Abstract
Purpose
To investigate the effects of anthelminthic drug niclosamide in renal cell carcinoma (RCC) and the underlying mechanisms of its action.
Methods
The effects of niclosamide on the proliferation and apoptosis of RCC cells were examined in vitro and in vivo by using MTS, colony formation assay, flow cytometry and xenograft cancer mouse model. Mechanism studies were performed by analyzing Wnt/β-catenin signaling and mitochondrial functions in a panel of RCC cell lines.
Results
We show that niclosamide effectively targets two RCC cell lines through inhibiting proliferation and anchorage-independent colony formation, and inducing apoptosis. It also enhances the inhibitory effects of chemotherapeutic drug cisplatin in two independent in vivo RCC xenograft mouse models. Mechanistically, niclosamide decreases β-catenin levels and therefore suppresses Wnt/β-catenin activities. Overexpression of β-catenin partially reverses the inhibitory effects of niclosamide in RCC cells, demonstrating that besides β-catenin, other mechanisms are involved in niclosamide’s anti-cancer activity. Indeed, we further show that niclosamide induces mitochondrial dysfunctions as shown by the decreased level of mitochondrial membrane potential and respiration, resulting in decreased ATP levels and increased reactive oxygen species (ROS) levels.
Conclusions
Our findings support the inhibitory effects of niclosamide in cancer and provide better understanding on its underlying mechanism. Our data suggests that niclosamide is a useful addition to the treatment armamentarium for RCC.
Similar content being viewed by others
Background
Renal cell carcinoma (RCC) is an epithelial tumour derived from the proximal tubules of nephrons (Thoenes et al. 1986). It is the most aggressive type of genitourinary cancer and resistant to chemotherapy, radiotherapy and targeted therapy (Bukowski 1997; Kanesvaran and Tan 2014). The molecular mechanisms leading to RCC development, progression and resistance are complex and involve abnormal genetic modifications and aberrant activation of oncogenic signaling pathways, such as Wnt/β-catenin, phosphatidylinositol 3-kinases (PI3 K)/Akt and hepatocyte growth factor (HGF)/c-MET pathways (Zhou et al. 2015; Cojocaru et al. 2015). The identification of therapeutic agents that targets these oncogenic pathways may have the potential to improve the efficacy for RCC treatment.
Niclosamide is an anthelmintic drug especially used for the treatment of cestodes infection (Tanowitz et al. 1993). It kills parasites through inhibiting mitochondrial oxidative phosphorylation (Weinbach and Garbus 1969). However, niclosamide has been recently identified as a novel type of anti-cancer drug. It suppresses growth of several tumor cell lines (Arend et al. 2014; Osada et al. 2011; Lu et al. 2011; Khanim et al. 2011). When niclosamide is combined with conventional chemotherapeutic drugs, the combination showed significant enhanced anti-cancer effects (Liu et al. 2016). Several work highlight niclosamide as a potent Wnt/β-catenin inhibitor and that inhibition of β-catenin signalling is the mechanism of its action on cancer (Arend et al. 2014; Osada et al. 2011; Lu et al. 2011; Chowdhury et al. 2016). Others believe that niclosamide’s anti-cancer activity is mediated through mitochondria, NF-κB pathway or androgen receptor signalling (Khanim et al. 2011; Liu et al. 2016; Jin et al. 2010). Nevertheless, the molecular mechanisms of niclosamide in cancer are not well defined.
The potent inhibitory effects on a panel of tumor cell lines and its known cytotoxicity as well as pharmacokinetic profiles make niclosamide an attractive candidate for cancer treatment. Although its anti-tumor activities had been reported in various tumors, the effectiveness of niclosamide in RCC is unknown. In this study, we investigated the effects of niclosamide and its underlying mechanisms in RCC cells using in vitro cellular culture system and in vivo xenograft mouse tumor model. To further explore the application potential of niclosamide in RCC, the combinatory effects of niclosamide and chemotherapeutic drugs were examined.
