Abstract
Background
Postoperative pulmonary complications (PPCs), which are not uncommon in one-lung ventilation, are among the main causes of postoperative death after lung surgery. Intra-operative ventilation strategies can influence the incidence of PPCs. High tidal volume (V T) and increased airway pressure may lead to lung injury, while pressure-controlled ventilation and lung-protective strategies with low V T may have protective effects against lung injury. In this meta-analysis, we aim to investigate the effects of different ventilation strategies, including pressure-controlled ventilation (PCV), volume-controlled ventilation (VCV), protective ventilation (PV) and conventional ventilation (CV), on PPCs in patients undergoing one-lung ventilation. We hypothesize that both PV with low V T and PCV have protective effects against PPCs in one-lung ventilation.
Methods
A systematic search (PubMed, EMBASE, the Cochrane Library, and Ovid MEDLINE; in May 2015) was performed for randomized trials comparing PCV with VCV or comparing PV with CV in one-lung ventilation. Methodological quality was evaluated using the Cochrane tool for risk. The primary outcome was the incidence of PPCs. The secondary outcomes included the length of hospital stay, intraoperative plateau airway pressure (Pplateau), oxygen index (PaO2/FiO2) and mean arterial pressure (MAP).
Results
In this meta-analysis, 11 studies (436 patients) comparing PCV with VCV and 11 studies (657 patients) comparing PV with CV were included. Compared to CV, PV decreased the incidence of PPCs (OR 0.29; 95 % CI 0.15–0.57; P < 0.01) and intraoperative Pplateau (MD −3.75; 95 % CI −5.74 to −1.76; P < 0.01) but had no significant influence on the length of hospital stay or MAP. Compared to VCV, PCV decreased intraoperative Pplateau (MD −1.46; 95 % CI −2.54 to −0.34; P = 0.01) but had no significant influence on PPCs, PaO2/FiO2 or MAP.
Conclusions
PV with low V T was associated with the reduced incidence of PPCs compared to CV. However, PCV and VCV had similar effects on the incidence of PPCs.
Similar content being viewed by others
Background
One-lung ventilation, used to isolate and protect the lung, has been widely used in thoracic surgery. Nonphysiologic tidal volumes, loss of normal functional residual capacity and hyperperfusion in the ventilated lung during one-lung ventilation result in alveolar damage and inflammation response in the lung. These changes make patients susceptible to PPCs (Lohser and Slinger 2015). Various ventilation strategies, such as pressure-controlled ventilation (PCV), volume-controlled ventilation (VCV), conventional ventilation (CV) and protective ventilation (PV), are used in one-lung ventilation. The ideal ventilation strategy should minimize the risk of PPCs while also benefitting both gas exchange and pulmonary mechanics.
High tidal volume (V T) is associated with increased areas of overinflation but decreased areas of atelectasis at end-inspiration (Guldner et al. 2015). Protective ventilation with low V T is thought to result in less ventilator-induced lung injury and has become a routine strategy in patients with ARDS (Petrucci and De Feo 2013). Recent studies have reported similar results in that low V T prevents postoperative complications in surgical patients (Serpa Neto et al. 2015a, b). However, the effect of low V T on patients undergoing one-lung ventilation remains unclear. In some studies, PV has been associated with a decreased oxygenation index and more dead space ventilation without decreases in the incidence of PPCs (Maslow et al. 2013; Jung et al. 2014; Blank et al. 2016; Neto et al. 2016). In other studies, PV was associated with a lower incidence of PPCs and satisfactory gas exchange (Schilling et al. 2005; Yang et al. 2011; Serpa Neto et al. 2015a, b). VCV and PCV are also used in one-lung ventilation. PCV may result in lower airway pressure and a more homogeneous distribution of the tidal volume; PCV also has less of an effect on cardiac function than VCV (Al Shehri et al. 2014). However, tidal volumes in PCV are highly variable (Della Rocca and Coccia 2013). The benefits of PCV in terms of oxygenation and protection against lung damage should be balanced.
This meta-analysis aims to investigate the association between ventilation strategies and PPCs; the length of hospital stay, intraoperative Pplateau, PaO2/FiO2 and MAP were compared as the secondary outcomes. We hypothesize that PV with low V T and PCV have protective effects on PPCs in one-lung ventilation.
