Abstract
Background
In the context of the knowledge of the functioning of estuaries and the evolution of abiotic factors on the basis of the marine dynamics, a study was conducted at the level of the Loukkos river estuary.
Results
Water samples were collected seasonally from March to September during 2006 in 5 stations, spread throughout the estuary. Monitoring of several parameters showed significant variations. Indeed, the temperature of water ranging from 19.20 at 29.70°C, the pH is alkaline fluctuating from 7.02 to 7.99, salinity reach 29.50 mg/l, dissolved oxygen fluctuated between 2.17 and 12.33 mg/l and conductivity which follows the profile of salinity indicating the degree of mineralization of mean ranging from 593 to 54700 μs/cm.
Conclusions
This research reveals to identify a spatial and temporal functional dynamism of the study estuary denoting the existence of two gradients: the first, upstream downstream, very marked at high tide, linked to marine and ocean inflow hydrodynamics and the second that in relation to the upstream water intake is very marked at low tide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Water is an indispensable natural resource on earth. All life including human being depends on water. Due to its unique properties water is of multiple uses for living organism (Majumder and Dutta [2014]; Singh [2014]). Thus, water is a natural resource with limited and uneven distribution in time and space. All forms of life and all human activities are dependent on water. Water resources are of great importance to human life and economy and are the main source of meeting the demand for drinking water, for irrigation of lands and industries. Lack of water is considered as a limiting factor of socio-economic development of a country (Këpuska [2013]; N’Diaye et al. [2013]).
The region of Larache endures a big problem of pollution of the surface waters. This pollution takes various origins, domestic, agricultural and industrial (El Morhit et al. [2013]). Indeed, the Loukkos basin is located in the Rif field. This area corresponds “to the Mediterranean Morocco”, i.e. to the northern part of Morocco who bears the geographical imprint and more geological Mediterranean influence (Durand Delga et al. [1962]; Marçais and Suter [1966]).
The basin of the Loukkos river extends on an area of 3750 km2. Its average altitude is 300 m. The highest points are djebels Alam (1670 m) and Khezena (1700 m).
The Loukkos river estuary is located in sub-humid upstairs of the Emberger classification (Emberger [1955]). Although it is located in the Atlantic coast. It has a regime of ‘Mediterranean’ type, characterized by the opposition of a wet season (November to April), and a dry season (from May to October). It is however tempered by the proximity of the ocean, but this moderating influence is hidden towards inside, by the presence of a plateau which is interposed between the plain and the ocean. The hydrology of the Loukkos river estuary, linked to seasonal variations, reflects those of precipitation. Upstream inputs currently regulated by the dam of guard. However, this moisture regime is confronted with the tide dynamics that conditions this contribution.
The Loukkos estuary is a river that meanders between the ancient city of Lorraine and the current city of Larache. Since the times of the Romans used means of communication between the Tingital Peninsula and the Mauritanian soil, as well as soon as a means of access to the Atlantic Ocean (Snoussi [1984]). This area of contact between fresh water and salt water, it is characterized by a dynamic tide we so pushed to focus our study on this estuary and to study some physical and chemical parameters and their spatial and temporal evolution.
Results and discussion
Spatiotemporal variation of hydrological parameters during 2006
Temperature
Air temperature
The temperature of the air is a very important factor in the determinism biocenotic also by its extreme levels than its diurnal or seasonal variations (Dakki [1997]). At the level of the water, it acts on density, viscosity, solubility of gases including oxygen and carbon dioxide (Bremond and Perrodon [1979]). But a certain threshold, it can play a negative role as soon as organisms by restricting the use of nutrients (Ennaji Dalal [2006]). Just as most vital chemical reactions are slowed down see arrested by a lowering of the temperature (Bremond and Perrodon [1979]).
During the Springer period (19.2°C), we recorded a decrease of temperature of 3°C between S1 and S5, while in summer, the temperature decreased to 2°C (Table 1).
The results of Figure 1 showed that the temporal variation of air temperatures in the studied sites, have identical appearance for the entire period of work with the exception the allure of S3. The magnitude of the variation between the campaigns does not exceed the value 27°C.
