Abstract
Background
The contact process preferably used vanadium pentaoxide as catalyst to increase the rate of reaction of producing sulphuric acid. Sulfuric acid plants regularly require catalyst replacement in order to cope with process improvements. The spent catalyst is considered as hazardous solid waste and cannot be discarded untreated owing to presence of high amount of vanadium and other associated metals. Because of significant environment implications of spent catalyst wastes, it is imperative to recover valuable metals present in them. The recovery of precious materials or metals from waste will not only help in mitigating environment problem due to metal pollution but also help improve the economy of the country. The purpose of this research work is to develop method to recover vanadium from spent V2O5 catalyst.
Results
The detailed study of extraction, separation and recovery of vanadium from leached spent catalyst solution of composition; V, 3.6% ; Al, 2.1%; Fe, 1.3%; Ti, 0.8% and less than 1 % of Cr and Pb is reported in this paper. Cyanex 272 (bis (2, 4, 4-trimethylpentyl) phosphinic acid) has been explored for the recovery of vanadium from spent V2O5 catalyst. The effects of different parameters like, pH, solvent concentration, organic to aqueous ratio etc. were optimised for the complete extraction and recovery of vanadium.
Conclusions
The proposed procedure gives high purity vanadium with almost a quantitative yield (~99%) and of course free from closely associated metals. The extractants could be reused up to ten cycles with no significant change in the extraction capability.
Similar content being viewed by others
Background
Vanadium is generally used for alloying steel and iron. Major uses of vanadium as oxide are in the production of oxidation catalysts for the manufacturing of sulphuric acid, in petroleum refinery for catalytic cracking of heavy oil and in many industrial processes. The vanadium has seen a steady rise in demand from the steel construction industry as building regulations increasingly call for improved strength and lighter construction materials. More recently the development of large-scale vanadium-redox flow batteries (VRFBs) for use in energy grid storage applications has opened up a new demand stream for vanadium (Gan and Dong 2010; Lazenby 2012).
The increased demand of vanadium by the industry has put extra pressure for the production of metal which has caused gradual depletion of natural resources containing V. This has encouraged researchers to look for alternative or secondary sources such as industrial or electronic wastes, spent catalysts and other by products. Among secondary resources, the metal recovery from spent catalyst is gaining interest due to both, its hazardous nature and stringent regulations associated with disposal methods (Furimsky 1996; Srichandan et al. 2013). The life of a catalyst varies from 3 to 6 years depending upon the impurities in the feed and number of cycles used (Park et al. 2006). The deactivated catalyst can be reactivated by a number of different mechanisms, both chemical and physical in nature. After a number of deactivation–activation cycles, the catalyst is discarded as waste (Furimsky 1996). Due to heavy metal content, spent catalyst is categorized as hazardous waste (Rapaport 2000) and therefore need to be processed for the total metal recovery prior to its safe disposal. Recycling of spent catalyst is also important from environmental and economical perspective. One of the major benefits is a significant energy saving using recycled materials compared to virgin materials. It also helps in waste management by limiting the amount of waste on landfilled (Fornalczyk 2012).
Main techniques for the separation and purification of vanadium in spent catalyst leach solutions are precipitation, carbon adsorption, ion exchange and solvent extraction. Precipitation offers low cost and simple operation, however, high purities of vanadium cannot be achieved by this (Zeng et al. 2006; Zeng and Yong Cheng 2009b). The loading capacities of activated carbon for vanadium are relatively low, resulting in no industrial application. The scale of application of ion exchange in industry is limited owing to high cost of exchangers although it can be used to separate vanadium from other associated impurities almost completely thereby producing high purity products. Solvent extraction is the well established cost effective operation for purification of vanadium in their aqueous solutions (Fornalczyk 2012 Marafi and Stanislaus 2008). Solvent extraction technique is one of the most attractive alternatives for this purpose. It has certain inherent advantages such as ease of continuous operation, high throughputs and improved economics coupled with flexibility of handling a variety of metal solutions from diverse sources (Zeng and Cheng 2009a).
A significant effort has been made to recover vanadium from aqueous solutions by solvent extraction technique using various extractants. During the latter half of the twentieth century, reagents like –diketones, oxines, oximes, dithizones, dithiocarbamates, dithiols, high molecular weight amines (HWWA) and organophosphorus compounds came into prominence. Among these HMWA and organophosphorus compounds emerged out as popular commercial extractants for vanadium. However, the problem of emulsion formation in the former prevented quantitative phase separation (Gupta and Krishnamurthy 1992; Nguyen and Lee 2013; Saily 1997; Tavakoli and Dreisinger 2014).
