Abstract
Background
Due to fast urban expansion and increased industrial activities, large quantities of solid and liquid wastes contaminated by trace metals are released into the environment of the Addis Ababa city, most often untreated. This study was conducted to investigate spatial distribution, seasonal variations and ecological risk assessment of selected trace metals (Cd, Cr, Cu, Fe, Mn, Pb, Ni and Zn) in the surface sediments from Akaki River catchment and Aba Samuel reservoir, Central Ethiopia.
Methods
Twenty-two surface sediment samples were collected, digested using the Mehlich-3 procedure and analyzed quantitatively using inductively coupled plasma optical emission spectrometer.
Results
The trace metals occurred in varying concentrations along the course of the sampling stations. The decreasing order of trace metal concentrations in the dry season was: Mn > Fe > Pb > Cr > Zn > Ni > Cu > Cd and in the rainy season was Mn > Fe > Pb > Cr > Ni > Zn > Cu > Cd. Little Akaki River contained a higher load of trace metals than the other regions, which is due to the existence of most of the industrial establishments and commercial activities. Relatively lower levels of trace metals were recorded at Aba Samuel reservoir due to the lower residence time of the sediment (reservoir rehabilitated recently). Ecological risk assessment using USEPA sediment guidelines, geo-accumulation index, contamination factor and pollution load index revealed the widespread pollution by Cd and Pb. These were followed by Mn, Ni and Zn.
Conclusion
The concentrations of Pb, Cd, Mn, Ni and Zn in sediments were relatively greater and at levels that may have adverse biological effects to the surrounding biota. Therefore, regular monitoring of these pollutants in water, sediment and biota would be required.
Similar content being viewed by others
Background
Contamination of the aquatic environment by trace metals in excess of the natural loads has become a problem of increasing concern. Large quantities of trace metals are discharged into the environment due to anthropogenic activities such as urbanization, industrialization and extension of irrigation and other agricultural practices. The situation is particularly alarming in developing countries, where most rivers, lakes and reservoirs are receiving untreated wastes due to poor setup of environmental sustainability (Mwanamoki et al. 2014; Awoke et al. 2016). The most vulnerable river-reservoir systems are those crossing large cities and densely populated areas, as well as near the industrial establishments (Mwanamoki et al. 2014; Yousaf et al. 2016). Trace metals are among the conservative pollutants that are not subject to degradation process and are permanent additions to aquatic ecosystems (Igwe and Abia 2006; El Nemr et al. 2016). As a result, higher levels of trace metals are found in soil, sediment and biota. Most of these trace metals are persistent, toxic, bioaccumulative and that they exert a risk for humans and ecosystems even when the exposure is low (Gao and Chen 2012; Diop et al. 2015; Tang et al. 2016).
Monitoring of trace metals in sediment is extremely important as it can serve as sources of information about the long term trends in geological and ecological conditions of the aquatic ecosystem and the corresponding catchment area (Mekonnen et al. 2012; Dhanakumar et al. 2015). The occurrence of high levels of these pollutants in sediments can be a good indicator of anthropogenic pollution, rather than natural enrichment of the sediment by geological weathering.
Trace metals, originating from man-induced pollution and geological sources, have low solubility in water, and thus they get adsorbed on suspended particles and strongly accumulate in the sediments (Li et al. 2017). Therefore, sediments are reservoirs for trace metal contaminants and help to characterize the degree of environmental contamination and thus are suitable targets for pollution studies (Iqbal and Shah 2014; Liang et al. 2015).
Addis Ababa, the capital city of Ethiopia and the headquarters of the African Union, with approximately 5 million population, is one of the fast expanding cities in the country. There are two major rivers draining the city from North to South. These are Greater Akaki River (GAR) and Little Akai River (LAR). Greater Akaki River and Little Akai River meet at Aba Samuel reservoir, 37 km South-West of Addis Ababa. Aba Samuel reservoir was built in 1939, for hydropower production. The reservoir was, however, abandoned for several years due huge pollution issues and siltation (Gizaw et al. 2004). It was rehabilitated recently and have come back to life in 2016. Since Addis Ababa is the country’s commercial, manufacturing and cultural center, large quantities of solid, liquid and gaseous wastes are released into the environment of the city, primarily nearby water bodies most often untreated (Alemayehu 2001, 2006; Awoke et al. 2016; Aschale et al. 2017). The water bodies around Addis Ababa receive increasing amounts of unlicensed discharge of effluents from industrial and domestic wastes and the water quality is deteriorating (Akele et al. 2016). The primary sources of trace metals pollution in the river system include metal finishing industries, tannery operations, textile industries, domestic sources, agrochemicals and leachates from landfills and contaminated sites (Melaku et al. 2007). The two Akaki Rivers and Aba Samuel reservoir, which are the main focuses of this study, serve as dumping grounds and pollutant sinks from upstream Addis Ababa and surrounding catchment areas.