Water temperature
The natural water temperature plays a key role in the distribution of the species also by its extreme levels and its diurnal or seasonal variations. It is an important factor in the life of a river estuary. The normal elevation of this setting is due to a direct input of heat from industries using water as refrigerant. The properties of water affected by temperature and which are important for its quality are: the density and the viscosity, two factors that control the speed of the sediment deposits and the formation of natural stratification, especially in lakes, ponds and the seas, the vapor pressure, so evaporation, the solubility of gases in water and in particular oxygen, thus the variation of the temperature of the water between 13°C and 20°C, causes a decrease in the concentration of oxygen in the order of 14% and finally the rates of chemical and biochemical reactions varied with temperature (Bremond and Perrodon [1979]).
We must remember that the three factors that control aquatic environments in general are on the one hand, the illumination, on the other hand temperature and depth. Indeed, if the illumination conditions in the first place the plant and animal life, the temperature adjusts the diffusion of gases (O2 and CO2) in water and subsequently influences the process of photosynthesis (Nisbet and Verneau [1970]). Therefore this parameter must be known accurately for all samples studied. As shown in Table 2, the temperature of the waters of the Loukkos river estuary varies between 19.20 and 29.70°C with a mean of 24.45°C.
During the Springer period, we recorded a decrease in temperature of 3°C between S 1 and S5, while in summer, the temperature decreased by 1°C.
The highest temperatures (29.70°C) are recorded during the months the hottest (July) by the year of 2006, while the (19.20°C) temperatures are recorded during the wet and cold months (March) of the same year.
The temporal variation of water temperatures of the studied sites, have identical appearance for the entire period of work (Figure 1). The amplitude of variation between the campaigns does not exceed in any case 30°C.
For stations in more downstream, the temperature increases by moving away from the mouth. This is due to the decrease of the influence of marine, generally colder waters, and the effect of continentality. This situation was made also in the Sebou River estuary (El Blidi et al. [2006]).
Potential hydrogen (pH)
pH of the water summarizes the stability of the balance between the different forms of carbonic acid. It is linked to the system buffer developed by carbonates and bicarbonates. It is a determinant factor in control of the process of relargage and the fixation of the phosphorus by sediment. It depends on the release of carbon dioxide from the atmosphere, the balance of respiratory and photosynthetic metabolism, as well as the origin of waters, the geological nature of the crossing environment and discharges of sewage (Goterman [1995]). It is inseparable from the values of temperature, salinity and the rate of CO2 (Nisbet and Verneau [1970]), thus it has been shown that the mineralization of organic matter causes a decreasing of the pH (Ezzaouaq [1991]).
In our study, pH valuesvary between 7.02 (during the month of March 2006) and 7.99 (during the month of September 2006). This small variation is due to the buffering effect of bicarbonate ions, indicating the good potability of the water.
The variation of pH between the campaigns generally exceeds the pH unit except the S4. Indeed, the S4 presents average pH (7.61) lower. However, as it is illustrated in Figure 1, the allure of the curves of temporal variation of the pH for the 5 study stations is very varied and does not follow a regular law.
The waters of the estuary are very anchors. The pH is about 8, due to the buffer system developed by carbonates and bicarbonates that are important in this environment.
The upstream waters are slightly more basic (pH = 7.72) than water near downstream of the mouth mostly at low tide. This observation was made also in the Bouregreg river estuary (El Blidi and Fekhaoui [2003]) and the Sebou River estuary (Ezzaouaq [1991]) in effect at low tide, upstream waters are dependent on freshwater and pollutant mass reduced by the Loukkos river estuary resulting in an increase of pH; linked to the degradation of organic matter (Table 3).
Salinity
Salinity explains the chlorosite of water, which is the percentage of chloride in water. We distinguish normal seawater salinity of 35 g/l of chlorides and fresh waters that contain up to 5 g/l. The salinity factor controls the distribution of fauna, flora and minerals. The normal salinity water shelters stenohaline forms (Brachiopods, crustaceans and lamellibranches). Brackish waters shelter to some euryhaline organisms and freshwater shelters to freshwater organisms.
Perisalins environments where chlorides are very high, which makes them toxic and therefore are not shelter any form of life.
