect of ensiling time; T × D, interaction of treatment and ensiling time; bar and SEM indicate standard error of means (df = 47)
As expected, inoculation of Lp A1 in silage increased DM digestibility compared with CON and Lp MTD/1 treatments (Table 4; P < 0.05), but no difference was found between Lp A1 and FM treatments. Silage treated with Lp A1 had higher in vitro total gas production, total VFA, total branched-chain VFA and NH3-N than CON and Lp MTD/1 treatments (P < 0.05), while no difference was observed in these parameters between CON and Lp MTD/1 treatments.
The correlation of rumen fermentation indicators and silage fiber components was conducted using a correlation heatmap (Fig. 2). All detected rumen fermentation indicators were negatively correlated with aNDF ADF and ADL (P < 0.05), except for butyrate and total branched-chain VFA (BCVFA). The concentration of cellulose was negatively correlated with total gas production and NH3-N concentration, but positively correlated with total BCVFA. The concentration of hemicellulose was negatively correlated with DM digestibility, butyrate and total BCVFA concentrations.
Effects of Lp A1 inoculation in silage on rumen microbial populations
Quantitative real-time PCR showed that ensiling increased (P < 0.05) the populations of ruminal total bacteria, methanogens and F. succinogenes, but reduced the populations of protozoa and fungi in CON compared to FM (Fig. 3). Compared with CON, the populations of total bacteria, fungi, R. albus and R. flavefaciens were increased by Lp A1 (P < 0.05) inoculation but not by Lp MTD/1. The population of methanogens was reduced by Lp MTD/1 inoculation (P < 0.05) compared to that of CON, while no effect was observed in Lp A1 treatment. Both Lp A1 and Lp MTD/1 inoculations in silage didn’t affect the populations of ruminal protozoa and F. succinogenes.
Effects of ferulic acid esterase-producing L. plantarum A1 inoculation in alfalfa silage on the population of total bacteria, protozoa, fungi, methanogens, Ruminococcus albus, Ruminococcus flavefaciens and Fibrobacter succinogenes (log10 copy number of the target genes/mL) in in vitro rumen cultures at 48 h. Treatment: FM = fresh material; CON = control (no additives); Lp A1 = silage inoculated with L. plantarum A1; Lp MTD/1 = silage inoculated with L. plantarum MTD/1. Means with different superscripts differ at P < 0.05
Change of rumen microbial community
The rumen microbial community was analyzed by Illumina sequencing of 16S rRNA gene. A total of 1,592,126 quality-checked sequences were generated as being bacterial across all 36 samples from the four treatments with average of 44,226 sequences for each sample were obtained. 99.96% of sequences had an average read length of 415 bp. Greater than 99% good’s coverage was achieved for all samples. The sequencing depth was also relatively comprehensive, as indicated by the rarefaction curve shown in Fig. S1.
The alpha diversity indices of the rumen microbial community were not influenced by ensiling or silage inoculants (P > 0.05; Fig. 4A). The Venn diagram showed the similarities and differences of ruminal microorganisms’ OUT detected in different treatment (Fig. 4B). A total of 949 OUT were simultaneously detected in 4 treatments. PCoA showed that microbial community structure tended to cluster based on the treatment (Fig. 4C). A clear separation was found between FM and silages along PC1, which explains > 30% of the total variation. In addition, the ruminal microbial communities of Lp A1 and Lp MTD/1 treatments were also separable with CON along PC1, while no distinction was noticed between Lp A1 and Lp MTD/1 treatments.
Bacterial community diversities of rumen fluid. A Alpha Diversity Index (including Shannon, Simpson, Chao 1, and ACE); B Venn diagram of ruminal microorganisms’ operational taxonomic unit (OTU); C Principal component analysis (PCoA) showing diversities of the bacterial communities in different treatments. Treatment: FM = fresh material; CON = control (no additives); Lp A1 = silage inoculated with L. plantarum A1; Lp MTD/1 = silage inoculated with L. plantarum MTD/1
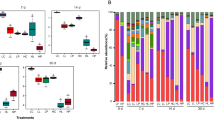
At the phylum level, a total of 7 bacteria phyla with an average relative abundance ≥ 1% were detected (Fig. 5A). Firmicutes (46.7%–56.5%) followed by Bacteroidetes (28.1%–32.1%) and Proteobacteria (5.91%–7.03%) were detected as the dominant bacterial phyla, together representing 85.7%–87.8% of all the sequences. Compared with FM, ensiling increased the relative abundance of Firmicutes in the culture of CON, but reduced the relative abundance of Bacteroidetes (P < 0.05). Both Lp A1 and Lp MTD/1 inoculated silages reduced the relative abundance of ruminal Firmicutes compared with CON, and a contrary result was observed in the relative abundance of Bacteroidetes (P < 0.05; Fig. 5a). Genus-level bacterial communities are shown in Fig. 5B and b. A total of 16 genera of bacteria were obtained to have a relative abundance greater than 1%, and these genera accounted for 75.1%–81.9% of the total sequences. Unclassified Ruminococcaceae (17.4%–20.0%) and unclassified Bacteroidales (13.9%–15.7%) were the most dominant bacteria. Although both Lp A1 and Lp MTD/1 inoculated silages increased the relative abundances of ruminal Prevotella and unclassified Succinivibrionaceae compared with CON, a greater effect was observed in Lp A1-treated group versus Lp MTD/1 treatment (P < 0.05). Besides, Lp A1- and Lp MTD/1-treated silages also had a parallel influence on some bacterial genera. The relative abundances of ruminal Ruminococcus, Treponema, Sphaerochaeta and Bacteroides were increased, while that of unclassified Lachnospiraceae and Pyramidobacter were decreased by Lp A1 and Lp MTD/1 inoculations.