Methods
Cells and drugs
Human renal carcinoma cell lines, A-498 and SW-839 were purchased from American Type Culture Collection. Cells were cultured in Eagle’s Minimal Essential Media (MEM) supplemented with a final concentration of 10 % fetal bovine serum (Hyclone, UK) and 100 u/ml penicillin–streptomycin (Invitrogen, US). Niclosamide (BioVision Inc. US) and cisplatin (Sigma, US) were dissolved in DMSO and water, respectively.
MTS proliferation assay
Cells at ten thousands were seeded in 96 well plates overnight. The next day, cells were treated with niclosamide for 72 h. 20 µl of tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] was added to each well and incubated for 2 h. The absorbance was measured at 490 nm.
Annexin V flow cytometry analysis
Cells at 1 × 105 were seeded at 12 well plate and treated with niclosamide for 72 h. After treatment, cells were detached using trypsin (Invitrogen) and stained with Annexin V-FITC (BD Pharmingen, US). The stained cells were analysed by flow cytometry on a Beckman Coulter FC500 and a minimum of 5000 events were counted. The percentage of Annexin V-positive cells was determined by CXP software.
Colony formation assay
A bottom layer of a final concentrating of 0.7 % Bacto agar was prepared in 12 well plates. Cells at 1000 together with niclosamide were seeded in a plate with a top layer of 0.3 % Bacto agar. 100 µl of culture medium was added to the top layer and replaced with fresh one twice a week. After 10–14 days, the colonies were stained with crystal violet and the number of colonies were scored.
Western blot (WB) analyses
Total protein were lysed by using radioimmunoprecipitation assay (RIPA) buffer (Invitrogen). Equal amount of proteins were resolved using denaturing SDS–PAGE and analyzed by WB using antibodies recognizing β-catenin and β-actin (Santa Cruz Biotechnology Inc, US).
Mitochondrial metabolic assay
Cells were treated with different concentrations of niclosamide for 24 h prior to mitochondrial function analysis. To measure mitochondrial membrane potential, cells were stained with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolylcarbocyanine iodide (JC-1, Invitrogen) and analysed by flow cytometry on a Beckman Coulter FC500. Oxygen Consumption Rate (OCR) was measured using a Seahorse XF24 extracellular flux analyser (Seahorse Bioscience, US) according to manufacturer’s instructions. Analyses were performed at basal conditions. ATP levels were measured by ATPlite Luminiescent Assay kit (Perkin Elmer, US) according to the manufacturer’s protocol. To measure intracellular ROS, cells were incubated with 10 µM CM-H2DCFDA (Invitrogen) and absorbance at ex/em of 495/525 nm were measured.
Plasmid transfection
Transfections were carried out in A-498 and SW-839 cells by using nucleofection (Lonza, US). The M50 Super 8× TOPFlash plasmid (a kind gift from Dr. Randall Moon) (Veeman et al. 2003) and Luciferase Reporter Assay System (Promega, US) were used for TOPflash assay. For β-catenin overexpression, cells were transfected with 1.5 μg pcDNA, or pcDNA-β-cat (human β-catenin pcDNA3 plasmid, a kind gift from Dr. Eric Fearon) (Kolligs et al. 1999). At 24 h after transfection, cells were used to perform WB or rescue experiments.
RCC xenograft in SCID mouse
The animal experiments were approved by the Institutional Animal Care and Use Committee of Xiangyang Central Hospital. SCID mice (Biocytogen Inc, China) were subcutaneously injected with 100 µl mixture (1:1) of 10 million cells and Matrigel (BD Biosciences, US). The mice were treated with vehicle control, intraperitoneal niclosamide at 10 mg/kg, cisplatin at 50 mg/kg or combination of both. Tumour volume were measured every three days.
Statistical analyses
The data are expressed as mean and standard deviation. Statistical analyses were performed by unpaired Student’s t test. p value <0.05 were considered statistically significant.