Previous studies on trace metals in sediment are hardly representative and sufficient in the catchment area. There exists an information gap regarding the systematic study of occurrence, distribution, ecological risk and seasonal distribution of trace elements in sediment. The available literature has focused on trace metal levels in water, soil/sediment and vegetables on LAR and not including GAR, Aba Samuel reservoir and downstream areas (Itanna 2002; Arficho 2009; Prasse et al. 2012; Akele et al. 2016; Aschale et al. 2017; Woldetsadik et al. 2017). To the best of our knowledge, no such comprehensive work has been done on the level of trace metals in sediment from Akaki River catchment and Aba Samuel reservoir. Moreover, this study presented one of the earliest set of environmental monitoring data for the reservoir from the feeder Rivers following the restoration of the reservoir in 2016.
Therefore, the objective of this work was to determine the occurrence, distribution, ecological risk and seasonal variation of trace metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in surface sediments from Akaki River catchment and Aba Samuel reservoir.
Materials and methods
Study area
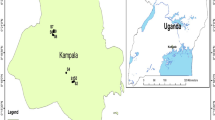
The Akaki catchment is located in central Ethiopia along the western margin of the main Ethiopian Rift Valley. The catchment is geographically bounded between 8°46′–9°14′N and 38°34′–39°04′E, covering an area of about 1500 km2 (Demlie and Wohnlich 2006). Addis Ababa, which lies within Akaki catchment, has a fast population growth, uncontrolled urbanization and industrialization, poor sanitation, uncontrolled waste disposal, which results in a serious deterioration of surface and ground water quality. As it is the country’s commercial, manufacturing and cultural center, large quantities of solid, liquid and gaseous wastes are generated and released into the environment of the city, most often untreated (Alemayehu 2006). There are two major rivers draining into the city from North to South, namely Greater Akaki River (GAR) (locally known as Tiliku Akaki River) and Little Akai River (LAR) (locally known as Tinishu Akaki River). GAR and LAR meet at Aba Samuel reservoir, 37 km South-West of Addis Ababa. Aba Samuel reservoir was built in 1939. It was the first hydropower station in Ethiopia, but it was abandoned in 1970s, because of many years of lack of maintenance, siltation and pollution issues (Gizaw et al. 2004). It was rebuilt and revived in 2016. The local people in the Akaki River catchment and Aba Samuel reservoir use the water for irrigation, drinking water for cattle, washing clothes, waste disposal site and other domestic needs without information on the level of water quality parameters (Melaku et al. 2007). Therefore, this study has been conducted in some parts of GAR, LAR, Aba Samuel reservoir and downstream to the reservoir (Fig. 1).
Sampling sites and sample collection
Twenty-two (22) sediment samples were collected in August, 2016 and January, 2017 representing the rainy and dry seasons respectively. Composite samples were collected at the following sampling stations: GAR at Entoto Kidanemihiret Monastery (S1, control site 1), GAR at Tirunesh Beijing hospital (S2), GAR below Akaki town (S3), LAR above Geferesa reservoir (S4, control site 2), LAR at Lafto bridge (S5), LAR at Jugan Kebele, boundary between Addis Ababa and Oromia Special zone (S6), Aba Samuel reservoir below the confluence point of GAR and LAR (S7), Aba Samuel reservoir at the midpoint (S8), Aba Samuel reservoir above the Dam (S9), downstream about 50 m from the reservoir (S10) and downstream about 1000 m from the reservoir (S11). The distribution of the sampling points was chosen based on topography, the purpose of the study and anthropogenic interference. Approximately 500 g of the top few centimeters of the sediment were collected using a stainless steel Ekman bottom Grab sampler. Each sample was obtained by mixing four randomly collected sediment samples. Samples were placed in clean polyethylene bags, labeled, stored in cooler box and transported into the laboratory. In the laboratory, coarse particles, leaves or large material was removed. Subsequently, sediment samples were air dried at ambient temperature and powdered using ceramic coated grinder. The dried and powdered samples were then sub-sampled and passed through a stainless steel sieve (45 μm mesh size) and transferred to labeled double-cap polyethylene bottles until further treatment.
Sample digestion and instrumental analysis
For determination of trace metals, 2 g sediment samples (< 45 μm) were digested using 20 ml of Mehlich 3 extractant [0.2 M CH3COOH, 0.25 M ammonium nitrate (NH4NO3), 0.015 M ammonium fluoride (NH4F), 0.013 M HNO3, and 0.001 M ethylene diamminetetraacetic acid (EDTA)] (Mehlich 1978). An inductively coupled plasma optical emission spectrometer, ICP-OES, (Arcos FHS2, Germany) was used for the determination of trace metal concentrations in sediment samples. Argon gas (99.99%) was used as a plasma with a flow rate of 81 l/min. Calibration curves were prepared using 10, 20, 30, 40 and 50 mg/l of Fe and Mn; 0.04, 0.08, 0.12, 0.20, 0.40, 0.80, 1.20, 1.60, and 2.00 for Cu and Zn; 0.5, 1, 2, 3, 4, 5 for Cd, Ni and Pb and 1, 2, 4, 6, 8, 10 for Cr. In all cases, standard purity was ≥99.8%. Quantification of the elements were recorded at 214.438, 267.716, 324.757, 262.567, 220.353, 257.611, 231.604 and 213.856 nm, which correspond to the most sensitive emission wavelengths of Cd, Cr, Cu, Fe, Pb, Mn, Ni, and Zn respectively. The calibration curve showed linearity (r > 0.995) by the detector response for the quantified elements. This indicates good correlation between concentration and emission intensities of the detected elements and thus proper calibration of the instrument.