Effects of ferulic acid esterase-producing Lp A1 inoculation in silage on relative abundance of ruminal bacteria at phylum (A and a) and genus level (B and b) that accounted for ≥ 1% of total sequences in at least one treatment. Treatment: FM = fresh material; CON = control (no additives); Lp A1 = silage inoculated with L. plantarum A1; Lp MTD/1 = silage inoculated with L. plantarum MTD/1. uncl, unclassified
Correlations of ruminal bacterial communities with rumen fermentation characteristics
Correlation analysis was conducted to evaluate the relationships of ruminal bacterial communities and rumen fermentation characteristics (Fig. 6). Total gas production was positively correlated with ruminal Ruminococcus, Bacteroides, Prevotella, unclassified Prevotellaceae, Treponema, Sphaerochaeta and unclassified Succinivibrionaceae, but negatively correlated with unclassified Lachnospiraceae, unclassified Ruminococcaceae and Pyramidobacter. Methane production, DM digestibility and VFA concentration were positively correlated with Butyrivibrio, Prevotella and unclassified Succinivibrionaceae. NH3-N was positively correlated with Ruminococcus, Bacteroides, Prevotella, Butyrivibrio, and unclassified Succinivibrionaceae, but negatively correlated with unclassified Lachnospiraceae.
Discussion
Conservation characteristics of alfalfa silage
Expectedly, inoculation with Lp A1 and Lp MTD/1 decreased silage pH as reported in a previous study [13], and the increases of lactic acid concentration in inoculated treatments account for the decrease of pH values [6, 18]. Compared with CON and Lp MTD/1-treated silages, inoculating Lp A1 in silage could produce FAE which acts as a biocatalytic agent to degrade lignocellulose structure during ensiling [14]. Afterwards, the water-soluble carbohydrates resulted from the lignocellulose degradation could be utilized by LAB to further promote fermentation [13, 33]. This was also confirmed by the substantial decrease in the fiber component and the increase of free ferulic acid concentration in Lp A1-treated silage. The results of DM loss further indicated that more nutrients were well preserved in Lp A1 and Lp MTD/1-treated silages compared with CON, which was consistent with previous studies [13, 18].
The Lp A1 is the major contributor to fiber degradation in silages of the present study due to its FAE producing ability. As previously reported, Lp A1 hydrolyzed the ester bond between lignin and structural polysaccharides by producing FAE during ensiling, thereby reducing the crystallinity and further enhancing the permeability of lignocellulose [10, 14]. Similar results had also been reported in our previous studies that lignocellulose was degraded in corn stalk, alfalfa and Pennisetum sinese silages when Lp A1 was used as an inoculant [12,13,14]. These results indicated that inoculation with both FAE-producing Lp A1 and the widely adopted commercial inoculant Lp MTD/1 in silage are effective treatments in improving alfalfa fermentation quality. But it is worth noting that Lp A1 also showed obvious fiber degradation performance at the same time compared with CON and Lp MTD/1-treated silage.
Effects of Lp A1 inoculation in silage on the major microbial groups and bacterial community involved in feed digestion and methane production
Consistent with previous studies, inoculating FAE-producing Lp A1 in alfalfa silage increased alfalfa silage DM digestibility [15, 16, 34], resulting in the digestibility of DM very similar to that of the alfalfa before ensiling. This could be attributed to the ability of Lp A1 to preserve more digestible DM during silage fermentation, which in turn provided more substrate available for degradation by rumen microbiome [35]. In addition, the difference of the DM digestibility between Lp A1 and other treated silages may be related to the enzymolysis of the cross-link between arabinoxylans and lignin by the action of FAE produced by Lp A1 during ensiling, resulting in fiber to be more susceptible to attack by enzymes or rumen microorganisms [12, 14]. It can also be easily seen from the correlation heatmap that the digestion of silage is highly associated with the silage fiber components except for cellulose.