As of now alkylphosphorus compounds are more or less dominating the market of extractants. Among organophosphorus, DEHPA (bis (2-ethylhexyl) phosphoric acid) and TOPO (Tri-n-octylphosphine oxide) have been extensively used for extraction behaviour of vanadium (Hughes and Biswas 1991; Islam and Biswas 1980; Juang and Lo 1993; Li et al. 2011; Sato et al. 1980). However, there are few problems with these solvents for example DEHPA readily form an emulsion or a third phase during stripping of Mo and V from the loaded solvent with the ammonia solution. Poor selectivity is another disadvantage of these extractants since they can co-extract several metals, including impurity elements such as iron and aluminium even at low pH (Sahu et al. 2013). TOPO shows high extraction coefficient but poor selectivity. It is known to extract approximately 30 metal ions from aqueous solutions (Shaeri et al. 2015).
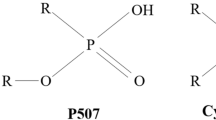
Cyanex reagents are structurally different than most of the commercially available organophosphorous reagents (e.g. DEHPA, DDPA, TBP, EHPEHPA etc.), where alkyl groups are bonded directly to the phosphorus atoms through P-C bonds rather than P-O-C bonding. The presence of hydrophobic P-C bonding in Cyanex reagents renders them to be less susceptible to hydrolysis and less soluble in water than other reagents (Chen and Wang 2010; Saily and Tandon 1998).

Extraction equilibria studies of vanadium using Cyanex 272 (bis (2, 4, 4-trimethylpentyl) phosphinic acid) have been reported by researchers (Li et al. 2012; Saily and Tandon 1998; Zang et al. 1996; Zeng and Cheng 2009a) and few studies have also been conducted on its recovery from wastes. Most of separation and recovery was done employing synergetic effects of two or more extractants (Noori et al. 2014; Shaeri et al. 2015; Tavakoli and Dreisinger 2014; Wu et al. 2012), however the occurrence of white turbidity and a third phase formation was the major problem observed during stripping of V from the loaded organic (Li et al. 2011; Sahu et al. 2013). The present study focussed on the extraction studies of V along with closely associated metal ions from nitrate solutions and its quantitative recovery from spent V2O5 catalyst employing Cyanex 272 without synergetic effect, which certainly enhance financial viability of methodology. A complete separation scheme for the recovery of vanadium from spent V2O5 catalyst was developed and designed to extract impurities in the organic phase leaving behind vanadium in raffinate to overcome the problem of turbidity and third phase formation during stripping.
Result and Discussion
Effect of solvent concentration on metal extraction
The concentration of Cyanex 272 was varied from 5% to 30% (v/v), to optimize the concentration of the solvent for the extraction of metals from leached spent V2O5 catalyst solution. The pH of leach solution and O/A ratio were maintained at a constant of 2 and 1, respectively. The percentage extraction of metal ions first shows an increase with the increasing solvent concentration up to 25% and then becomes constant (Figure 1). Cyanex 272 shows a greater affinity for titanium over iron at a lower solvent concentration. For all other experiments 30% solvent was used.
Effect of pH on the extraction
Extraction efficiency of vanadium is highly dependent on pH because it forms different anionic complexes at low pH (Zeng and Yong Cheng 2009b). The extraction behaviour was studied at different pH ranging from 0 to 3. The other experimental conditions were fixed as O/A 1:1, Cyanex 272 concentration 30% (v/v). The extraction behaviour of V along with Al, Fe, Ti, Cr and Pb is shown in Figure 2. The extraction of Fe and Al increased with increasing pH, however, V follows reverse pattern that is extraction efficiency increased with decreasing pH. Extraction of Ti is constant (80%) at lower pH and shows a quantitative increase at higher pH from 1 to 3. Co-extraction of Cr and Pb is almost negligible (<5 %) and is not shown. At the equilibrium pH of 3, the distribution ratios for V, Al, Fe and Ti were found to be 0.04, 21, 64 and 37 respectively. The separation factor of Ti/V, Al/V and Fe/V were determined to be 880, 500 and 1523, respectively, which is a good separation value. To separate V from Fe Al and Ti from the leached solution the pH was fixed to 3.