Results
Niclosamide is active against renal carcinoma cells
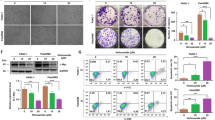
Two human RCC cell lines, A-498 and SW-839, were used in this study. These cell lines were treated with various concentrations of niclosamide for 3 days prior to MTS and Annexin V analysis. We found that niclosamide significantly inhibited proliferation in a dose-dependent manner in A-498 and SW-839 cells (Fig. 1a). In addition, niclosamide dose-dependently induced apoptosis of these two cell lines (Fig. 1b). Anchorage-independent growth in soft agar is often predictive of tumorigenicity as colonies formed in soft agar are from cells displaying both highly proliferative and invasive phenotypes (Gao et al. 2005). We found that niclosamide was also effective in inhibiting anchorage-independent growth of RCC cells (Fig. 1c, d). The IC50 of niclosamide on A-498 is similar to the one in SW-839, suggesting that niclosamide is active against RCC cells regardless of their cellular origin.
Niclosamide inhibits proliferation and anchorage-independent colony formation and induces apoptosis in RCC cell lines. a Niclosamide decreases proliferation of A-498 and SW-839 cells. b Niclosamide induces apoptosis of A-498 and SW-839. c Representative images taken at 14 days of an anchorage-independent colony formation assay. d Quantification of colonies show the dose-dependent inhibitory effect of niclosamide on colony formation in RCC cells. *p < 0.05, compared to control
The inhibitory effects of niclosamide on renal carcinoma cells are partially through suppressing Wnt/β-catenin pathway
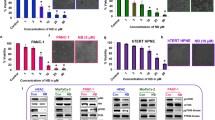
Several studies have demonstrated the potent inhibitory effects of niclosamide on Wnt/β-catenin signaling pathway in various types of cancers (Arend et al. 2014; Osada et al. 2011; Lu et al. 2011). Consistent with these findings, our work demonstrated that niclosamide decreased levels of intracellular β-catenin in RCC cells (Fig. 2a). To confirm the inhibitory effect of niclosamide on Wnt/β-catenin signaling in RCC cells, we performed Wnt/β-catenin reporter assay by transiently transfected with TOPFlash plasmid. Niclosamide dose-dependently inhibited the TOPFlash activity in RCC cells (Fig. 2b), which correlates well with the decreased levels of β-catenin in RCC cells exposed to niclosamide (Fig. 2a).
Niclosamide inhibits Wnt/β-catenin signaling in RCC cells. a Niclosamide decreases intracellular β-catenin levels in A-498 and SW-839 cells. Cells were treated with niclosamide for 24 h prior to WB analysis. b Niclosamide inhibits TOPflash activation. RCC cells transfected with TOPflash plasmid were treated with niclosamide as indicated. RLU, relative light units. c β-Catenin level is increased n A-498 and SW-839 cells transfected with β-catenin overexpression plasmid. Overexpression of β-catenin partially rescued the effects of niclosamide in inhibiting proliferation (d) and colony formation (e) and inducing apoptosis (f) in RCC cells. *p < 0.05, compared to control or p-Vector
To investigate whether β-catenin stabilization can abolish the effects of niclosamide, we rescued β-catenin levels by overexpressing β-catenin in RCC cells (Fig. 2c). In β-catenin-overexpressing A-498 and SW-839 cells, niclosamide is less effective in inhibiting proliferation and colony formation, and inducing apoptosis compared to control cells (Fig. 2d–f). These data demonstrate that inhibition of Wnt/β-catenin signalling is the molecular mechanism of niclosamide’s action in RCC cells. It is also noted that β-catenin-overexpression failed to completely rescue the inhibitory effects of niclosamide, suggesting that other mechanisms are involved for the observed biological effects of niclosamide in RCC.
Niclosamide induces mitochondrial dysfunctions
It is known that niclosamide kills the tapeworm through inhibiting oxidative phosphorylation (Weinbach and Garbus 1969). Khanim et al. recently demonstrated that niclosamide targets mitochondria in multiple myeloma cells by reducing free light chain production (Khanim et al. 2011). We therefore investigated the effects of niclosamide on mitochondrial functions in RCC cells.
We found that niclosamide decreased mitochondrial membrane potential at the same effective concentrations (e.g., 1, 5, and 10 µM) that inhibits proliferation and induces apoptosis (Fig. 3a). In addition, niclosamide significantly inhibited OCR, decreased ATP levels and increased ROS levels in RCC cells (Fig. 3b–d). Taken together these data show that niclosamide impairs mitochondrial functions to induce an energy crisis and oxidative damage in RCC cells.