Quality control and quality assurance
All the glassware used were thoroughly washed with detergent, soaked in 10% HNO3 for 24 h and rinsed with de-ionized water. All reagents used were analytical grade. In order to validate and evaluate the accuracy of the method used, certified reference material (ISE-952) obtained from Wageningen University Environmental Sciences section, Netherlands, was employed. Blank analyses were carried out to check interference from the laboratory. Mean recovery rates of the 4 metals were: Zn, 100.52%; Fe, 106.69%; Mn, 118.52%; Cu, 74.14%. The limit of detection (LOD) based on three times the standard deviation (3σ) of the blank and the limit of quantification (LOQ) based on ten times the standard deviation (10σ) of the blank for the ICP-OES were calculated for each analyte ions. The results are summarized in Table 1. The LOD was found to be in the range of 0.07–1.06 mg/kg, whereas LOQ ranged between 0.23 and 3.52 mg/kg. These ranges were found satisfactory for the determination of analyte ions in sediment samples.
Assessment of sediment contamination
The excessive accumulation of trace metals in sediments posed a potential ecological risk to freshwater ecosystems (Olivares-Rieumont et al. 2005; Chen et al. 2007). Different pollution assessment methods of trace metals were applied to evaluate the pollution degree and potential ecological risk posed by trace metals in sediment of Akaki River catchment and Aba Samuel reservoir. To this end, USEPA sediment guidelines, geo-accumulation index (Igeo), contamination factor and pollution load index were used (Wang et al. 2014).
Geo-accumulation index (Igeo): the geo-accumulation index (Igeo) is a geochemical criterion used to assess heavy metal accumulation in surface sediment studies (Muller 1981; Singh 2001; Aschale et al. 2017). It is expressed as
where Cn is the measured total concentration of the element n in the sediment and Bn is the average concentration of element n in shale (background) value. The constant 1.5 is introduced to include possible variations of the background values due to lithogenic effects in sediments (Loska et al. 2004). Thus, the background concentrations (mg/kg) of 0.3 for Cd, 90.0 for Cr, 45.0 for Cu, 46,700.0 for Fe, 20.0 for Pb, 850.0 for Mn, 68.0 for Ni and 95.0 for Zn are used in this study (Turekian and Wedepohl 1961). The background values were used to assess the degree of contamination and to understand the distribution of elements of anthropogenic origin in the study areas. According to Muller (1981), the corresponding relationships between Igeo and the pollution level are given as follows: unpolluted (Igeo ≤ 0), unpolluted to moderately polluted (0 < Igeo ≤ 1), moderately polluted (1 < Igeo ≤ 2), moderately to heavily polluted (2 < Igeo ≤ 3), heavily polluted (3 < Igeo ≤ 4), heavily to extremely polluted (4 < Igeo ≤ 5) and extremely polluted (Igeo > 5).
Contamination factor
The assessment of sediment contamination was also carried using the contamination factor (CF). The CF is the single element index and is represented by the following equation
where Co is the mean content of metals from at least five sampling sites and Cn is the background value of the individual metal. The CF may indicate low contamination (CF < 1), moderate contamination (1 < CF < 3), considerable contamination (3 < CF < 6) and very high contamination (CF > 6) (Hakanson 1980).
Pollution load index (PLI)
Pollution load index (PLI) was examined to assess the overall pollution status of a sampling site. The index was determined by calculating the geometrical mean of the concentrations of all the trace elements in the particular sampling site (Usero et al. 1997; Chakravarty and Patgiri 2009).
The PLI is computed by the formula:
\({\text{PLI }} = \, \left( {{\text{CF}}_{ 1} \times {\text{CF}}_{ 2} \times {\text{CF}}_{ 3} \times \cdots \times {\text{CF}}_{\text{n}} } \right)^{{ 1/{\text{n}}}},\) where n is the number of metals investigated and CF is the contamination factor. The PLI value > 1 is polluted whereas PLI value < 1 indicates no pollution (Chakravarty and Patgiri 2009).
Statistical analysis
Analysis of variance (ANOVA) was applied to assess significant differences in trace element concentrations at the various sampling sites. Multivariate analysis of the element concentration was performed through cluster analysis technique. It was performed to classify elements of different sources on the basis of their concentration similarities using dendrograms, and identify relatively homogeneous groups of variables with similar properties. Pearson’s correlation coefficient was used to determine the association and possible sources of trace metals. Statistical analyses of the results were carried out using Origin Pro (version 9.4, 2017) and Microsoft Excel 2007.