Saturation loading capacity of Extractant
At pH 3 the 30% (v/v) Cyanex 272 was contacted with the leach solution of composition V- 366 mg/L, Al-215 mg/L, Fe-129 mg/L and Ti-75 mg/L, in a multiple contact mode. The O/A phase ratio was maintained at 1:1 to study the saturation loading capacity of the solvent. The metal uptake from the aqueous phase continued to increase after each contact, however, from third contact onwards, the extracted metal showed precipitation at the interface of aqueous and organic layer (Figure 3). The solvent was loaded with about 660 mg/L of Al, 453 mg/L of Fe and 256 mg/L of Ti after the fourth cycle, which was assumed as the saturated loading capacity of 30% Cyanex 272 for Al, Fe and Ti. At pH 3, the vanadium uptake was found less than 5% (Figure 2) in multiple contact modes and hence it is the most enabled condition for the separation of vanadium from Al, Fe and Ti.
Effect of O/A phase ratio
The distribution of V, Al, Fe and Ti present in leached solution was carried out at a different O/A ratio (organic to aqueous) using 30% Cyanex 272 at pH 3 to determine the condition for the maximum extraction and subsequent separation of these metals. The O/A phase ratio was varied from 1:5 to 5:1. The solution containing V- 366 mg/L, Al-215 mg/L, Fe-129 mg/L and Ti-75 mg/L with pH 3 was used for the experiment. It was observed that with increase in O/A phase ratio from 0.2 to 3.0, extraction of Fe, Ti and Al also increased (Table 1). In order to determine the extraction isotherms, the McCabe –Thiele plot was constructed from phase ratio variation study for Fe and Al. The McCabe –Thiele plot represented in Figures 4 and 5 for Fe and Al, respectively, predicted requirement of 2 theoretical stages at O/A ratio at 1:2 for complete extraction of associated metal ions (Al, Fe, and Ti) and subsequent separation of vanadium from leached solution of spent V2O5 catalyst.
In order to validate the number of stages determined from the McCabe –Thiele plots (Figures 4 and 5), counter current simulation study for the complete extraction of Fe and Al from the leached solution was carried out. It was observed that 99% of Fe and 95% of Al could be extracted in two stages leaving behind vanadium in aqueous phase using 30% Cyanex 272 with O:A ratio of 1:2 at an aqueous phase pH of 3. The problem in stripping of vanadium from loaded Cyanex 272 encountered by other researchers due to turbidity and third phase formation could be overcome in this investigated process as vanadium remains in aqueous phase.
Stripping of iron, titanium and aluminium from loaded Cyanex 272
Leached solution of spent V2O5 catalyst containing V, Al, Fe and Ti loaded to the Cyanex 272 by two stage counter current process as determined by McCabe –Thiele plot (Figures 4 and 5) using O/A phase ratio of 1:2. The loaded organic containing Al; 730 mg/L, Fe; 262 mg/L, and Ti 155 mg/L was washed first with 5% (NH4)2CO3 for selective stripping of Ti from Fe and Al. After the removal of Ti, Fe was stripped from organic phase by washing it first with10 ml of 3.0 M HCl and then with 10 ml of 0.10 M oxalic acid. Al left behind after Ti and Fe removal was stripped from organic phase by washing it with 10 ml of 0.1 M tartaric acid. The percentage recovery of Al, Fe and Ti was 98, 99 and 99, respectively.
The organic phase, after removal of Fe, Ti and Al can be regenerated by washing it twice with water. The regeneration capacity of this extraction system was tested by carrying out successive extraction stripping cycles of kerosene solution of Cyanex 272. The results show no significant change in the extractability of reagents upto ten cycles.
Process flow sheet of vanadium recovery from spent V2O5 catalyst
Based on above investigation, a process flow sheet was developed for the recovery of vanadium from leached solution of spent V2O5 catalyst (Figure 6). In this flow sheet, vanadium can be effectively separated from Al, Fe and Ti in two stages counter current extraction process using 30% Cyanex 272. Vanadium rich raffinate, which was almost free from Al, Fe and Ti was precipitated with NH4OH at a pH about 8. The precipitate was filtered and calcined at 500°C for 1 hr to produce corresponding oxide in pure form.
Conclusions
The above investigations project the potential of Cyanex 272 for the recovery of vanadium from spent V2O5 catalyst of composition V- 366 mg/L, Al-215 mg/L, Fe-129 mg/L and Ti-75 mg/L. In the proposed extraction process vanadium remains in aqueous phase which has two major advantages. First, it not only succeeded in dealing with the problem of turbidity but also eliminated the problem of third phase formation, which was encountered by past researchers during the stripping of vanadium from loaded Cyanex 272 and second the possibility of loss of vanadium is negligible. The formation of emulsion and crud is the severe disadvantage in solvent extraction operations as this constitutes a major solvent loss which affects the operating costs. Further, the extraction process using Cyanex 272 also recovered aluminium, iron and titanium besides vanadium, which are present in significant amount in spent V2O5 catalyst.