It has been proven that changes of forage fiber affect the rumen microbial community composition, which in turn affect fermentation products and ruminal pH [36, 37]. Therefore, increasing the knowledge of the rumen microbiomes and their transition in response to the alteration of feed’s fiber structure could help to improve the feed utilization efficiency of the host animal [37]. R. albus, R. flavefaciens and F. succinogenes are considered the predominant rumen fibrolytic bacteria due to their high cellulose digestion ability [29, 38]. Moreover, protozoa and fungi have also been reported to take function in fiber degradation [29, 37, 39]. Consistent with previous findings, we detected more total bacteria, R. albus, R. flavefaciens and fungi in Lp A1 treatment, compared with CON and Lp MTD/1 treated group [40,41,42]. Thus, the increased total bacteria, R. albus, R. flavefaciens and fungi might have resulted in the improved silage DM digestibility in the Lp A1 treatment [29, 42]. In addition, based on 16S rRNA sequencing analysis, the main changes in the bacterial community as a result of Lp A1 inoculation were significant increases in the abundances of Prevotella, Butyrivibrio and unclassified Succinivibrionaceae. Butyrivibrio is recognized as fibrolytic bacterial genera which was increased in Lp A1 treatment [29, 43]. As a core genus in the rumen, Prevotella was also increased in Lp A1 treatment, and the relative abundance was similar to that of FM. Studies have shown that Prevotella app. are highly active hemicellulolytic and proteolytic bacteria [42, 44]. Furthermore, ruminal Prevotella is also known to actively take part in the breakdown of starch, xylan and pectin during digestion and utilization [44, 45]. The increase in these bacteria is also crucial for the efficient degradation and utilization of cellulosic and non-cellulosic plant polysaccharide and protein in the rumen.
The results of the present study indicated that the silage inoculation with Lp A1 increased DM digestibility with a concomitant increase in methanogenesis, which is consistent with the notion that the higher methane production could be due to the higher in vitro DM digestibility [46]. Thus, this finding largely associates with the increased amounts of fermentable substrates in the in vitro fermenters [47]. However, it is worth noting that the methane production in Lp A1 treatment is similar to that of the CON on the basis of per gram of digestible DM. On the contrary, methane production was reduced by Lp MTD/1 inoculation in silage without affecting the in vitro DM digestibility, which was consistent with previous studies [35, 48, 49]. The rumen microbial mechanism of methanogenesis from Lp A1 and Lp MTD/1 inoculated silages might be different. In the current study, silages inoculated with Lp A1 had no effect on the population of methanogens, but it had been reduced in Lp MTD/1 treated group. Muck et al. [50] reported that some inoculant-treated silages reduced gas production compared with the untreated silage, suggesting that a modification had occurred in the ruminal fermentation process. But the specific reason for the inhibition of methanogens is not clearly known, which require further investigation for verification.
Effects of Lp A1 inoculation in silage on the major microbial groups and bacterial community involved in rumen fermentation
Acetate, propionate and butyrate are the key VFA produced in the rumen from the fermentation of dietary carbohydrates, and these could provide ruminants with up to 70%–80% of all their energy requirements [51]. The greater total VFA concentration in the rumen generally resulted from the higher DM degradation of the diet [2, 35], which supplied more fermentation substrate for rumen microbes. In the current study, silage inoculated with Lp A1 increased the ruminal acetate, propionate, and butyrate concentrations after 48 h of in vitro incubation, but it did not affect the ratio of acetate to propionate. The increased VFA concentration in Lp A1 treatment was probably related to the increase of Gram-positive fibrolytic bacteria belonging to Ruminococcus spp. [29, 52, 53]. Therefore, we posited that more structural carbohydrates were disintegrated to hexoses or pentoses, which were then rapidly converted to pyruvate and finally to acetate, propionate, and butyrate in Lp A1 treatment [35].
The cleavage of the acetyl linkages during the hydrolysis of the hemicellulose may have actuated the removal of acetyl groups, resulting in the formation of more free acetate in the culture [14]. An unsubstantiated proposition by Shen et al. [29] suggested that some Gram-positive bacteria, such as those unclassified bacteria in Ruminococcaceae and Lachnospiraceae were also positively correlated with acetate concentration. However, our results did not concur with the above proposition. Prevotella is a genus consisting of proteolytic, amylolytic, and hemicellulolytic bacteria, dominating the rumen of adult ruminants and producing succinate and acetate [44, 54]. Therefore, the higher abundance of Prevotella in Lp A1 treatment might be another reason for the increased acetate and propionate concentration. The present results suggested that the production of acetate was basically related to the fiber degrading bacteria of the rumen, while the efficiency of fiber-degrading bacteria was facilitated by the change in the fiber structure during silage fermentation.