Results and discussion
Concentrations of trace metals in sediment samples
The average concentrations along with standard deviations of 8 selected trace metals in sediment samples from the Akaki River catchment and Aba Samuel reservoir, Ethiopia in two seasons are presented in Table 2. Based on the elemental concentrations, the pattern in sediment was: Mn > Fe > Pb > Cr > Zn > Ni > Cu > Cd in the dry season and Mn > Fe > Pb > Cr > Ni > Zn > Cu > Cd in the rainy season. In both seasons, a similar pattern was observed. The concentration (mg/kg) ranges of trace metals in the dry season were 2.1–2.9 for Cd, 16.2–43.7 for Cr, 1.6–15.3 for Cu, 406.4–844.8 for Fe, 124.4–256.4 for Pb, 335.5–1319.2 for Mn, 15.6–36.2 for Ni and 4–110 for Zn. Similarly, in the rainy season the concentrations (mg/kg) were in the range of 2.5–3.1 for Cd, 18.3–29.4 for Cr, 2.1–6.2 for Cu, 415.2–1442 for Fe, 101.4–133.7 for Pb, 385.1–1833.4 for Mn, 14.6–24 for Ni and 4.8–38.2 for Zn. In both seasons, the minimum concentration was observed for the known toxic elements (such as Cd and Cu) while the highest concentration is observed for Mn and Fe. Iron and manganese pollution of the catchment area, possibly arises from effluents from iron and steel manufacturing industries established within the catchment area of Akaki River (Melaku et al. 2007). The highest concentrations of Mn and Fe observed could also be related to the geological sources in addition to anthropogenic inputs (Alemayehu 2006). The geology of Addis the Ababa area is characterized by basaltic volcanic rocks with minor amounts of Quaternary alluvial sediments (Demlie et al. 2008). The rocks underlying the city and its environs were altered by intensive hydrothermal activity resulting in the characteristics reddish color of the residual soils (Gizaw 2002). Kaolin deposits found in many parts of the city are particularly good evidence of hydrothermal activity on lava flows. Alemayehu (2006) indicated that rock and soil outcrops of the Addis Ababa area are anomalously rich in trace metals derived from hydrothermal activity, which are related to geologic sources. From the hydrogeological point of view, the major rock types forming a reservoir of groundwater in the Addis Ababa area are considered to be the volcanic rocks consisting of basalts, trachytes, rhyolites, scoriaes and trachy-basalts. Studies indicated that the main aquifers in the Addis Ababa area include: shallow aquifers, deep aquifers and thermal aquifers (located at depths greater than 300 m) (Alemayehu 2006; Demlie et al. 2007).
Table 3 compares the results obtained from Akaki River Catchment and Aba Samuel reservoir and those from other freshwater ecosystems to understand the extent of trace metal pollution of the study area. A comparison between a study in LAR (Aschale et al. 2016) and this study indicates that samples from the latter showed higher concentrations of Cd and Pb and lower average concentrations of Cr, Cu, Fe, Mn and Zn. The Pb contamination in the sediments of this study was relatively higher than values from all the other studies. The concentrations of Cd, Cr, and Ni were within the ranges observed in the other polluted sediments. The levels of Cu and Zn were generally lower than values for other sediments. Overall, this comparison indicated that there was high accumulation of trace metals in the sediments of Akaki River and Aba Samuel reservoir and that it requires special care and management interventions such as proper waste collection, treatment and disposal.
Spatial distribution and seasonal variations of Trace metals in sediment
The spatial distribution of trace elements in the river and reservoir sediment depends on many factors including distance of the element sources to the reservoir, the chemical characteristics of the element and the hydrological conditions of the river and reservoir system (Zhang et al. 2013). The level of trace elements in surface sediments of Akaki River catchment and Aba Samuel reservoir is shown in Figs. 2 and 3. In order to better understand the distribution and seasonal variation of trace metals, the study area was divided into four regions: GAR, LAR, reservoir and Downstream to the reservoir. Overall, LAR is more contaminated than GAR (Melaku et al. 2007; Akele et al. 2016). Statistical analyses of the results (p < 0.05) indicated that there were no significant spatial variations of the trace metal among the sampling stations in both seasons except Pb. The concentration of Pb varied significantly from upstream to downstream area in both seasons (p = 0.02). Dry season trace metal concentrations were slightly higher than the rainy season for Pb, Cr, Zn, Ni, and Cu. This might be attributed to the lower dilution process in the dry season than in the rainy season. During the dry season, the highest concentrations of Cr, Pb, Mn, and Ni were observed at S6 and Cu at S5 (Figs. 2 and 3). Both sampling sites lie along the Little Akaki River (LAR), which is in agreement with the previous results (Melaku et al. 2007; Akele et al. 2016). The average levels of Mn and Fe were higher in the rainy than in the dry season, which might be attributed to the anthropogenic and geologic inputs (Alemayehu 2006). It is presumed that pollutants from GAR and LAR finally ended up at the Aba Samuel reservoir. However, the levels of most trace metals investigated were lower at the Aba Samuel reservoir (S7, S8 and S9) than the upstream areas (Figs. 2 and 3). The relatively lower concentration of sediment-bound trace metals in the reservoir might have been due to less accumulated metals in the sediment because reservoir was rehabilitated in 2016. Furthermore, the natural processes that can attenuate the concentration of the chemicals/pollutants on their pathway (mixing, dilution, volatilization and biological degradation) might have contributed to the attenuation.