Methods
We used the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) recommended by the PRISMA working group (http://www.prisma-statement.org/) in this meta-analysis (Moher et al. 2009). This meta-analysis was registered on PROSPERO (Prospective Register of Ongoing Systematic Reviews, http://www.crd.york.ac.uk/prospero, Registration No. CRD42015022087).
Eligibility criteria
We compared 2 types of interventions with 2 control groups. In the comparison of PCV and VCV, the intervention group was the PCV group and the control group was the VCV group. PCV was defined as ventilation under pressure control with or without PEEP in one-lung ventilation. VCV was defined as ventilation under volume control with or without PEEP in one-lung ventilation. In the comparison of PV and CV, the intervention group was the PV group and the control group was the CV group. According to previous studies, PV was defined as ventilation using low V T (V T ≤ 6 ml/kg predicted body weight) with or without PEEP and with or without alveolar recruitment strategies in one-lung ventilation. CV was defined as ventilation using V T ≥ 7 ml/kg predicted body weight with or without PEEP and without recruitment maneuvers in one-lung ventilation (Lohser 2008; Della Rocca and Coccia 2013).
The included studies met the following criteria: randomized controlled trials of patients aged 18 years or older who were undergoing one-lung ventilation during a surgical procedure. Randomized clinical trials (RCTs) were excluded if they did not involve a surgical procedure, if they included patients undergoing cardiac surgery, if they included patients with cardiac diseases, sepsis or ARDS before surgery, if they were conference abstracts or if full-text articles could not be obtained, if they did not focus on the comparisons of different ventilation strategies in the dependent lung, if the intervention group and control group had different ventilation settings during two-lung ventilation (TLV), or if the RCTs did not report any outcomes mentioned above. Animal studies were also excluded.
The primary outcome of interest was the development of PPCs during follow up, defined as the development of atelectasis, lung infiltration, pneumonia or ARDS. The secondary outcomes included the length of hospital stay, intraoperative Pplateau, PaO2/FiO2 and MAP. PaO2/FiO2 at 20–40 min in one-lung ventilation in randomized parallel studies was included in the analysis of PaO2/FiO2. Cross-over studies were not used to evaluate the effect of ventilation strategies on PPCs, the length of hospital stay or PaO2/FiO2.
Information sources
We performed a literature search in PubMed, EMBASE, the Cochrane Library, and Ovid MEDLINE in May 2015. The last search was performed on May 14th, 2015.
Search strategy
The terms ‘anesthesia’, ‘anaesthesia’, ‘surgery’, ‘surgical’, ‘operative’, ‘surgical operations’, ‘intra-operative care’, ‘postoperative care’, ‘preoperative care’, ‘perioperative care’, ‘one lung ventilation’, ‘single-lung ventilation’, ‘single-lung ventilations’, ‘lung separation techniques’, ‘lung separation technique’ were used in various combinations. The search was limited to clinical trials. The detailed search strategy is provided as Additional file 1.
Study selection
Two reviewers (ZL, XWL) conducted the systematic search and independently reviewed the titles and abstracts of the studies. Only reports meeting the criteria listed above were included for data extraction, trial quality assessment and the analysis of results. Any disagreements among reviewers was resolved by discussion with a third author (JZ).
Data collection process
Data were extracted independently by two reviewing authors (ZL, XWL). Authors of the original studies were contacted to provide additional information if necessary.
Data items
The following information was extracted: study design (randomized parallel studies, randomized cross-over studies), number of patients, ventilation strategies, type of surgery, duration of one-lung ventilation, outcomes and preoperative FEV1 (% of predicted).
Risk of bias
The Cochrane tool for risk of bias was used to assess the risk of bias for all studies (Higgins et al. 2011). The risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and others was evaluated and classified as “low”, “high”, or “unclear” risk. We used funnel plots to assess reporting bias, and these plots are available in the Additional file 1. The risk of bias evaluation was conducted independently by two authors (ZL, XWL).
Synthesis of results
Review-Manager software (RevMan, version 5.3; The Cochrane Collaboration, Oxford, UK) was used to conduct the data analysis. For binary outcomes, PPCs were summarized using odds ratios and their 95 % confidence intervals (CIs). Mean difference and 95 % CI were reported for continuous outcomes. We used I2 to estimate heterogeneity within the studies (Higgins et al. 2003). A fixed-effect model was used to analyze the data. If I2 was greater than 50 %, we utilized the random-effects model.