Niclosamide inhibits growth of renal carcinoma in vivo and enhances the effects of chemotherapeutic drug
We next tested the in vivo efficacy of niclosamide and investigated whether its combination with cisplatin (common chemotherapeutic drug) resulted in greater efficacy than single drug alone. We established two RCC xenograft mouse models by subcutaneous injection of A-498 or SW-839 cells into nude mice. When mice tumor reached 200 mm3, mice were treated with vehicle control, intraperitoneal niclosamide, cisplatin or combination of niclosamide and cisplatin for 3 weeks. No significant body weight loss and abnormal behavior in all groups (data not shown) indicate that the mice tolerated the treatment well. We further show that niclosamide inhibited growth of tumors derived from both A-498 and SW-839 cells (Fig. 4). It is noted that niclosamide at 10 mg/kg achieved similar efficacy as chemotherapeutic drug cisplatin at 40 mg/kg. More importantly, the combination of niclosamide and cisplatin further inhibited tumor growth in both RCC xenograft models (Fig. 4). Our in vivo data are consistent with in vitro data, and confirmed the anti-cancer activities of niclosamide in RCC.
Niclosamide inhibits RCC tumor growth and enhances the inhibitory effect of cisplatin in vivo. Niclosamide inhibited growth of RCC tumors derived from A-498 (a) and SW-839 (b) cells as a single drug. Combination of niclosamide and cisplatin significantly inhibited much more tumor growth throughout the duration of treatment in two xenograft RCC models. *p < 0.05, compared to control or single arm
Discussion
Current therapies including chemotherapy, radiotherapy and targeted therapy have not yielded durable remissions in RCC patients (Kanesvaran and Tan 2014; Lisa et al. 2016). There are many ongoing research efforts to discover drugs to eradicate RCC cells. The discovery, development and registration of novel compounds can be a long and laborious process. An alternative to new drug development is drug repositioning. For example, thalidomide has been successfully repurposed for myeloma treatment (Mutsaers 2007; Chong and Sullivan 2007). In this study, we evaluated anthelmintic drug niclosamide as a potential candidate for RCC treatment. We are the first to demonstrate the suppression of Wnt/β-catenin and induction of mitochondrial dysfunctions in RCC cells by niclosamide, leading to proliferation inhibition and cell death.
Two cell lines, A-498 and SW-839, selected for demonstration of the biological effects of niclosamide are derived from the most frequent clear-cell tumor subtype of RCC (accounting for >80 % of all RCCs) (Kovacs et al. 1997). Our study show that niclosamide significantly inhibits proliferation and induces apoptosis in both A-498 and SW-839 cells (Fig. 1a, b), suggesting the therapeutic efficacy of niclosamide in the majority of RCCs. Niclosamide also significantly inhibits anchorage-independent colony formation of RCC cells (Fig. 1c, d), further demonstrating its inhibitory effects on RCC subpopulations with highly proliferative and invasive properties (Gao et al. 2005). The efficacy of niclosamide have been demonstrated in various cancers, such as colon, cancer, ovarian cancers and myeloma (Arend et al. 2014; Osada et al. 2011; Khanim et al. 2011). Our data supports the previous studies on the inhibitory effects of niclosamide in cancer and adds RCC to the growing list of niclosamide-targeted cancers.
Consistent with the in vitro data, niclosamide inhibits growth of two independent RCC cancer xenograft mouse models established by using A-493 and SW-839 cells (Fig. 4), confirming its in vivo efficacy. Notably, niclosamide further enhanced the inhibitory effect of cisplatin in RCC tumor growth (Fig. 4), demonstrating the synergism of niclosamide and chemotherapeutic drugs in RCC. In addition, our data show that mice tolerate very well to niclosamide at 10 mg/kg given by intraperitoneal injection (data not shown). Although niclosamide has little absorption from gastrointestinal tract, pharmacokinetic analysis of niclosamide following oral administration in mice by Osada et al. show the efficient and reliable destruction of niclosamide from blood to tumor tissue (Osada et al. 2011). Our data together with previous studies suggest that greater absorption of niclosamide can be achieved either by drug modifications or by using different administration route.