Correlation analysis
Pearson’s correlation coefficients were computed to see if the elements were interrelated with each other in the sediment samples from the different sampling sites in both dry and rainy seasons. Examination of correlations also provides clues on the source(s) of pollution, distribution and similarity of behaviors of trace metals (Zhang et al. 2013; Diop et al. 2015). Table 4 shows the correlation matrix of the determined elements. A significant positive correlation was observed for Pb with Cr (r = 0.85), Mn with Cr (r = 0.81), Mn with Fe (r = 0.73), Mn with Pb (r = 0.60), Ni with Cr (r = 0.97), Ni with Pb (r = 0.85) and Ni with Mn (r = 0.83) in the dry season. Similarly, a significant positive correlation was observed between Fe with Cd (r = 0.75), Fe with Cr (r = 0.67), Mn with Cd (r = 0.66), Mn with Cr (r = 0.66), Mn with Fe (r = 0.99), Ni with Cr (0.81), Ni with Fe (r = 0.61) in the rainy season. This significant positive correlation suggests that the elements might have a common origin. Concentration of Zn was not significantly correlated with any of the studied trace metals. Significant negative correlations were also found between Cd with Cr (r = − 0.75), Cd with Pb (r = − 0.72), Cd with Mn (r = − 0.72), Cd with Ni (r = − 0.71) in the dry season and Pb with Fe (r = − 0.78), Mn with Pb (r = − 0.82) in the rainy season.
Cluster analysis
The hierarchical clustering by applying group average method and Euclidean distances for similarities in the variables was performed on the dataset. Altogether, 8 variables (Cd, Cr, Cu, Fe, Pb, Mn, Ni and Zn) from 11 sampling sites in dry and rainy seasons were subjected to the cluster analysis. The dendrogram derived from the cluster analysis is shown in Fig. 4. In both seasons, similar types of clusters of the elements were observed. When we cut the dendrogram at an imaginary distance between 1000 and 2000 cm, it leaves two major clusters. Cluster 1 (Cd, Cu, Cr, Ni, Zn and Pb) and cluster 2 (Fe and Mn). From the dendrogram, there are two distinct source factors; one that relates to the soil/geologic inputs (which may introduce Fe and Mn) and another that relates to a variety of activities in the catchment including anthropogenic sources, which collectively contributed to the remaining metals in the sediment. Elements belonging to the same clusters or groups are likely to have originated from common sources (Faisal et al. 2014). The source factors for the cluster analysis of some of the trace metals are presented as follows. Chromium (Cr) contamination of the study area might have originated from one or some of the industries including electroplating and tannery industries, paints and inks, wood preservatives, textile and refractoriness. The highest concentration of Cr was observed at S6 where the majority of Tannery industries were located on the bank of Little Akaki River. The reason for the elevated concentration of Cr at S4 (control site), is not clear. Ni pollution in the study area might arise from sources like domestic wastes, municipal sewage, electroplating coal, oil combustion, pigments and batteries (Aschale et al. 2017). Zn pollution in the study area might arise from the expected sources such as textile and metal works/iron and steel works. In addition to the geological sources, anthropogenic Pb pollution in the study area may arise as a result of activities such as industrial discharge from smelters, paints and ceramics, through vehicular emissions, runoff from contaminated land areas and sewage effluent.
Assessment of trace metals pollution
Assessment of sediment pollution using sediment contamination guidelines
After generating reliable data on the level of trace metals in sediment, interpretive tools are required to relate sediment chemistry information to the risk. To this end, numerical sediment quality guidelines (SQGs) established based on biological tests can be used (Macdonald et al. 1996). Most recent SQGs are derived from matching chemistry and toxicity data. The average concentration of trace metals in surface sediments and guideline values is presented in Table 5. Based on the guideline, the river system and reservoir were non-polluted with Cd and Cu, but non-to moderately polluted with Cr, Ni and Zn. All sampling sites were heavily polluted with Pb.
The concentration of Ni in the analyzed samples were within the same range or slightly higher than the background values for sediment quality guidelines. Only one site, S11 in the dry season is moderately polluted with Zn. In both seasons, all the sampling stations were heavily polluted with Pb. The Federal Democratic Republic of Ethiopia (FDRE) has formulated three proclamations that are directly and/or indirectly related to the environment and pollution (Mekonnen et al. 2015). However, based on the results obtained in this study, the proclamations seem to have not properly implemented. The river system and reservoir need immediate attention of those trace metals having higher concentrations. Unless control measures are made possible, the situation could be worsened and affect biota in Akaki River system and Aba Samuel reservoir and downstream Awash River, which is the most productive inland river in Ethiopia.
“Effects range low” (ERL) and “effects range median” (ERM) developed by the National Oceanic and Atmospheric Administration are another sediment toxicity guidelines for trace metals and other contaminants (Long et al. 1995; Macdonald et al. 1996). ERL and ERM values identify threshold concentrations that, if exceeded, are expected to have adverse ecological or biological effects (Mekonnen et al. 2015). Based on the ERL- ERM range the level of Pb at S6 in LAR in the dry season could be toxic to bottom dwelling aquatic organisms (Table 6), while for Cr, Cu and Ni are less than the ERL range. Some of the results from the sampling sites lie between ERL-ERM range for Cd, Pb and Ni.