Propionate is produced via succinate (randomizing pathway) or acrylate (non-randomizing pathway) in the rumen, and the succinate pathway is regarded as the major pathway [29, 55]. In addition to Prevotella, which fermentation products include succinate [44, 56], previous studies have also shown that fermentation end-products of F. succinogenes and R. flavefaciens are mainly succinate, which could be used for propionate production in the rumen [52, 55]. In the current study, silage inoculated with Lp A1 did not affect the population of ruminal F. succinogenes compared with CON and Lp MTD/1 treatments. Thus, a possible reason for the higher propionate concentration in Lp A1 treatment is that the population of R. flavefaciens was greater in this group. Consistent with previous studies, higher abundance of unclassified Succinivibrionaceae had been found in the rumen culture of Lp A1 treatment, indicating that this taxon might also play an important role in the increased propionate production [56, 57]. Besides, it's worth noting that the higher propionate concentration in Lp A1 treatment probably associated with the high lactic acid concentration of the silage, since lactate could be converted to propionate in the rumen by lactate-utilizing bacteria such as Megasphaera elsdenii and Selenomonas ruminantium [52].
Butyrivibrio and Pseudobutyrivibrio are regarded as important butyrate-producing genera in the rumen [29], but the relative abundances of Pseudobutyrivibrio was not different among treatments. Additionally, Pseudobutyrivibrio was not considered due to the abundance of Pseudobutyrivibrio (< 0.1%) was much less than that of Butyrivibrio. Hence, based on the positive correlations between Butyrivibrio and butyrate concentration, we speculated that the high abundance of Butyrivibrio in the Lp A1 treatment caused the higher butyrate production. Valerate and branched-chain VFA (i.e., isovalerate and isobutyrate) in the rumen primarily originate from ruminal oxidative-deamination and decarboxylation of valine, leucine, and isoleucine [36, 58]. Therefore, the increased valerate and branched-VFA concentration in Lp A1 treated group may result from increased amino acid deamination by microbes if the utilization rate was not changed. Even though we did not determine the main hyper-ammonia-producing bacteria in our study, it could be substantiated by the higher ammonia concentration in the Lp A1 treatment. The higher concentration of ammonia in FM group might attribute to the low total bacterial populations that reduced ammonia utilization by microbes [29].
Conclusions
Inoculating alfalfa silage with either Lp A1 or Lp MTD/1 improved the fermentation quality. However, Lp A1 exerted greater effects on fiber degradation as indicated by the low fiber content and high free ferulic acid concentration of the treated silages. Noteworthily, alfalfa silage inoculated with Lp A1 improved in vitro DM digestibility than CON and Lp MTD/1 treatments. Additionally, although inoculating alfalfa silage with Lp A1 had little effect on the rumen’s microbial alpha diversity, it had triggered different modifications of the rumen microbiota resulting in superior rumen fermentation characteristics. Therefore, our findings suggest that alfalfa silage inoculated with FAE-producing Lp A1 could be practically more effective in improving silage quality and digestibility as well as modulating the rumen fermentation which ultimately translate to efficient feed utilization. Our results provide an important basis for deeper understandings and further research on practical application of FAE-producing LAB as silage inoculants.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Raw sequencing files have been deposited in the NCBI database (https://www.ncbi.nlm.nih.gov/sra/PRJNA793346).
Change history
07 April 2023
A Correction to this paper has been published: https://doi.org/10.1186/s40104-023-00871-y
Abbreviations
- ADF:
-
Acid detergent fiber
- ADL:
-
Acid detergent lignin
- BCVFA:
-
Branched-chain volatile fatty acid
- CP:
-
Crude protein
- CON:
-
Control (no additives)
- DM:
-
Dry matter
- FAE:
-
Ferulic acid esterase
- FM:
-
Fresh material
- LAB:
-
Lactic acid bacteria
- Lp A1:
-
Silage inoculated with Lactiplantibacillus plantarum A1
- Lp MTD/1:
-
Silage inoculated with Lactiplantibacillus plantarum MTD/1
- NDF:
-
Neutral detergent fiber
- NH3-N:
-
Ammonia nitrogen
- OUT:
-
Operational taxonomic units
- PcoA:
-
Principal coordinate analysis
- PCR:
-
Polymerase chain reaction
- qPCR:
-
Quantitative real-time PCR
- VFA:
-
Volatile fatty acid
- WSC:
-
Water soluble carbohydrates
References
Klopfenstein TJ, Erickson GE, Berger LL. Maize is a critically important source of food, feed, energy and forage in the USA. Field Crop Res. 2013;153:5–11. https://doi.org/10.1016/j.fcr.2012.11.006.