The mechanism of action of niclosamide seems to be in a cancer cell-type specific manner. Some have reported that niclosamide suppresses Wnt/β-catenin signalling via inducing lipoprotein receptor-related protein-6 (LRP6) degradation in prostate and breast cancer cells (Lu et al. 2011). Others have demonstrated that niclosamide induces free light chain production in myeloma cells (Khanim et al. 2011). We found that niclosamide decreased levels of β-catenin and therefore suppressed Wnt/β-catenin activities in A-498 and SW-839 cells (Fig. 2a, b). Furthermore, overexpression of β-catenin levels partially abolished the inhibitory effects of niclosamide in RCC cells (Fig. 2c–f). These data agree with the previous work that niclosamide targets RCC cells through inhibiting Wnt/β-catenin signalling pathways. Aberrant Wnt/β-catenin signalling pathway caused by APC deficiency or HIF1α play important roles in RCC progression (Cojocaru et al. 2015; Majid et al. 2012). Wnt/β-catenin signalling pathway has been the target of major drug discovery programs in the past decade. In line with these efforts, our work agrees with the essential roles of Wnt/β-catenin in RCC and further demonstrates that it can be effectively targeted by niclosamide.
However, the partial but not complete rescue by β-catenin overexpression suggests that other mechanisms of action might be involved in the cells treated with niclosamide. Indeed, we found that niclosamide induces mitochondrial dysfunctions via decreasing mitochondrial membrane potential, OCR and ATP levels (Fig. 3a, b, d). A consequence of impaired mitochondrial function induced by niclosamide is the increased oxidative damage as shown by the increased levels of ROS (Fig. 3c). Targeting cancer metabolism, such as mitochondrial metabolism, has recently become an attractive therapeutic strategy in cancer due to their unique dependence on oxidative phosphorylation (Jaras and Ebert 2011; Loureiro et al. 2013; Moreno-Sanchez et al. 2007; Funes et al. 2007). Various antibiotics or anthelminthic drugs that target mitochondria have been reported to be effective in inhibiting a large panel of cancer cells (Lamb et al. 2015). Our work together with the previous work suggest that induction of mitochondria dysfunctions might be a common mechanism of the action of those antibiotics in cancers.
In conclusion, our results show that niclosamide effectively targets RCC cells through both suppressing Wnt/β-catenin signalling and inducing mitochondrial dysfunctions. Importantly, niclosamide significantly enhances the effect of chemotherapeutic drug cisplatin in inhibiting tumour growth in two-independent RCC xenograft models. Our findings suggest that niclosamide may have utility in treating patients with RCC.
References
Arend RC, Londono-Joshi AI, Samant RS, Li Y, Conner M, Hidalgo B et al (2014) Inhibition of Wnt/beta-catenin pathway by niclosamide: a therapeutic target for ovarian cancer. Gynecol Oncol 134(1):112–120. doi:10.1016/j.ygyno.2014.04.005
Bukowski RM (1997) Natural history and therapy of metastatic renal cell carcinoma: the role of interleukin-2. Cancer 80(7):1198–1220
Chong CR, Sullivan DJ Jr (2007) New uses for old drugs. Nature 448(7154):645–646. doi:10.1038/448645a
Chowdhury MK, Wu LE, Coleman JL, Smith NJ, Morris MJ, Shepherd PR et al (2016) Niclosamide blocks glucagon phosphorylation of Ser552 on beta-catenin in primary rat hepatocytes via PKA signalling. Biochem J 473(9):1247–1255. doi:10.1042/BCJ20160121
Cojocaru E, Lozneanu L, Giusca SE, Caruntu ID, Danciu M (2015) Renal carcinogenesis—insights into signaling pathways. Rom J Morphol Embryol = Revue roumaine de morphologie et embryologie 56(1):15–19
Funes JM, Quintero M, Henderson S, Martinez D, Qureshi U, Westwood C et al (2007) Transformation of human mesenchymal stem cells increases their dependency on oxidative phosphorylation for energy production. Proc Natl Acad Sci USA 104(15):6223–6228. doi:10.1073/pnas.0700690104
Gao CF, Xie Q, Su YL, Koeman J, Khoo SK, Gustafson M et al (2005) Proliferation and invasion: plasticity in tumor cells. Proc Natl Acad Sci USA 102(30):10528–10533. doi:10.1073/pnas.0504367102
Jaras M, Ebert BL (2011) Power cut: inhibiting mitochondrial translation to target leukemia. Cancer Cell 20(5):555–556. doi:10.1016/j.ccr.2011.10.028