Chlorides exist in all waters at concentrations very variables whose origin may be a percolation through dirty areas, infiltration of seawater into groundwater aquifers or deep, of human waste (urine), extractive industries (petroleum industries, coal…) and especially the industries of salt (saline), of soda and potash. Chlorides levels founded in brackish waters are generally 10 to 20 mg/l chloride ions, but can reach a few grams per liter in contact with certain geological formations (Bremond and Perrodon [1979]).
The extreme salinity values were recorded during various campaigns. Indeed, higher concentrations of salinity were detected in station 1 during the campaign of the may 2006. However, lower values of salinity were noted in station 5 during the campaigns of September 2006 (Table 4).
At low tide, in all the campaigns, there is a very pronounced descending gradient downstream-upstream for salinity (Figure 1). This is explained by the arrival of the mass of upstream fresh water, which lowers the salinity of this zone (episodic release of dam and ecological flow); in some campaigns, we note the decrease of the salinity for example during the campaign July 2006.
Thus, during the rainy season we are witnessing a phenomenon of dilution by precipitation inputs. However there is a high concentration of salts during the dry season because of high evaporation of water bodies. This concentration is more pronounced during 2006 where climatic conditions were more severe. This observation was made in the wet lands of Mohammedia (Serghini [2003]).
Dissolved oxygen
Dissolved Oxygen is a particularly useful parameter for water and is an excellent indicator of quality. Its presence in surface water plays a key role in the self-purification and maintenance of aquatic life. However, its presence in urban water is seen as troublesome due to the possibility of corrosion of metal distributors (MC Bride and Rutherford [1983]).
Dissolved Oxygen is one of the fundamental factors of life. It enters the composition of atmospheric air with 21%, and represents approximately 35% of the dissolved gas in water at normal pressure (Bremond and Perrodon [1979]).
Results temporal, shows a low quantity of dissolved oxygen (2.17 mg/l) was found in station 1 during the month of July 2006 and high concentrations of DO were recorded during the month September (12.33 mg/l) (Figure 1).
The allure of the curves of temporal variation of dissolved oxygen for all stations is identical except the S1 where there is low dissolved oxygen during the month of July 2006 (Table 5).
The highest values being stored at the level of the mouth, submitted the direct influences of oxygenated seawater. The lowest values were found in upstream. This is explained by the pollutant load conveyed by the Loukkos river, mainly at low tide to the ocean. This observation was made also in the Sebou river estuary (El Blidi et al. [2006]).
Waters of the S2, very near to upstream, subject to a mainly fluvial influence, have relatively high dissolved oxygen levels. This situation could be explained by the absence of organic input of the upstream (guard dam prevents their decent to the mouth) and originally a very important in this deduction fish mortality. This finding was made also by several authors (MC Bride and Rutherford [1983]).
Conductivity
The conductivity of water is an indicator of changes in the composition of materials and their overall concentrations. It is proportional to the quality of dissolved ionisable salts (Nisbet and Verneau [1970]).
The conductivity informs on the degree of global mineralization of the surface waters. High temperatures affect the conductivity (Goterman [1995]). Natural waters are used as solvent of a considerable number of solutes, which in aqueous solution are either completely associated to ions or partially ionized. High conductivity informs either to normal pH, either the most often a high salinity (Fekhaoui [1983]).
The results presented on Table 6 showed that the recorded values oscillate between 593 μs/cm (S1) during the month of March 2006 and 54700 μS/cm (S5) during the month September 2006. Indeed, these values of 593–54700 μS/cm recorded in our study are lower to those reported by Behera et al. ([2014]) in Mangrove Ecosystem of Mahanadi River Delta, Odisha of India which the electrical conductivity was detected of the order 5160–17330 μS/cm.
The upstream to downstream the conductivity increases gradually. This increase is normal. Indeed, the Loukkos river estuary receives at these level releases of rice. For S2, the conductivity is very varied and the values registered in this site are between 5100 and 10800 μs/cm. For S3, the conductivity decreases gradually during the Springer period while during the summer, there is does increase the conductivity.
Figure 1 shows the curves of variation of conductivity of the raw water of S5. The allure of the curves is not regular. Indeed, for S2 and S4, the allure of the curves is almost identical. It presents a low conductivity in the month September.