Cantalapiedra-Hijar G, Yáñez-Ruiz DR, Martín-García AI, Molina-Alcaide E. Effects of forage: concentrate ratio and forage type on apparent digestibility, ruminal fermentation, and microbial growth in goats. J Anim Sci. 2009;87:622–31. https://doi.org/10.2527/jas.2008-1142.
Muck RE, Nadeau EMG, McAllister TA, Contreras-Govea FE, Santos MC, Kung L Jr. Silage review: recent advances and future uses of silage additives. J Dairy Sci. 2018;101:3980–4000. https://doi.org/10.3168/jds.2017-13839.
Weinberg ZG, Shatz O, Chen Y, Yosef E, Nikbahat M, Ben-Ghedalia D, et al. Effect of lactic acid bacteria inoculants on in vitro digestibility of wheat and corn silages. J Dairy Sci. 2007;90:4754–62. https://doi.org/10.3168/jds.2007-0176.
Oliveira AS, Weinberg ZG, Ogunade IM, Cervantes AA, Arriola KG, Jiang Y, et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J Dairy Sci. 2017;100:4587–603. https://doi.org/10.3168/jds.2016-11815.
Filya I, Muck RE, Contreras-Govea FE. Inoculant effects on alfalfa silage: fermentation products and nutritive value. J Dairy Sci. 2007;90:5108–14. https://doi.org/10.3168/jds.2006-877.
Yu P, McKinnon JJ, Christensen DA. Hydroxycinnamic acids and ferulic acid esterase in relation to biodegradation of complex plant cell walls. Can J Anim Sci. 2005;85:255–67. https://doi.org/10.4141/A04-010.
Aboagye IA, Lynch JP, Church JS, Baah J, Beauchemin KA. Digestibility and growth performance of sheep fed alfalfa hay treated with fibrolytic enzymes and a ferulic acid esterase producing bacterial additive. Anim Feed Sci Tech. 2015;203:53–66. https://doi.org/10.1016/j.anifeedsci.2015.02.010.
de Oliveira DM, Finger-Teixeira A, Rodrigues Mota T, Salvador VH, Moreira-Vilar FC, Correa Molinari HB. Ferulic acid: a key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotechnol J. 2015;13:1224–32. https://doi.org/10.1111/pbi.12292.
Usman S, Li F, An D, Shou N, Deng J, Zhang Y, et al. Lignocellulose degradation and enzymatic hydrolysis of soybean incorporated sorghum silage inoculated with feruloyl-esterase producing Lactobacillus plantarum. Fermentation. 2022;8:70. https://doi.org/10.3390/fermentation8020070.
Nsereko VL, Smiley BK, Rutherford WM, Spielbauer A, Forrester KJ, Hettinger GH, et al. Influence of inoculating forage with lactic acid bacterial strains that produce ferulate esterase on ensilage and ruminal degradation of fiber. Anim Feed Sci Technol. 2008;145:122–35. https://doi.org/10.1016/j.anifeedsci.2007.06.039.
Li F, Ke W, Ding Z, Bai J, Zhang Y, Xu D, et al. Pretreatment of Pennisetum sinese silages with ferulic acid esterase-producing lactic acid bacteria and cellulase at two dry matter contents: fermentation characteristics, carbohydrates composition and enzymatic saccharification. Bioresour Technol. 2020;295:122261. https://doi.org/10.1016/j.biortech.2019.122261.
Li FH, Ding ZT, Chen XZ, Zhang YX, Ke WC, Zhang X, et al. The effects of Lactobacillus plantarum with feruloyl esterase-producing ability or high antioxidant activity on the fermentation, chemical composition, and antioxidant status of alfalfa silage. Anim Feed Sci Tech. 2021;273:114835. https://doi.org/10.1016/j.anifeedsci.2021.114835.
Li F, Ding Z, Ke W, Xu D, Zhang P, Bai J, et al. Ferulic acid esterase-producing lactic acid bacteria and cellulase pretreatments of corn stalk silage at two different temperatures: ensiling characteristics, carbohydrates composition and enzymatic saccharification. Bioresour Technol. 2019;282:211–21. https://doi.org/10.1016/j.biortech.2019.03.022.
Kang TW, Adesogan AT, Kim SC, Lee SS. Effects of an esterase-producing inoculant on fermentation, aerobic stability, and neutral detergent fiber digestibility of corn silage. J Dairy Sci. 2009;92:732–8. https://doi.org/10.3168/jds.2007-0780.