Geo-accumulation index, contamination factor and pollution load index
Geo-accumulation index
In this study, the calculated value of the geo-accumulation index (Igeo) is presented in Table 7. According to the Muller scale, the calculated results of Igeo values (Table 7) indicated that the sediments from the 11 sampling sites were found to be in class 0, thus are uncontaminated with Cr, Cu, Fe, Ni, and Zn. Mn concentrations represent unpolluted conditions at all stations except S6 (Igeo = 0.05) in the dry season and S2 (Igeo = 0.26) and S4 (Igeo = 0.52) in the rainy seasons. However, all the sediment samples were moderately to strongly contaminated with Cd. Similarly, sediment samples were moderately to strongly contaminated with Pb.
Contamination factor (CF) and pollution load index (PLI)
Pollution severity and its variation along the sites were determined with the use of pollution load index (PLI). This index is a quick tool to compare the pollution status of different sampling locations. The contamination factor values (Table 8) for Cr, Cu, Fe and Ni were (< 1) at all the sampling sites in both seasons indicating low contamination. The CF of Zn represents low contamination at all the sampling sites except S11 (CF = 1.16) in the dry season. CF values for Cd in both dry and rainy seasons were > 6 at all the sampling sites, indicating very high contamination. Cadmium is one of the highly toxic, non-essential elements. Therefore, even at low concentrations, Cd could be harmful to living organisms. This amount of Cd from the study area may be attributed to the release of chemicals from sewage and industrial wastes from the nearby Addis Ababa city. The CF value of Pb > 6 in all the sampling sites except S1 (CF = 5.58), S2 (CF = 5.72) and S4 (CF = 5.07) in the rainy season, indicating very high contamination. The study area might be exposed to Pb pollution from various activities such as industrial discharge from smelters, paints and ceramics, through vehicular emissions, runoff from contaminated land areas and sewage effluent. CF values for Mn at S2 (CF = 1.30) and S10 (CF = 1.25) in the dry season and S2 (CF = 1.80) and S4 (CF = 2.16) in the rainy season suggesting moderate contamination, while values of CF < 1 in all other sites indicate low contamination. The relatively higher CF value of Mn in the rainy season is in agreement with the cluster analysis result that may in turn suggest soil runoff/geologic inputs as the potential source of the trace metal. The values of PLI (Table 7) were found to be generally low (< 1) in all the studied stations. The higher value of PLI (0.59 at S5 and 0.58 at S11 in the dry season) implies appreciable input of trace metals from anthropogenic sources (Table 8).
Conclusions
This study was targeted at generating up-to-date data on the spatial and seasonal variation and contamination levels of trace metals in surface sediments from Akaki River catchment and Aba Samuel reservoir, Central Ethiopia. The decreasing order of trace metal concentrations in the dry season was: Mn > Fe > Pb > Cr > Zn > Ni > Cu > Cd and in the rainy season was Mn > Fe > Pb > Cr > Ni > Zn > Cu > Cd. When comparing the sampling regions in Akaki River Catchment and Aba Samuel reservoir, Little Akaki River contained a higher trace metal load than the other regions. From the result, it can be concluded that the catchment area have high influx of trace metals as a result of uncontrolled urbanization, industrialization, poor sanitation and uncontrolled waste disposal from municipal, industrial and agricultural sources in the upstream Addis Ababa city. However, relatively lower concentration of sediment-bound trace metals were recorded in the reservoir which might be due to less accumulated metals in the sediment because the reservoir was rehabilitated in 2016. Ecological risk assessment using the USEPA guideline, Igeo, CF and PLI revealed the widespread pollution by Cd and Pb. These were followed by Mn, Ni and Zn. Hence, high level of trace metals in sediments probably have adverse effects to the bottom dwelling aquatic organisms as well as to the health of the people who depend on the water for various activities. Therefore, strict policy measures are required to decrease the degree of contamination since some of the elements are known to be toxic to biota. Furthermore, regular monitoring of these pollutants in water, sediment and biota is recommended.