Additional analysis
When comparing the clinical effect of PCV with VCV, we performed subgroup analyses to determine if outcomes could be influenced by the setting of V T or the type of PCV. The setting of V T was divided into V T ≤ 6 ml/kg and V T ≥ 7 ml/kg predicted body weight. The type of PCV was divided into conventional pressure-controlled ventilation and PCV-VG. Subgroup analysis was performed only when there were no less than 3 studies providing information for one outcome. If I2 > 50 %, we performed a sensitivity analysis by removing trials and reanalyzing the remaining studies.
Quality assessment
A Measure Tool to Assess Systematic Reviews (AMSTAR) was applied to assess the methodological quality. Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used to assess the evidence quality.
Results
Study selection
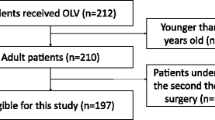
A total of 467 studies were screened and assessed for eligibility. Of these, 446 records were excluded for a variety of reasons as shown in Fig. 1. The remaining 21 randomized controlled trials (reporting on 22 comparisons) involving 1083 one-lung ventilation patients undergoing non-cardiac surgery were included in this meta-analysis. Eleven studies including 436 patients compared PCV with VCV, and 11 studies including 657 patients compared PV with CV. Data shown in the histogram were collected by contacting the corresponding authors by e-mail for one trial (Jung et al. 2014).
Study characteristics
Eleven of the 21 studies included in this meta-analysis were randomized parallel studies and 10 studies were randomized cross-over studies. The study sample sizes ranged from 18 to 120 patients. The trials involved both open thoracic surgery and video-assisted thoracic surgery. The duration of one-lung ventilation ranged from 73.4 to 109 min. The characteristics and risk of bias in each study are shown in Tables 1 and 2, respectively.
Results of individual studies and synthesis of results
Postoperative pulmonary complications
Two of the 11 studies including 147 patients comparing PCV with VCV reported PPCs as an outcome (Pardos et al. 2009; Boules and Ghobrial 2011). Both studies found that PCV did not have any advantages over VCV in terms of decreased incidence of PPCs (OR 1.05; 95 % CI 0.25–4.34; I2 = 0; P = 0.95) (Fig. 2a).
Four of the 12 studies including 285 patients comparing PV with CV reported PPCs as an outcome (Michelet et al. 2006; Yang et al. 2011; Maslow et al. 2013; Shen et al. 2013). PV showed a protective effect over CV on respiratory complications after one-lung ventilation (OR 0.29; 95 % CI 0.15–0.57; I2 = 0; P < 0.01) (Fig. 2b).
Length of hospital stay
Four studies including 272 patients comparing PV with CV reported the length of hospital stay as an outcome (Yang et al. 2011; Maslow et al. 2013; Shen et al. 2013; Qutub et al. 2014). No advantages in terms of the length of hospital stay were found in the PV group (MD −0.65; 95 % CI −1.59 to 0.30; I2 = 27 %; P = 0.18) (Fig. 3).
Plateau airway pressure
Eight of the 11 studies including 359 patients comparing PCV with VCV reported Pplateau as an outcome (Tugrul et al. 1997; Unzueta et al. 2007; Choi et al. 2009; Pardos et al. 2009; Montes et al. 2010; Boules and Ghobrial 2011; Al Shehri et al. 2014; Pu et al. 2014). PCV showed decreased Pplateau compared to VCV (MD −1.46; 95 % CI −2.58 to −0.34; I2 = 72 %; P = 0.01) (Fig. 4a).
Eight of the 12 studies including 497 patients comparing PV with CV reported Pplateau as an outcome (Michelet et al. 2006; Lin et al. 2008; Sungur Ulke et al. 2011; Yang et al. 2011; Roze et al. 2012; Maslow et al. 2013; Végh et al. 2013; Jung et al. 2014). PV decreased Pplateau compared to CV (MD −3.57; 95 % CI −5.74 to −1.76; I2 = 94 %; P < 0.01) (Fig. 4b).
PaO2/FiO2
Three randomized parallel trials including 167 patients comparing PCV and VCV reported PaO2/FiO2 at 20–30 min after OLV as an outcome (Pardos et al. 2009; Boules and Ghobrial 2011; Ye and Li 2011). No differences in PaO2/FiO2 were found in those 2 groups (MD 47.56; 95 % CI −7.67 to 102.79; I2 = 91 %; P = 0.09) (Fig. 5).