Jin L, Duniere L, Lynch JP, McAllister TA, Baah J, Wang Y. Impact of ferulic acid esterase producing lactobacilli and fibrolytic enzymes on conservation characteristics, aerobic stability and fiber degradability of barley silage. Anim Feed Sci Tech. 2015;207:62–74. https://doi.org/10.1016/j.anifeedsci.2015.06.011.
Li F, Zhang B, Zhang Y, Zhang X, Usman S, Ding Z, et al. Probiotic effect of ferulic acid esterase-producing Lactobacillus plantarum inoculated alfalfa silage on digestion, antioxidant, and immunity status of lactating dairy goats. Anim Nutr. 2022;11:38–47. https://doi.org/10.1016/j.aninu.2022.06.010.
Hu W, Schmidt RJ, McDonell EE, Klingerman CM, Kung L Jr. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J Dairy Sci. 2009;92:3907–14. https://doi.org/10.3168/jds.2008-1788.
Ding ZT, Xu DM, Bai J, Li FH, Adesogan AT, Zhang P, et al. Characterization and identification of ferulic acid esterase-producing Lactobacillus species isolated from Elymus nutans silage and their application in ensiled alfalfa. J Appl Microbiol. 2019;127:985–95. https://doi.org/10.1111/jam.14374.
Zhang YX, Ke WC, Vyas D, Adesogan AT, Franco M, Li FH, et al. Antioxidant status, chemical composition and fermentation profile of alfalfa silage ensiled at two dry matter contents with a novel Lactobacillus plantarum strain with high-antioxidant activity. Anim Feed Sci Tech. 2021;272:114751. https://doi.org/10.1016/j.anifeedsci.2020.114751.
Association of Official Analytical Chemists (AOAC). In: Cuniff P, editor. Official methods of analysis of AOAC international. 16th ed., 1. Washington: Association of Office Analytical Chemists International; 1999. 5th revision
Coblentz WK, Akins MS, Kalscheur KF, Brink GE, Cavadini JS. Effects of growth stage and growing degree day accumulations on triticale forages: 1) Dry matter yield, nutritive value, and in vitro dry matter disappearance. J Dairy Sci. 2018;101:8965–85. https://doi.org/10.3168/jds.2018-14868.
Thomas TA. An automated procedure for the determination of soluble carbohydrates in herbage. J Sci Food Agric. 1977;28:639–42. https://doi.org/10.1002/jsfa.2740280711.
Zhao S, Yao S, Ou S, Lin J, Wang Y, Peng X, et al. Preparation of ferulic acid from corn bran: its improved extraction and purification by membrane separation. Food Bioprod Process. 2014;92:309–13. https://doi.org/10.1016/j.fbp.2013.09.004.
Theodorou MK, Williams BA, Dhanoa MS, Mcallan AB. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Tech. 1994;48:185–97. https://doi.org/10.1016/0377-8401(94)90171-6.
Wang W, Ungerfeld EM, Degen AA, Jing X, Guo W, Zhou J, et al. Ratios of rumen inoculum from Tibetan and Small-tailed Han sheep influenced in vitro fermentation and digestibility. Anim Feed Sci Tech. 2020;267:114562. https://doi.org/10.1016/j.anifeedsci.2020.114562.
Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. 1980;63:64–75. https://doi.org/10.3168/jds.S0022-0302(80)82888-8.
Wang T, Jiao J, Wang H, Degen AA, Gou N, Li S, et al. The effects of supplementing sweet sorghum with grapeseeds on dry matter intake, average daily gain, feed digestibility and rumen parameters and microbiota in lambs. Anim Feed Sci Tech. 2021;272:114750. https://doi.org/10.1016/j.anifeedsci.2020.114750.
Shen J, Liu Z, Yu Z, Zhu W. Monensin and nisin affect rumen fermentation and microbiota differently in vitro. Front Microbiol. 2017;8:1111. https://doi.org/10.3389/fmicb.2017.01111.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8. https://doi.org/10.1038/nmeth.2604.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microb. 2007;2007(73):5261–7. https://doi.org/10.1128/AEM.00062-07.
Saro C, Ranilla MJ, Tejido ML, Carro MD. Influence of forage type in the diet of sheep on rumen microbiota and fermentation characteristics. Livest Sci. 2014;160:52–9. https://doi.org/10.1016/j.livsci.2013.12.005.
Lynch JP, Baah J, Beauchemin KA. Conservation, fiber digestibility, and nutritive value of corn harvested at 2 cutting heights and ensiled with fibrolytic enzymes, either alone or with a ferulic acid esterase-producing inoculant. J Dairy Sci. 2015;98:1214–24. https://doi.org/10.3168/jds.2014-8768.