Abbreviations
- ANOVA:
-
analysis of variance
- CF:
-
contamination factor
- CRM:
-
certified reference material
- ERL:
-
effects range low
- ERM:
-
effects range median
- GAR:
-
Greater Akaki River
- ICP-OES:
-
inductively coupled plasma optical emission spectrometer
- Igeo:
-
geo-accumulation index
- LAR:
-
Little Akai River
- LOD:
-
limit of detection
- LOQ:
-
limit of quantification
- PLI:
-
pollution load index
- SD:
-
standard deviation
- SQGs:
-
sediment quality guidelines
References
Akele M, Kelderman P, Koning C, Irvine K (2016) Trace metal distributions in the sediments of the Little Akaki River, Addis Ababa, Ethiopia. Environ Monit Assess 188:389
Alemayehu T (2001) The impact of uncontrolled waste disposal on surface water quality in Addis Ababa, Ethiopia. SINET 24:93–104
Alemayehu T (2006) Heavy metal concentration in the urban environment of Addis Ababa, Ethiopia. Soil Sediment Contam 15:591–602
Arficho DF (2009) Status, Distribution, and Phytoavailability of Heavy Metals and Metalloids in Soils Irrigated with Wastewater from Akaki River, Ethiopia: Implications for environmental management of heavy metal/metalloid affected soils. Addis Ababa University, Addis Ababa
Aschale M, Sileshi Y, Kelly-Quinn M, Hailu D (2016) Evaluation of potentially toxic element pollution in the benthic sediments of the water bodies of the city of Addis Ababa, Ethiopia. J Environ Chem Eng 4:4173–4183
Aschale M, Sileshi Y, Kelly-Quinn M, Hailu D (2017) Pollution assessment of toxic and potentially toxic elements in agricultural soils of the city Addis Ababa, Ethiopia. Bull Environ Contam Toxicol 98:234–243
Awoke A, Beyene A, Kloos H, Goethals PL, Triest L (2016) River water pollution status and water policy scenario in Ethiopia: raising awareness for better implementation in developing countries. Environ Manag 58:694–706
Chakravarty M, Patgiri AD (2009) Metal pollution assessment in sediments of the Dikrong River, NE India. J Hum Ecol 27:63–67
Chen CW, Kao CM, Chen CF, Dong CD (2007) Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 66:1431–1440
Demlie M, Wohnlich S (2006) Soil and groundwater pollution of an urban catchment by trace metals: case study of the Addis Ababa region, central Ethiopia. Environ Geol 51:421–431
Demlie M, Wohnlich S, Wisotzky F, Gizaw B (2007) Groundwater recharge, flow and hydrogeochemical evolution in a complex volcanic aquifer system, central Ethiopia. Hydrogeol J 15:1169–1181
Demlie M, Wohnlich S, Ayenew T (2008) Major ion hydrochemistry and environmental isotope signatures as a tool in assessing groundwater occurrence and its dynamics in a fractured volcanic aquifer system located within a heavily urbanized catchment, central Ethiopia. J Hydrol 353:175–188
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Saf 113:145–151
Diop C, Dewaelé D, Cazier F, Diouf A, Ouddane B (2015) Assessment of trace metals contamination level, bioavailability and toxicity in sediments from Dakar coast and Saint Louis estuary in Senegal, West Africa. Chemosphere 138:980–987
Dirbaba NB, Xue Y, Wu H, Wang J (2018) Occurrences and Ecotoxicological Risk Assessment of Heavy Metals in Surface Sediments from Awash River Basin, Ethiopia. Water 10:535
El Nemr A, El-Said GF, Ragab S, Khaled A, El-Sikaily A (2016) The distribution, contamination and risk assessment of heavy metals in sediment and shellfish from the Red Sea coast, Egypt. Chemosphere 165:369–380
Faisal B, Majumder RK, Uddin MJ, Abdul M (2014) Studies on heavy metals in industrial effluent, river and groundwater of Savar industrial area, Bangladesh by principal component analysis. Int J Geomatics Geosci 5:182–191
Gao X, Chen CTA (2012) Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res 46:1901–1911
Gizaw B (2002) Hydrochemical and environmental investigation of the Addis Ababa region, Ethiopia. Unpublished PhD Thesis, Ludwig Macmillan, University of Munich, Munich
Gizaw E, Legesse W, Haddis A, Deboch B, Birke W (2004) Assessment of factors contributing to eutrophication of Aba Samuel Water reservoir in Addis Ababa, Ethiopia. Ethiop J Health Sci 14:112–223
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Halli M, Sari E, Kurt MA (2014) Assessment of arsenic and heavy metal pollution in surface sediments of the Ergene River, Turkey. Pol J Environ Stud 23:1581
Igwe J, Abia A (2006) A bioseparation process for removing heavy metals from waste water using biosorbents. Afr J Biotechnol 5:11
Iqbal J, Shah MH (2014) Occurrence, risk assessment, and source apportionment of heavy metals in surface sediments from Khanpur Lake, Pakistan. J Anal Sci Technol 5:28
Itanna F (2002) Metals in leafy vegetables grown in Addis Ababa and toxicological implications. Ethiop J Health Dev 16:295–302
Kishe M, Machiwa J (2003) Distribution of heavy metals in sediments of Mwanza Gulf of Lake Victoria, Tanzania. Environ Int 28:619–625
Li N, Tian Y, Zhang J, Zuo W, Zhan W, Zhang J (2017) Heavy metal contamination status and source apportionment in sediments of Songhua River Harbin region, Northeast China. Environ Sci Poll Res 24:3214–3225
Liang J, Liu J, Yuan X, Zeng G, Lai X, Li X, Wu H, Yuan Y, Li F (2015) Spatial and temporal variation of heavy metal risk and source in sediments of Dongting Lake wetland, mid-south China. J Environ Sci Health 50:100–108
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Loska K, Wiechuła D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30:159–165
Macdonald DD, Carr RS, Calder FD, Long ER, Ingersoll CG (1996) Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology 5:253–278