Furthermore, temporal variations in S1 and S5 are characterized by a decrease in conductivity mainly during the month of May 2006, while they reach the maximum during the months of July and September 2006.
According the analysis of the results of the evolution of the electrical conductivity in all study the stations, there is spatial and temporal variations of this factor similar to those of salinity (Figure 1). Eventually, we can deduce that at the level of each station, the conductivity follows proportionally the same fluctuations as the salinity.
From downstream to upstream, there is a gradient of decreasing of the conductivity. This is explained by the far of the marine influences (tide and intrusion) and dilution by groundwater.
The contribution of marine waters and the nature of the soil rich in chlorides and sulphates are the probable cause of this intense mineralization. This same observation was made in the wet sites of Mohammedia (Serghini [2003]).
State of stratification of water and air temperature
Marked thermal stratification of temperature was detected at the spatial scale.
It begins to settle from S4 due to increased surface water temperatures followed by a decline that began between S4 and S5 to be diffuse total mixing until the S5. This is proportional to that observed in the same stations in air temperature (Figure 2).
The increase of temperature in the basin will lead to the increase of evaporation. Therefore, the decrease of precipitation and increase of evaporation have resulted in the decrease of river runoff of the Loukkos River (Wu and Xia [2014]).
The decreasing trend of river runoff of the Loukkos River and the increase of temperature were mainly caused by the climate change (Zeng et al. [2004]; Liu [2007]; Jia et al. [2008]). Therefore, the results obtained in this research implicates that climate change will affect the water quality including major ion concentrations, conductivity and pH value of rivers (Wu and Xia [2014]).
State of stratification of salinity and conductivity
Marked thermal stratification of salinity was detected at the spatial scale.
She begins to settle from S4 due to increased salinity followed by a decline that began between S4 and S5 to be diffuse total mixing until the S5. This is proportional to that observed in the same stations in conductivity (Figure 3).
The solubility of oxygen or its ability to dissolve in water decreases as the water temperature and salinity increases (Saravanakumar et al. [2008]).
Conclusion
In the present study disrupts normal functioning of the ecosystem, causing a variety of problems such as a lack of oxygen in the water, needed for fish and shellfish to survive. During the rainy season, the increased flow of freshwater results in the appearance of freshwater species. However, the majority of dry season species cannot survive in these low salinities and migrate to higher salinity areas offshore. Salinity is extremely important from the standpoint of monitoring water quality. There was no significant change in the pH value during the observation period. Indeed, the observed values were an alkaline in all stations. There was a considerable decrease in dissolved oxygen from the deep marine region towards inner riverine downstream Station (S5) to upstream (S1). Salinity and conductivity decreased from upstream S1 to downstream S5. However, the air and water temperature increased from downstream to upstream.
As water originating from rain and snow is the main medium shaping soil processes and transport of minerals, particular attention has been paid to variations in atmospheric precipitation during the analyzed time period. Increased human activities over the recent past are imposing a greater stress on these ecosystems, resulting on changing their water quality and loss of biodiversity. In the present study tidal and diurnal variations in a large spectrum of physico-chemical fraction was irrigates for this coastal environment.
Methods
Study site
For the study of the hydrology of the Loukkos river estuary, several physical and chemical tracers have been the subject of a temporal and spatial monitoring: temperature (air and water), pH, salinity, dissolved oxygen, and conductivity. Five stations selected and visited in Loukkos river estuary for four periods as soon as March, May, July and September during 2006 (Table 7).
Water samples collected at the surface of the water and the various parameters were measured on the ground.
Presentation of sampling stations
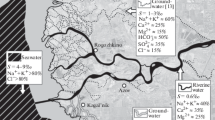
For our study 5 stations distributed along the estuary were chosen taking into account various activities identified in the area (Figure 4).
Dhiria (S1): it is located upstream from the estuary, at the level of the dam’s guard and it is a 21 km away from the mouth, characterized by: rice fields, modern agriculture (fertilizer use), hunting (waterfowl)…
Ain Chouk (S2): it is near a channel and a distance of 16 km from the mouth.