Addah W, Baah J, Okine EK, McAllister TA. A third-generation esterase inoculant alters fermentation pattern and improves aerobic stability of barley silage and the efficiency of body weight gain of growing feedlot cattle1. J Anim Sci. 2012;90:1541–52. https://doi.org/10.2527/jas.2011-4085.
Guo G, Chen S, Liu Q, Zhang SL, Shao T, Wang C, et al. The effect of lactic acid bacteria inoculums on in vitro rumen fermentation, methane production, ruminal cellulolytic bacteria populations and cellulase activities of corn stover silage. J Integr Agr. 2020;19:838–47. https://doi.org/10.1016/S2095-3119(19)62707-3.
Patra AK, Yu Z. Effects of vanillin, quillaja saponin, and essential oils on in vitro fermentation and protein-degrading microorganisms of the rumen. Appl Microbiol Biot. 2014;98:897–905. https://doi.org/10.1007/s00253-013-4930-x.
Iqbal MW, Zhang Q, Yang Y, Zou C, Li L, Liang X, et al. Ruminal fermentation and microbial community differently influenced by four typical subtropical forages in vitro. Anim Nutr. 2018;4:100–8. https://doi.org/10.1016/j.aninu.2017.10.005.
Carberry CA, Kenny DA, Han S, McCabe MS, Waters SM. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl Environ Microb. 2012;78:4949–58. https://doi.org/10.1128/AEM.07759-11.
Thoetkiattikul H, Mhuantong W, Laothanachareon T, Tangphatsornruang S, Pattarajinda V, Eurwilaichitr L, et al. Comparative analysis of microbial profiles in cow rumen fed with different dietary fiber by tagged 16S rRNA gene pyrosequencing. Curr Microbiol. 2013;67:130–7. https://doi.org/10.1007/s00284-013-0336-3.
Giraldo LA, Tejido ML, Ranilla MJ, Ramos S, Carro MD. Influence of direct-fed fibrolytic enzymes on diet digestibility and ruminal activity in sheep fed a grass hay-based diet. J Anim Sci. 2008;86:1617–23. https://doi.org/10.2527/jas.2007-0343.
Gado HM, Salem AZM, Robinson PH, Hassan M. Influence of exogenous enzymes on nutrient digestibility, extent of ruminal fermentation as well as milk production and composition in dairy cows. Anim Feed Sci Tech. 2009;154:36–46. https://doi.org/10.1016/j.anifeedsci.2009.07.006.
Adesogan AT, Arriola KG, Jiang Y, Oyebade A, Paula EM, Pech-Cervantes AA, et al. Symposium review: Technologies for improving fiber utilization. J Dairy Sci. 2019;102:5726–55. https://doi.org/10.3168/jds.2018-15334.
Palevich N, Kelly WJ, Leahy SC, Denman S, Altermann E, Rakonjac J, et al. Comparative genomics of rumen Butyrivibrio spp. uncovers a continuum of polysaccharide-degrading capabilities. Appl Environ Microb. 2019;86:e01993-19. https://doi.org/10.1128/AEM.01993-19.
Emerson E, Weimer P. Fermentation of model hemicelluloses by Prevotella strains and Butyrivibrio fibrisolvens in pure culture and in ruminal enrichment cultures. Appl Microbiol Biot. 2017;101:4269–78. https://doi.org/10.1007/s00253-017-8150-7.
Kabel MA, Yeoman CJ, Han Y, Dodd D, Abbas CA, de Bont JA, et al. Biochemical characterization and relative expression levels of multiple carbohydrate esterases of the xylanolytic rumen bacterium Prevotella ruminicola 23 grown on an ester-enriched substrate. Appl Environ Microb. 2011;77:5671–81. https://doi.org/10.1128/AEM.05321-11.
Ungerfeld EM. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: a meta-analysis. Front Microbiol. 2015;6:37. https://doi.org/10.3389/fmicb.2015.00037.
Kaewpila C, Gunun P, Kesorn P, Subepang S, Thip-Uten S, Cai Y, et al. Improving ensiling characteristics by adding lactic acid bacteria modifies in vitro digestibility and methane production of forage-sorghum mixture silage. Sci Rep. 2021;11:1–9. https://doi.org/10.1038/s41598-021-87311-x.
Cao Y, Cai Y, Takahashi T, Yoshida N, Tohno M, Uegaki R, et al. Effect of lactic acid bacteria inoculant and beet pulp addition on fermentation characteristics and in vitro ruminal digestion of vegetable residue silage. J Dairy Sci. 2011;94:3902–12. https://doi.org/10.3168/jds.2010-3623.