Mehlich A (1978) New extractant for soil test evaluation of phosphorus, potassium, magnesium, calcium, sodium, manganese and zinc. Commun Soil Sci Plant Anal 9:477–492
Mekonnen KN, Ambushe AA, Chandravanshi BS, Abshiro MR, McCrindle RI, Panichev N (2012) Distribution of mercury in the sediments of some freshwater bodies in Ethiopia. Toxicol Environ Chem 94:1678–1687
Mekonnen KN, Ambushe AA, Chandravanshi BS, Redi-Abshiro M, McCrindle RI (2015) Occurrence, distribution, and ecological risk assessment of potentially toxic elements in surface sediments of Lake Awassa and Lake Ziway, Ethiopia. J Environ Sci Health 50:90–99
Melaku S, Wondimu T, Dams R, Moens L (2007) Pollution status of Tinishu Akaki River and its tributaries (Ethiopia) evaluated using physico-chemical parameters, major ions, and nutrients. Bull Chem Soc Ethiop 21:13–22
Mohiuddin K, Alam M, Ahmed I, Chowdhury A (2015) Heavy) metal pollution load in sediment samples of the Buriganga river in Bangladesh. J Bangladesh Agril Univ 13:229–238
Muller G (1981) The heavy metal pollution of the sediments of Neckars and its tributary: a stocktaking. Chem Ztg 105:157–164
Mwanamoki PM, Devarajan N, Thevenon F, Birane N, de Alencastro LF, Grandjean D, Mpiana PT, Prabakar K, Mubedi JI, Kabele CG (2014) Trace metals and persistent organic pollutants in sediments from river-reservoir systems in Democratic Republic of Congo (DRC): spatial distribution and potential ecotoxicological effects. Chemosphere 111:485–492
Olivares-Rieumont S, de la Rosa D, Lima L, Graham DW, Katia D, Borroto J, Martínez F, Sánchez J (2005) Assessment of heavy metal levels in Almendares River sediments—Havana City, Cuba. Water Res 39:3945–3953
Prasse C, Zech W, Itanna F, Glaser B (2012) Contamination and source assessment of metals, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons in urban soils from Addis Ababa, Ethiopia. Toxicol Environ Chem 94:1954–1979
Shanbehzadeh S, Vahid Dastjerdi M, Hassanzadeh A, Kiyanizadeh T (2014) Heavy metals in water and sediment: a case study of Tembi River. J Environ Public Health 2014:858720
Singh M (2001) Heavy metal pollution in freshly deposited sediments of the Yamuna River (the Ganges River tributary): a case study from Delhi and Agra urban centres, India. Environ Geol 40:664–671
Tang W, Shan B, Zhang H, Zhu X, Li S (2016) Heavy metal speciation, risk, and bioavailability in the sediments of rivers with different pollution sources and intensity. Environ Sci Poll Res 23:23630–23637
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192
Usero J, Gonzalez-Regalado E, Gracia I (1997) Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic Coast of Southern Spain. Environ Int 23:291–298
Wang L, Wang Y, Zhang W, Xu C, An Z (2014) Multivariate statistical techniques for evaluating and identifying the environmental significance of heavy metal contamination in sediments of the Yangtze River, China. Environ Earth Sci 71:1183–1193
Woldetsadik D, Drechsel P, Keraita B, Itanna F, Gebrekidan H (2017) Heavy metal accumulation and health risk assessment in wastewater-irrigated urban vegetable farming sites of Addis Ababa, Ethiopia. Int J Food Contam 4:9
Xu D, Wang Y, Zhang R, Guo J, Zhang W, Yu K (2016) Distribution, speciation, environmental risk, and source identification of heavy metals in surface sediments from the karst aquatic environment of the Lijiang River, Southwest China. Environ Sci Poll Res 23:9122–9133
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SM, Ishizuka M (2013) Occurrence, distribution, and ecological risk assessment of DDTs and heavy metals in surface sediments from Lake Awassa—Ethiopian Rift Valley Lake. Environ Sci Poll Res 20:8663–8671
Yousaf B, Liu G, Wang R, Imtiaz M, Zia-Ur-rehman M, Munir MA, Niu Z (2016) Bioavailability evaluation, uptake of heavy metals and potential health risks via dietary exposure in urban-industrial areas. Environ Sci Poll Res 23:22443–22453
Zhang D, Zhang X, Tian L, Ye F, Huang X, Zeng Y, Fan M (2013) Seasonal and spatial dynamics of trace elements in water and sediment from Pearl River Estuary, South China. Environ Earth Sci 68:1053–1063
Authors’ contributions
All authors have contributed at different stages of this study. ABK designed the study, collected and analyzed samples and interpreted the data. He also wrote the draft manuscript. SLA and TBE involved on the design of the study, supervised the progress and provide comments on the manuscript. JOO supervised the work and provided comments on the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The first author would like to thank Addis Ababa University for financial support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The dataset and materials used for this manuscript is available and can be shared whenever necessary. The data was generated by the author from the field sample collection, processing and laboratory analysis.
Consent for publication
Note applicable.
Ethics approval and consent to participate
Note applicable.
Funding
The authors greatly acknowledge Addis Ababa University Vice President office for Research and Technology Transfer for Financial support via thematic research project.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kassegne, A.B., Esho, T.B., Okonkwo, J.O. et al. Distribution and ecological risk assessment of trace metals in surface sediments from Akaki River catchment and Aba Samuel reservoir, Central Ethiopia. Environ Syst Res 7, 24 (2018). https://doi.org/10.1186/s40068-018-0127-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40068-018-0127-8