The extraction isotherm, constructed at pH 3 with O:A phase ratio of 1:2 through McCabe-Thiele plot predicted two stage counter current process. Vanadium free from Al, Fe and Ti was selectively recovered as their ammonium salt, which was calcined at 500°C to get the vanadium as oxide (V2O5). Recovery of vanadium as V2O5 was found to be 99%. The extractants could be reused simply by washing with water upto ten cycles with no significant change in the extraction capability.
The present bench level study successfully showed pure and quantitative recovery of vanadium as oxide. However, few additional experiments like leaching process time, phase contact time and effect of different stripping agents along with deep understanding of extraction mechanism need to be considered as future work before it can be scaled up from bench level to plant scale.
Methods
Cyanex 272 was obtained from Cytec, USA and was used without further purification. The sample of spent V2O5 catalyst was obtained from Projects and Development India Limited (PDIL), Sindri (India). All chemicals used were of analytical purity. Stock solutions of metal ions were standardized by usual complexometric titrations (Meites 1963; Schwarzenbach and Flaschka 1969).
1 g of the powdered spent V2O5 catalyst (V, 3.5% ; Al, 2.0%; Fe, 1.3% ,Ti, 0.8%; and less than 1% of Cr and Pb) was repeatedly (four times) digested with aqua-regia. The residue was treated with 10 ml of concentrated HNO3 and filtered. The filtrate was boiled with nitric acid, cooled and made upto a final volume of 100 ml. The metal ion concentrations in the leach solution of catalyst agree fairly well with the composition data provided by the supplier. In order to get a representative value five separate samples of the catalyst were dissolved and processed by the proposed extraction procedure.
The solvent extraction and stripping experiments were carried out by shaking the required volumes of aqueous and organic phase in a glass -stopper separatory funnel at room temperature (25 ± 2 °C) for 3 minutes to ensure equilibration. After phase separation the metal concentration in aqueous and stripped organic phase were analysed by Inductive Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES). The distribution ratio (D) and per cent extraction (% E) of metal ions were calculated by the usual method.
where, [M]org is the concentration of a metal in the organic phase and [M]aq is concentration in the aqueous phase. Vorg and Vaq are the volume of the organic and aqueous phases, respectively. The different results reported in the paper are the average of minimum of two determinations. Blank determinations were carried out wherever necessary and the corrections were made if required. During the analysis for different parameters blanks, duplicates, spikes and standards were processed on 5% basis. The percentage recovery for spiked samples in metal determinations ranged from 94% to 104%, which indicate that the results are accurate and unbiased. Relative percent difference of duplicate measurements was less than 10%, which is a satisfactory precision.
References
Chen L, Wang YZ (2010) Aryl polyphosphonates: useful halogen-free flame retardants for polymers. Materials 3:4746–4760
Fornalczyk A (2012) Industrial catalysts as a source of valuable metals. JAMME 55:864–869
Furimsky E (1996) Spent refinery catalysts: environment, safety and utilization. Catal Today 30:223–286
Gan Y, Dong H (2010) Review of Applications of Vanadium in Steels. Proceedings of International Seminar on Production and Application of High Strength Seismic Grade Rebar Containing Vanadium Beijing, China
Gupta CK, Krishnamurthy N (1992) Extractive metallurgy of vanadium (process metallurgy). Elsevier, Netherlands
Hughes MA, Biswas RK (1991) The kinetics of V(IV) extraction in the acidic sulphate DEHPA-n- hexane system using the rotating diffusion cell technique. Hydrometallurgy 26:281
Islam F, Biswas RK (1980) The solvent Extraction of V (IV) with HDEHP in benzene and kerosene. The solvent extraction of V (IV) from sulphuric acid solutions with bis(2-ethylhexyl)phosphoric acid in benzene and kerosene. J Inorg Nucl Chem 42:415
Juang RS, Lo RH (1993) Stoichimetery of vanadium (IV) extraction from sulphate solutions with bis(2-ethylhexyl)phosphoric acid dissolved in kerosene. J Chem Eng Jpn 26:219
Lazenby H (2012) High-tech uses for vanadium to drive demand, prices higher. Minning weekly Creamer Media’s, Johannesburg, South Africa
Li X, Wei C, Deng Z, Li M, Li C, Fan G (2011) Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy 105:359–363