Ellis J, Bannink A, Hindrichsen IK, Kinley RD, Pellikaan WF, Milora N, et al. Effect of lactic acid bacteria inoculants on in vitro rumen organic matter digestibility, total gas and methane production. Anim Feed Sci Tech. 2016;34–9. https://doi.org/10.1016/j.anifeedsci.2015.10.016.
Muck RE, Filya I, Contreras-Govea FE. Inoculant effects on alfalfa silage: in vitro gas and volatile fatty acid production. J Dairy Sci. 2007;90:5115–25. https://doi.org/10.3168/jds.2006-878.
Van Houtert MFJ. The production and metabolism of volatile fatty acids by ruminants fed roughages: a review. Anim Feed Sci Tech. 1993;43:189–225. https://doi.org/10.1016/0377-8401(93)90078-X.
Jeyanathan J, Martin C, Morgavi DP. The use of direct-fed microbials for mitigation of ruminant methane emissions: a review. Animal. 2014;8:250–61. https://doi.org/10.1017/S1751731113002085.
Candyrine SCL, Mahadzir MF, Garba S, Jahromi MF, Ebrahimi M, Goh YM, et al. Effects of naturally-produced lovastatin on feed digestibility, rumen fermentation, microbiota and methane emissions in goats over a 12-week treatment period. PloS One. 2018;13:e0199840. https://doi.org/10.1371/journal.pone.0199840.
Stevenson DM, Weimer PJ. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biot. 2007;75:165–74. https://doi.org/10.1007/s00253-006-0802-y.
Chen L, Dong Z, Li J, Shao T. Ensiling characteristics, in vitro rumen fermentation, microbial communities and aerobic stability of low-dry matter silages produced with sweet sorghum and alfalfa mixtures. J Sci Food Agr. 2019;99:2140–51. https://doi.org/10.1002/jsfa.9406.
Xue M, Sun H, Wu X, Guan LL, Liu J. Assessment of rumen microbiota from a large dairy cattle cohort reveals the pan and core bacteriomes contributing to varied phenotypes. Appl Environ Microb. 2018;84:e00970-e1018. https://doi.org/10.1128/AEM.00970-18.
Xue MY, Sun HZ, Wu XH, Guan LL, Liu JX. Assessment of rumen bacteria in dairy cows with varied milk protein yield. J Dairy Sci. 2019;102:5031–41. https://doi.org/10.3168/jds.2018-15974.
Tedeschi LO, Fox DG, Russell JB. Accounting for ruminal deficiencies of nitrogen and branched-chain amino acids in the structure of the Cornell net carbohydrate and protein system. In: Proceedings of Cornell nutrition conference for feed manufacturers. New York, USA: Cornell University; 2000. p. 224–38.
Acknowledgements
The authors are grateful for the financial support provided by the National Natural Science Foundation of China (31901390), China Postdoctoral Science Foundation (2022M711451) and Natural Science Foundation of Gansu Province, China (22JR5RA527).
Funding
This work was funded by National Natural Science Foundation of China (project no. 31901390), China Postdoctoral Science Foundation (project no. 2022M711451) and Natural Science Foundation of Gansu Province, China (22JR5RA527).
Author information
Authors and Affiliations
Contributions
XG contributed to securing financial support, designing the study; FL contributed to do this study, and preparing the first manuscript draft; SU, ZAK, TR, ZD and FL revised the manuscript draft; WH, MJ and FL performed data collection and statistical analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The whole animal-handing protocols were reviewed and approved by Animal Ethics Committee of Lanzhou University (file No. 2010–1 and 2010–2), following the Chinese Standards for the Use and Care of Research Animals, and confirming that all experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
The original version of this article was revised: “'[amylase-treated neutral detergent fiber (ADF), acid detergent lignin (ADL)]' on page 3 in the right column has been changed to '[amylase-treated neutral detergent fiber (aNDF), acid detergent fiber (ADF), acid detergent lignin (ADL)]'.
Supplementary Information
Additional file 1:
Table S1. Primers and corresponding amplification conditions used for quantitative real-time PCR in this study.
Additional file 2:
Fig. S1. Rarefaction of four treatments. Treatment: FM = fresh material; CON = control (no additives); Lp A1 = silage inoculated with L. plantarum A1; Lp MTD/1 = silage inoculated with L. plantarum MTD/1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, F., Usman, S., Huang, W. et al. Effects of inoculating feruloyl esterase-producing Lactiplantibacillus plantarum A1 on ensiling characteristics, in vitro ruminal fermentation and microbiota of alfalfa silage. J Animal Sci Biotechnol 14, 43 (2023). https://doi.org/10.1186/s40104-023-00837-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-023-00837-0