Mean arterial pressure
Six of the 11 studies including 181 patients comparing PCV and VCV reported MAP as an outcome (Tugrul et al. 1997; Choi et al. 2009; Boules and Ghobrial 2011; Al Shehri et al. 2014; Hu et al. 2014; Pu et al. 2014). No differences in MAP were found in those 2 groups (MD 0.26; 95 % CI −2.28–2.79; I2 = 0; P = 0.84) (Fig. 6a).
Four of the 12 studies including 222 patients comparing PV and CV reported MAP as an outcome (Michelet et al. 2006; Sungur Ulke et al. 2011; Végh et al. 2013; Qutub et al. 2014). No differences in MAP were found in those 2 groups (MD −0.89; 95 % CI −3.20 to 1.41; I2 = 0; P = 0.45) (Fig. 6b).
Subgroup analysis
Subgroup analysis regarding the volume of V T showed a decrease in Pplateau (MD −2.58; 95 % CI −4.74 to −0.43; I2 = 85 %; P = 0.02) in patients undergoing PCV with V T 6 ml/kg predicted body weight compared to VCV. In the groups with V T ≥ 7 ml/kg, no significant benefit was found in patients undergoing PCV compared to VCV (MD −0.58; 95 % CI −1.37–0.20) (Table 3).
Subgroup analysis on the type of PCV showed decreases in Pplateau (MD −2.78; 95 % CI −4.21 to −1.35; I2 = 0 %; P < 0.01) in patients undergoing volume guaranteed pressure-controlled ventilation (PCV-VG) compared to VCV, while traditional PCV showed no significant benefits in Pplateau (MD −1.06; 95 % CI −2.37–0.24; I2 = 75 %; P = 0.11). With respect to MAP, no significant differences or heterogeneity were found in the subgroup analysis.
Sensitivity analyses of Pplateau and PaO2/FiO2 were also performed. When comparing PCV with VCV, heterogeneity in Pplateau could be resolved by excluding the study by Al Shehri et al. (2014) (MD −0.89; 95 % CI −1.50 to −0.28; I2 = 37 %; P < 0.01). This change had no effect on the final result. Heterogeneity in PaO2/FiO2 could be resolved by excluding the study by Pardos et al. (Pardos et al. 2009) (MD 74.01; 95 % CI 60.04–87.98; I2 = 0 %; P < 0.01). This change affected the final result and showed PCV benefited PaO2/FiO2 in comparison with VCV. In the comparison of PV with CV on Pplateau, heterogeneity and the final result could not be resolved by the exclusion of any study involved in this meta-analysis.
Discussion
This meta-analysis suggests that PV but not PCV can decrease the incidence of PPCs. Although both PV and PCV can decrease the Pplateau, subgroup analyses show that PCV-VG (but not traditional PCV) can decrease Pplateau. Currently available data are insufficient to identify differences between PV and CV or PCV and VCV on the length of hospital stay, PaO2/FiO2 or MAP.
Our result suggesting that PV with low V T can protect surgical patients from PPCs is consistent with recently published studies (Hemmes et al. 2015, Serpa Neto et al. 2015a, b). However, the definition of PV in these studies is ventilation with V T ≤ 8 ml/kg, and they also include all surgical patients under general anesthesia (Hemmes et al. 2015). The definition of V T and the conclusions from these studies might not be suitable in one-lung ventilation. Our results suggest that PV with V T ≤ 6 ml/kg can benefit surgical patients in one-lung ventilation. A high quality retrospective study published recently found that low V T does not prevent PPCs, which contradicts our results (Blank et al. 2016). In this retrospective study, fewer than half (47 %) of the patients received PEEP ≥ 5 cmH2O (Blank et al. 2016). Atelectasis should be considered in all general anesthetized patients. And it is of great importance to avoiding the occurrence of atelectasis during OLV (Lohser and Slinger 2015). Low V T with low PEEP can cause increased amounts of atelectasis (Guldner et al. 2015). Only one study with a sample size of 40 patients used PV with PEEP ≤ 5 cmH2O (Lin et al. 2008). In this study performed by Lin et al., PPCs were not included in the outcomes (Lin et al. 2008). To achieve a protective effect on PPCs, PEEP ≥ 5 cmH2O may be necessary when PV is used in surgical patients undergoing one-lung ventilation.