Baggara (S3): it is a 9 km away from the mouth and is characterized by overgrazing (causing erosion of the land), a good extension of agricultural land, vegetation cutting, hunting…
Grangha (S4): it is at a distance of 3 km from the mouth, just near urban discharges from the town of Larache.
Port (S5): located at the mouth of the Loukkos river estuary.
Sampling and sample preparation
The water samples were collected at the level of each station, during each campaign, from a boat. In outside, the various parameters were measured.
Analysis method
Air temperature
The temperature of the air surveys were conducted using a Pulse Oximeter.
Water temperature
The temperature of the water surveys were conducted using a Mercury thermometer graduated in 1/10 of a degree Celsius.
Potential hydrogen (pH)
The measurements were made using a pH meter Orion Research, Ionalyeser model 607 with specific electrode Orion pH 91–05.
Salinity
The measurements of salinity (expressed in mg/l) were conducted using an YSI (model 33) type salinometers. S.C.T Meter.
Dissolved oxygen
Expressed in mg/l, it is a parameter was measured in the field using a Pulse Oximeter ORION Research, Ionalyser model 607 with specific electrode O2.
Conductivity
The conductivity is measured using a conductimeter.
References
Bremond R, Perrodon C: Paramètres de la qualité des eaux. Ministère de l’environnement et cadre de vie. 1979.
Behera BC, Mishra RR, Patra JK, Dutta SK, Thatoi HN: Physico chemical properties of water sample collected from mangrove ecosystem of Mahanadi River Delta, Odisha, India. Am J Mar Sci 2014,2(1):19–24.
Dakki M (1997) Recherches hydrobiologiques sur un cour d’eau du moyen Atlas (Maroc) ». Thèse de 3ème cycle. Uni Aix Marseille Fac Sci Tech, St Jérôme ᅟ:126 Dakki M (1997) Recherches hydrobiologiques sur un cour d’eau du moyen Atlas (Maroc) ». Thèse de 3ème cycle. Uni Aix Marseille Fac Sci Tech, St Jérôme ᅟ:126
Durand Delga M, Hottinger L, Marcais J, Mattauer M, Milliard Y, Suter G (1962) Données actuelles sur la structure du Rif (extrait du livre à la mémoire du professeur Paul Fallot). Soc Géol de France; Hors-série, Tome I ᅟ:399–422, , [http://books.google.co.ma/books?id=oEAftH_vN_8C&pg=PA172&lpg=PA172&dq] Durand Delga M, Hottinger L, Marcais J, Mattauer M, Milliard Y, Suter G (1962) Données actuelles sur la structure du Rif (extrait du livre à la mémoire du professeur Paul Fallot). Soc Géol de France; Hors-série, Tome I ᅟ:399–422,
El Blidi S, Fekhaoui M: Hydrologie et dynamique marégraphique de l’estuaire du Sebou (Gharb, Maroc). Bulletin de l’Institut Scientifique, Rabat, section Sciences de la Vie 2003, 25: 57–65.
El Blidi S, Fekhaoui M, Serghini A, El Abidi A: Rizières de la plaine du Gharb (Maroc): qualité des eaux superficielles et profondes. Bulletin de l’Institut Scientifique, Rabat, section Sciences de la Vie 2006, 28: 55–60.
El Morhit M, Fekhaoui M, El Morhit A, Élie P, Yahyaoui A: Hydrochemical characteristics and metallic quality in fish in the Loukkos river estuary of Morocco. J Mater Environ Sci 2013,4(6):893–904.
Emberger L: Une classification biogéographique des climats. Rec Trav Lab Bot Géol Zool Fac Sci Monpelier, série Bot 1955, 7: 3–43.
Ennaji Dalal EF (2006) Niveau de pollution dans le bas Beht et suivi par le système d’information géographique. Mémoire de 3ème cycle pour l’obtention du Diplôme d’Ingénieur d’Etat en Agronomie. Institut agronomique et vétérinaire Hassan II, Rabat., pp 86–94 Ennaji Dalal EF (2006) Niveau de pollution dans le bas Beht et suivi par le système d’information géographique. Mémoire de 3ème cycle pour l’obtention du Diplôme d’Ingénieur d’Etat en Agronomie. Institut agronomique et vétérinaire Hassan II, Rabat., pp 86–94
Ezzaouaq M: Caractérisation hydrodynamique, physico-chimiques et bactériologiques des eaux superficielles des l’estuaire du Bouregreg (Maroc) soumis aux rejets des villes de Rabat-Salé. Thèse D.E.S. Univ. Mohammed V. Fac. Science, Rabat; 1991.