Jin Y, Lu Z, Ding K, Li J, Du X, Chen C et al (2010) Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res 70(6):2516–2527. doi:10.1158/0008-5472.CAN-09-3950
Kanesvaran R, Tan MH (2014) Targeted therapy for renal cell carcinoma: the next lap. J Carcinog 13:3. doi:10.4103/1477-3163.127638
Khanim FL, Merrick BA, Giles HV, Jankute M, Jackson JB, Giles LJ et al (2011) Redeployment-based drug screening identifies the anti-helminthic niclosamide as anti-myeloma therapy that also reduces free light chain production. Blood Cancer J 1(10):e39. doi:10.1038/bcj.2011.38
Kolligs FT, Hu G, Dang CV, Fearon ER (1999) Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol 19(8):5696–5706
Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B et al (1997) The Heidelberg classification of renal cell tumours. J Pathol 183(2):131–133. doi:10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G
Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE et al (2015) Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget 6(7):4569–4584. doi:10.18632/oncotarget.3174
Lisa D, Laurence A, Christophe M, Yohann L, Karim F, Bernard E (2016) Safety of available treatment options for renal cell carcinoma. Expert Opin Drug Saf. doi:10.1080/14740338.2016.1184643
Liu C, Armstrong C, Zhu Y, Lou W, Gao AC (2016) Niclosamide enhances abiraterone treatment via inhibition of androgen receptor variants in castration resistant prostate cancer. Oncotarget. doi:10.18632/oncotarget.8493
Loureiro R, Mesquita KA, Oliveira PJ, Vega-Naredo I (2013) Mitochondria in cancer stem cells: a target for therapy. Recent Pat Endocr Metab Immune Drug Discov 7(2):102–114
Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y (2011) Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/beta-catenin pathway. PLoS ONE 6(12):e29290. doi:10.1371/journal.pone.0029290
Majid S, Saini S, Dahiya R (2012) Wnt signaling pathways in urological cancers: past decades and still growing. Mol Cancer 11:7. doi:10.1186/1476-4598-11-7
Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E (2007) Energy metabolism in tumor cells. FEBS J 274(6):1393–1418. doi:10.1111/j.1742-4658.2007.05686.x
Mutsaers AJ (2007) Chemotherapy: new uses for old drugs. Vet Clin N Am Small Anim Pract 37(6):1079–1090. doi:10.1016/j.cvsm.2007.07.002
Osada T, Chen M, Yang XY, Spasojevic I, Vandeusen JB, Hsu D et al (2011) Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res 71(12):4172–4182. doi:10.1158/0008-5472.CAN-10-3978
Tanowitz HB, Weiss LM, Wittner M (1993) Diagnosis and treatment of intestinal helminths. I. Common intestinal cestodes. Gastroenterologist 1(4):265–273
Thoenes W, Storkel S, Rumpelt HJ (1986) Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas). The basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract 181(2):125–143. doi:10.1016/S0344-0338(86)80001-2
Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT (2003) Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol 13(8):680–685
Weinbach EC, Garbus J (1969) Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature 221(5185):1016–1018
Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S et al (2015) Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. doi:10.1038/onc.2015.343
Authors’ contributions
JZ and LC designed the experiments, interpreted the data and wrote the manuscript. ZJ, QSH, ZMG and SC performed the experiments. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by research Grants provided by Xiangyang Central Hospital (Grant No. 201366816) and Science and Technology Department of Hubei Province (Grant No. EK2013D16011668122).
Competing interests
The authors declare that they have no competing interests.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Research involving Human Participants and/or Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Juan Zhao and Qiushan He contributed equally to this work
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhao, J., He, Q., Gong, Z. et al. Niclosamide suppresses renal cell carcinoma by inhibiting Wnt/β-catenin and inducing mitochondrial dysfunctions. SpringerPlus 5, 1436 (2016). https://doi.org/10.1186/s40064-016-3153-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-3153-x