Li X, Wei C, Wu J, Li C, Li M, Deng Z (2012) Thermodynamics and mechanism of vanadium(IV) extraction from sulphate medium with D2EHPA, EHEHPA and CYANEX 272 in kerosene. Trans Nonferrous Metals Soc China 22:461–466
Marafi M, Stanislaus A (2008) Spent hydrprocessing catalyst management: A review Part II Advances in metal recovery and safe disposal methods. Resour Conserv Recycl 53:1–26
Meites L (1963) Hand book of analytical chemistry. Mc Graw-Hill, New York
Nguyen HT, Lee MS (2013) Recovery of molybdenum and vanadium from acidic leaching solution of spent catalysts by solvent extraction. J Korean Inst Resour Recycl 22:3–11
Noori M, Rashchi F, Babakhani A, Vahidi E (2014) Selective recovery and separation of nickel and vanadium in sulfate media using mixtures of D2EHPA and Cyanex 272. Sep Purif Technol 136:265–273
Park KH, Mohapatra D, Reddy BR (2006) Selective recovery of molybdenum from spent HDS catalyst using oxidative soda ash leach/carbon adsorption method. J Hazard Mater 138:311–316
Rapaport D (2000) Are spent hydrocracking catalysts listed hazardous wastes? Hydrocarb Process 79:49–53
Sahu KK, Agrawal A, Mishra D (2013) Hazardous waste to materials: recovery of molybdenum and vanadium from acidic leach liquor of spent hydroprocessing catalyst using alamine. J Environ Manage 125:68–73
Saily A (1997) Studies on liquid-liquid extraction of molybdenum, tugsten and vanadium using alkylphosphine extractants. Indian Institute of Technology, Roorkee
Saily A, Tandon SN (1998) Liquid-liquid extraction behaviour of V (IV) using phosphinic acids as extractants. Fresenius J Anal Chem 360:266–270
Sato T, Ikoma S, Nakamura T (1980) Solvent extraction of vanadium (IV) from hydrochloric acid solutions by neutral organophosphorus compounds. Hydrometallurgy 6:13–23
Schwarzenbach G, Flaschka H (1969) Complexometric Titrations. Methuen, London
Shaeri M, Torab-Mostaedi M, Rahbar Kelishami A (2015) Solvent extraction of thorium from nitrate medium by TBP, Cyanex272 and their mixture. J Radioanal Nucl Chem 303(3):2093–2099
Srichandan H, Singh S, Kim DJ, Lee S (2013) A comparative study of metal extraction from spent catalyst using acidithiobacillus ferrooxidans. Eng Technol 7:112–116
Tavakoli MR, Dreisinger DB (2014) Separation of vanadium from iron by solvent extraction using acidic and neutral organophosporus extractants. Hydrometallurgy 141:17–23
Wu J, Wei C, Li X, Wang S, Wang M, Li C (2012) Selective extraction of Mo using Cyanex-272 and tributyl phosphate from low grade Ni-Mo ore leach liquor. Sep Purif Technol 99:120–126
Zang P, Inoue K, Tsuyama H (1996) Solvent extraction of V(IV) from H2SO4 acid solution by bis(2,4,4-trimethylpentyl) phosphinic acid by ExxsolD80. J Chem Eng Jpn 29:82
Zeng L, Cheng CY (2009a) A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part I: Metallurgical processes. Hydrometallurgy 98: 1–9
Zeng L, Yong Cheng C (2009b) A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part II: Separation and purification. Hydrometallurgy 98: 10–20
Zeng L, Xiao LS, Li QG, Xiang XY (2006) Study of separation of vanadium from ammonium molybdate solution by ion exchange. Rare Metal and Hard Alloy 3:1–4, in Chinese
Acknowledgements
The author is grateful to Cytec Industries, USA for providing the gift of Cyanex extractants. Assistance from the Projects and Development India Limited (PDIL), Sindri (India) is highly appreciated. The financial assistance from the Council of Scientific and Industrial Research (CSIR), New Delhi, India is gratefully acknowledged. Author would like to express thanks and appreciation to the Chancellor, Pro Chancellor and Vice Chancellor of Shri Ramswaroop Memorial University, India for their help and encouragement to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that there are no identified conflicts of interest associated with this publication and there has been no major financial support for this work that could have influenced its outcome.
Authors’ contributions
ASP has designed the research plan and carried out the experiments. The analysis and data interpretation has done by author. The author has written and approved the final manuscript.
Authors’ information
Dr. Archana Saily Painuly is a researcher in Shri Ramswaroop Memorial University, India. Dr. Painuly’s research interest cover broad spectrum of hydrometallurgical and bio-hydrometallurgical methods with interest from basic mechanism to industrial applications.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Painuly, A.S. Separation and recovery of vanadium from spent vanadium pentaoxide catalyst by Cyanex 272. Environ Syst Res 4, 7 (2015). https://doi.org/10.1186/s40068-015-0032-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40068-015-0032-3