Fekhaoui M (1983) Toxico-cinétique de trois polluants métalliques majeurs chez la truite arc-en-ciel (Salmo gairdneri), le chrome, le cuivre et le zinc. Thèse de 3ème cycle. Univ. De Claude Bernard, Lyon, p 125
Goterman HL (1995) Toxico cinétique de trois polluants métalliques majeurs chez la truite arc-en-ciel (Salmo gairdneri), le chrome, le cuivre et le zinc. Thèse de 3ème cycle. Univ. De Claude Bernard, Lyon, p 125
Jia YW, Gao H, Niu CW, Qiu YQ: Impact of climate change on runoff process in headwater area of the Yellow River in Chinese. Shuili Xuebao 2008,39(1):52–58.
Këpuska X: Study of physico-chemical parameters of water quality in the Lumbardh Deçani. Anglisticum J (IJLLIS) 2013,2(3):154–158.
Liu CZ: The advances in studying detection of stream flow trend influenced by climate change. Adv Earth Sci 2007,22(8):777–782.
Majumder S, Dutta TK: Studies on seasonal variations in physico-chemical parameters in Bankura segment of the Dwarakeshwar River (W.B.) India. Int J Adv Res 2014,2(3):877–881.
Marçais J, Suter G: Aperçu structurel sur la région du rif oriental. Notes. Service Géologique. Maroc, T. 27, N° 198. pp 35–42, 1 carte H.T. 1966.
MC Bride GB, Rutherford JC: Handbook on estimating dissolved oxygen depletion in polluted rivers. Water and Soil Misc Publ, Wellington 1983, 51: 1–69.
N’Diaye AD, El Kory MB, Kankou M, Ould SAO: Study of water quality during rainy season using physico-chemical and metallic parameters of Senegal River in Mauritania. Sci-Afric J Sci Issues, Res Essays 2013,1(1):01–10.
Nisbet M, Verneau J: Composantes chimiques des eaux courantes. Annales de limnologie, T6 Fasc 1970, 2: 161–190. 10.1051/limn/1970015
Saravanakumar A, Rajkumar M, Sesh Serebiah J, Thivakaran GA: Seasonal variation in physicochemical characteristics of water, sediment and soil texture in arid zone mangroves of Kachchh-Gujarat. J Environ Biol 2008,29(5):725–732.
Serghini A: Diagnose écologique du complexe zones humides de Mohammedia : étude qualitative et quantitative des composantes physique (eau – sédiment) et biologique de l’écosystème. Thèse de Doctorat d’Etat Es Science. Univ. Mohammed V Agdal, Rabat; 2003.
Singh P: Studies on seasonal variations in physico-chemical parameters of the river Gomti (U.P.) India. Int J Adv Res 2014,2(2):82–86.
Snoussi M: Comportement du Pb, Zn, Ni et Cu dans les sédiments de l’estuaire de Loukkos et du proche plateau continental (côte Atlantique marocaine). Bulletin de l’Institut géologique du bassin d’aquitaine 1984, 35: 23–30.
Wu Q, Xia X: Trends of water quantity and water quality of the Yellow River from 1956 to 2009: implications for the effect of climate change. Environ Syst Res 2014,3(1):1–6. 10.1186/2193-2697-3-1
Zeng T, Hao ZC, Wang JH: Modelling the runoff under changing climate. J Glaciol Geocryol 2004,26(3):324–332.
Acknowledgments
This work was performed under collaboration between the Scientific Institute and the National Institute of Health (Rabat). The authors thank the anonymous reviewers for comments and correction that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ME carried out the data analysis and drafted the manuscript. LM provided the conceptual guidance and polished the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
El Morhit, M., Mouhir, L. Study of physico-chemical parameters of water in the Loukkos river estuary (Larache, Morocco). Environ Syst Res 3, 17 (2014). https://doi.org/10.1186/s40068-014-0017-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40068-014-0017-7