Pplateau is part of the driving pressure and contributes to ventilator-induced lung injury (Neto et al. 2016). Our results suggest that PV has lower Pplateau compared to CV, which might explain the mechanism of decreased PPCs in the PV group. Although PCV can also decrease the Pplateau compared to VCV, current data are insufficient to identify any difference between PCV and VCV on PPCs. It should be noted that Pplateau in PV is lower than Pplateau in PCV on average (Choi et al. 2009; Sungur Ulke et al. 2011). Differences in Pplateau may be caused by the differences in V T. The V T in PCV is usually 8 ml/kg or higher, while the V T in PV is no more than 6 ml/kg (Michelet et al. 2006; Jung et al. 2014; Pu et al. 2014). PCV-VG is a novel mode of ventilation which has been used in recent years. Although present data suggest that PCV-VG can decrease Pplateau, more studies are still needed.
A combination of low V T and PEEP is generally used in PV. PEEP can cause cardiac compromise, which can be reflected by MAP. Low V T may induce hypoxemia. In this meta-analysis, the effects of different ventilation strategies on PaO2/FiO2 and MAP were compared. Decreasing alveolar oxygen tension could induce HPV and resulted in the redistribution of pulmonary blood flow (Moudgil et al. 2005). HPV had a rapid-onset phase and a delayed phase in response to alveolar hypoxia. The rapid-onset phase reached a plateau at 20–40 min. The delayed phase begins at 40 min and takes more than 2 h to reverse (Lumb and Slinger 2015). PaO2/FiO2 can be affected by HPV. Because most thoracic surgery can be completed in 2 h, only the results of PaO2/FiO2 at 20 to 40 min after one-lung ventilation are compared in this meta-analysis. Our results suggest that there is no difference between PV and CV or PCV and VCV on PaO2/FiO2 and MAP. This result is consistent with clinical studies published previously (Boules and Ghobrial 2011; Qutub et al. 2014).
The studies included in this meta-analysis are all RCTs and the overall quality of their reporting is good. Random sequence generation and allocation concealment are utilized in most studies. This meta-analysis is of high methodological quality assessed by AMSTAR. However, limited by the number of patients, the overall strength of the evidence provided by this meta-analysis is moderate (Additional file 2).
This meta-analysis has some limitations. First, PPCs include a combination of various lung injuries after surgery. The incidence of atelectasis, volutrauma, barotrauma and ARDS may not be the same with different ventilation strategies. However, this definition of PPCs is thought to be a stronger outcome than a single complication analysis (Hemmes et al. 2015). Second, the surgical procedure is one of the factors that could influence the incidence of PPCs (Licker et al. 2003). Some studies in this meta-analysis did not report the exact surgical procedure and currently available data cannot meet the criteria of subgroup analysis on surgical procedure. However, the differences in surgical procedure can be minimized by randomization. All studies included in this meta-analysis were of randomized design. Third, the length of hospital stay was a combination of ICU days and non-ICU days in many studies included in the meta-analysis. Therefore, the results on the length of hospital stay should be interpreted with caution.
Conclusions
This meta-analysis suggests that protective ventilation with low V T protects surgical patients against PPCs in one-lung ventilation. Further trials are needed to define the role of PCV in preventing PPCs in one-lung ventilation.
Abbreviations
- ARDS:
-
respiratory distress syndrome
- CV:
-
conventional ventilation
- HPV:
-
hypoxic pulmonary vasoconstriction
- MAP:
-
mean arterial pressure
- PaO2/FiO2 :
-
oxygen index
- PCV:
-
pressure-controlled ventilation
- PCV-VG:
-
volume guaranteed pressure-controlled ventilation
- PEEP:
-
positive end expiratory pressure
- Pplateau :
-
intraoperative plateau airway pressure
- PV:
-
protective ventilation
- VCV:
-
volume-controlled ventilation
- V T :
-
tidal volume
References
Al Shehri AM, El-Tahan MR, Al Metwally R, Qutub H, Ghoneimy YF, Regal MA, Zien H (2014) Right ventricular function during one-lung ventilation: effects of pressure-controlled and volume-controlled ventilation. J Cardiothorac Vasc Anesth 28:892–896
Blank RS, Colquhoun DA, Durieux ME, Kozower BD, McMurry TL, Bender SP, Naik BI (2016) Management of one-lung ventilation: impact of tidal volume on complications after thoracic surgery. Anesthesiology 124(6):1286–1295
Boules NS, Ghobrial HZ (2011) Efficiency of the newly introduced ventilatory mode “pressure controlled ventilation-volume guaranteed” in thoracic surgery with one lung ventilation. Egypt J Anaesth 27:113–119
Choi YS, Shim JK, Na S, Hong SB, Hong YW, Oh YJ (2009) Pressure-controlled versus volume-controlled ventilation during one-lung ventilation in the prone position for robot-assisted esophagectomy. Surg Endosc 23:2286–2291
Della Rocca G, Coccia C (2013) Acute lung injury in thoracic surgery. Curr Opin Anaesthesiol 26(1):40–46
Guldner A, Kiss T, Serpa Neto A, Hemmes SN, Canet J, Spieth PM, Rocco PR, Schultz MJ, Pelosi P, Gama de Abreu M (2015) Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology 123(3):692–713
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Hu X, Shen H, Li X, Chen J (2014) Effects of volume-controlled ventilation and pressure-controlled volume-guaranteed mode during one-lung ventilation on circulation, pulmonary function and lung injury. [Chinese]. Zhonghua Yi Xue Za Zhi 94:1006–1009
Jung JD, Kim SH, Yu BS, Kim HJ (2014) Effects of a preemptive alveolar recruitment strategy on arterial oxygenation during one-lung ventilation with different tidal volumes in patients with normal pulmonary function test. Korean J Anesthesiol 67:96–102
Licker M, de Perrot M, Spiliopoulos A, Robert J, Diaper J, Chevalley C, Tschopp JM (2003) Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 97(6):1558–1565
Lin WQ, Lu XY, Cao LH, Wen LL, Bai XH, Zhong ZJ (2008) Effects of the lung protective ventilatory strategy on proinflammatory cytokine release during one-lung ventilation. Chin J Cancer 27:870–873
Lohser J (2008) Evidence-based management of one-lung ventilation. Anesthesiol Clin 26(2):241–272
Lohser J, Slinger P (2015) Lung injury after one-lung ventilation: a review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg 121(2):302–318
Lumb AB, Slinger P (2015) Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology 122(4):932–946
Maslow AD, Stafford TS, Davignon KR, Ng T (2013) A randomized comparison of different ventilator strategies during thoracotomy for pulmonary resection. J Thorac Cardiovasc Surg 146:38–44
Michelet P, D’Journo XB, Roch A, Doddoli C, Marin V, Papazian L, Decamps I, Bregeon F, Thomas P, Auffray JP (2006) Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 105:911–919
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Montes FR, Pardo DF, Charrís H, Tellez LJ, Garzón JC, Osorio C (2010) Comparison of two protective lung ventilatory regimes on oxygenation during one-lung ventilation: a randomized controlled trial. J Cardiothorac Surg 5:99
Moudgil R, Michelakis ED, Archer SL (2005) Hypoxic pulmonary vasoconstriction. J Appl Physiol (1985) 98(1):390–403
Neto AS, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, Futier E, Gajic O, El-Tahan MR, Ghamdi AA, Gunay E, Jaber S, Kokulu S, Kozian A, Licker M, Lin WQ, Maslow AD, Memtsoudis SG, Miranda DR, Moine P, Ng T, Paparella D, Ranieri VM, Scavonetto F, Schilling T, Selmo G, Severgnini P, Sprung J, Sundar S, Talmor D, Treschan T, Unzueta C, Weingarten TN, Wolthuis EK, Wrigge H, Amato MB, Costa EL, de Abreu MG, Pelosi P, Schultz MJ (2016) Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med
Pardos PC, Garutti I, Piñeiro P, Olmedilla L, Gala F (2009) Effects of ventilatory mode during one-lung ventilation on intraoperative and postoperative arterial oxygenation in thoracic surgery. J Cardiothorac Vasc Anesth 23:770–774
Petrucci N, De Feo C (2013) Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev 2:Cd003844
Prospective Register of Ongoing Systematic Reviews. http://www.crd.york.ac.uk/prospero. Accessed 18 June 2015
Pu J, Liu Z, Yang L, Wang Y, Jiang J (2014) Applications of pressure control ventilation volume guaranteed during one-lung ventilation in thoracic surgery. Int J Clin Exp Med 7:1094–1098
Qutub H, El-Tahan MR, Mowafi HA, El Ghoneimy YF, Regal MA, Al Saflan AA (2014) Effect of tidal volume on extravascular lung water content during one-lung ventilation for video-assisted thoracoscopic surgery: a randomised, controlled trial. Eur J Anaesthesiol 31(9):466–473
Roze H, Lafargue M, Perez P, Tafer N, Batoz H, Germain C, Janvier G, Ouattara A (2012) Reducing tidal volume and increasing positive end-expiratory pressure with constant plateau pressure during one-lung ventilation: effect on oxygenation. Br J Anaesth 108(6):1022–1027
Schilling T, Kozian A, Huth C, Buhling F, Kretzschmar M, Welte T, Hachenberg T (2005) The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg 101(4):957–965
Serpa Neto A, Hemmes SN, Barbas CS, Beiderlinden M, Biehl M, Binnekade JM, Canet J, Fernandez-Bustamante A, Futier E, Gajic O, Hedenstierna G, Hollmann MW, Jaber S, Kozian A, Licker M, Lin WQ, Maslow AD, Memtsoudis SG, Reis Miranda D, Moine P, Ng T, Paparella D, Putensen C, Ranieri M, Scavonetto F, Schilling T, Schmid W, Selmo G, Severgnini P, Sprung J, Sundar S, Talmor D, Treschan T, Unzueta C, Weingarten TN, Wolthuis EK, Wrigge H, Gama de Abreu M, Pelosi P, Schultz MJ (2015a) Protective versus conventional ventilation for surgery: a systematic review and individual patient data meta-analysis. Anesthesiology 123(1):66–78
Serpa Neto A, Schultz MJ, Gama de Abreu M (2015b) Intraoperative ventilation strategies to prevent postoperative pulmonary complications: systematic review, meta-analysis, and trial sequential analysis. Best Pract Res Clin Anaesthesiol 29(3):331–340
Shen Y, Zhong M, Wu W, Wang H, Feng M, Tan L, Wang Q (2013) The impact of tidal volume on pulmonary complications following minimally invasive esophagectomy: a randomized and controlled study. J Thorac Cardiovasc Surg 146:1267–1274
Song SY, Jung JY, Cho MS, Kim JH, Ryu TH, Kim BI (2014) Volume-controlled versus pressure-controlled ventilation-volume guaranteed mode during one-lung ventilation. Korean J Anesthesiol 67(4):258–263
Sungur Ulke Z, Kose B, Camci E, Yavru A, Koltka K, Toker A, Dilge S, Senturk M (2011) Effects of lower tidal volumes on oxygenation during one-lung ventilation. Gogus-Kalp-Damar Anestezi ve Yogun Bakim Dernegi Dergisi 17:5–11 (in Turkish)
Tugrul M, Camci E, Karadeniz H, Senturk M, Pembeci K, Akpir K (1997) Comparison of volume controlled with pressure controlled ventilation during one-lung anaesthesia. Br J Anaesth 79(3):306–310
Unzueta MC, Casas JI, Moral MV (2007) Pressure-controlled versus volume-controlled ventilation during one-lung ventilation for thoracic surgery. Anesth Analg 104:1029–1033
Végh T, Juhász M, Szatmári S, Enyedi A, Sessler DI, Szegedi LL, Fülesdi B (2013) Effects of different tidal volumes for one-lung ventilation on oxygenation with open chest condition and surgical manipulation: a randomised cross-over trial. Minerva Anestesiol 79:24–32
Yang M, Ahn HJ, Kim K, Kim JA, Yi CA, Kim MJ, Kim HJ (2011) Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 139:530–537
Ye FF, Li LW (2011) Effects of different ventilation modes for one-lung ventilation anesthesia on respiratory function and F(A)/F(I) changes during sevoflurane inhalation. Nan Fang Yi Ke Da Xue Xue Bao 31(4):714–717
Authors’ contributions
ZL helped design the study, conduct the study, analyze the data, and write the manuscript. XL helped conduct the study and analyze the data. YH helped conduct the study. JZ helped design the study, conduct the study, and write the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank the authors of all the studies included in this meta-analysis.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional files
40064_2016_2867_MOESM1_ESM.doc
Additional file 1. Subgroup analysis, publication bias, GRADE system assessment and search strategies of this meta-analysis.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, Z., Liu, X., Huang, Y. et al. Intraoperative mechanical ventilation strategies in patients undergoing one-lung ventilation: a meta-analysis. SpringerPlus 5, 1251 (2016). https://doi.org/10.1186/s40064-016-2867-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-2867-0