Abstract
Background
The optimal length of proximal margin for Siewert type II/III adenocarcinoma of the esophagogastric junction (AEJ) is still need to be clarified. The aim of the present study was to investigate the appropriate length of proximal margin for Siewert type II/III AEJ through transhiatal approach.
Methods
From September 2009 to December 2014, a total of 693 consecutive patients with Siewert type II/III AEJ were retrospectively analyzed. All patients received transhiatal R0 resection. The proximal margin length was measured immediately after resection. The prognostic value of proximal margin length on Siewert type II/III AEJ with transhiatal approach was analyzed.
Results
There were 404 cases of Siewert type II AEJ (58.3 %) and 289 cases of Siewert type III AEJ (41.7 %). Total gastrectomy was performed in 526 patients (75.9 %), and proximal gastrectomy was performed in 167 patients (24.1 %). The median length of the gross proximal margin was 2.4 (range 0.1–5.0) cm. Lymph node metastasis was the only independent prognostic predictor for Siewert type II AEJ. Tumor size and lymph node metastasis were independent prognostic predictors for Siewert type III AEJ.
Conclusions
For Siewert type II/III AEJ with esophageal invasion of 3 cm or less, proximal margin length does not influence the prognosis of patients after transhiatal curative gastrectomy.
Similar content being viewed by others
Background
Adenocarcinoma of the esophagogastric junction (AEJ) is defined as a tumor with an epicenter within the 5 cm proximal and distal of the esophagogastric junction (Keeney and Bauer 2006). The incidence of AEJ has been increasing in both Western and Asian countries (Hasegawa and Yoshikawa 2010). AEJ is classified into three types by Siewert in 1998 (Siewert and Stein 1998). Type I is defined as tumors with an epicenter of 1–5 cm above the junction, type II as epicenter within 1 cm above and 2 cm below the junction, type III as epicenter within 2–5 cm below the junction (Parry et al. 2015). There are many unresolved issues on surgical management. Curative surgical resection is considered the mainstay of therapy. The current trend of surgical resection for Siewert type III AEJ was radical gastrectomy (Gertler et al. 2011). However, the optimal surgical treatment for Siewert type II AEJ remains controversial. Previously, two phase III clinical trials performed in Japan and Netherlands demonstrated that transthoracic approach could not improve the prognosis of patients with Siewert type II compared with transhiatal approach (Sasako et al. 2006; Hulscher et al. 2002). Thus, transhiatal approach is considered sufficient for Siewert type II AEJ.
The optimal length of proximal margin for Siewert type II/III AEJ is still need to be clarified. Several reports have advocated that a resection margin of up to 10 cm is necessary to prevent local recurrence (Ito et al. 2004; Mariette et al. 2003). Barbour et al. reported that the prognosis of T2+ patients could be significantly improved if the length of proximal margin was greater than 3.8 cm (Barbour et al. 2007). A recent study revealed that proximal margin length of more than 2 cm seemed satisfactory for patients with type II/III AEJ (Mine et al. 2013). Thus, the aim of the present study was to investigate the appropriate length of proximal margin for Siewert type II/III AEJ through transhiatal approach.
Patients and methods
This study was performed in the Xijing Hospital of Digestive Diseases affiliated to the Fourth Military Medical University. From September 2009 to December 2014, a total of 693 consecutive patients with AEJ were retrospectively analyzed. The inclusion criteria were: (1) Siewert type II or III AEJ, (2) length of esophageal invasion was less than 3 cm, (3) underwent radical proximal or total gastrectomy, (4) with negative proximal margin, (5) pathological T2-4N0-3M0 tumor. The exclusion criteria were: (1) with neoadjuvant therapy, (2) with malignant tumor in other location, (3) underwent left thoracoabdominal approach. This study was approved by the Ethics Committee of Xijing Hospital, and written informed consent was obtained from all patients before surgery.
All patients were treated with proximal or total gastrectomy with a combined D2 lymphadenectomy via a transhiatal approach. The proximal margin was sent for frozen-section pathological examination when the proximal margin length was considered inadequate. If the proximal margin was positive, further resection of distal esophagus with an additional 1–2 cm was performed. All the surgical procedure and the extent of lymph node clearance were based on the recommendations of the Japanese Gastric Cancer Treatment Guidelines (Japanese Gastric Cancer Association 2011).
The fresh specimen was cut open longitudinally immediately after resection. Then the specimen was stretched maximally and fixed on a board. The length of proximal margin was measured and recorded by the surgeon. Additional proximal margin length was also measured if further resection of distal esophagus was performed. Then, specimens were fixed in 10 % neutral formalin immediately and embedded routinely for pathological examination.
Clinicopathological features including age, gender, tumor size, differentiation status, Bormann type, length of proximal margin, tumor depth, lymph node metastasis, lymphatic–vascular invasion (LVI), neural invasion, operation time, blood loss and postoperative complications were collected. The patients were followed up till October 2015 by enhanced chest and abdominal CT every 6 months after discharge to evaluate tumor recurrence and distant metastasis.
Data were processed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Discrete variables were analyzed using the Chi square test or Fisher’s exact test. Numerical variables were expressed as the mean ± SD unless otherwise stated. Significant predictors for survival identified by univariate analysis were further assessed by multivariate analysis using the logistic regression analysis. The P value was considered to be statistically significant at 5 % level.
Results
The clinicopathological and surgical related characteristics of patients were summarized in Table 1. There were 603 male (87.0 %) and 90 female (13.0 %). The patient age ranged from 22 to 87 years (median 61 years; mean 60.4 years). There were 404 cases of Siewert type II AEJ (58.3 %) and 289 cases of Siewert type III AEJ (41.7 %). Total gastrectomy was performed in 526 patients (75.9 %), and proximal gastrectomy was performed in 167 patients (24.1 %). The median length of the gross proximal margin was 2.4 (range 0.1–5.0) cm. The 5-year overall survival of Siewert type II and III was 54.7 and 50.8 %, respectively.
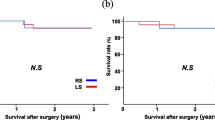
The prognostic factors for Siewert type II AEJ were summarized in Table 2. The results showed that tumor size, Borrmann type, tumor depth, lymph node metastasis, TNM stage, lymphatic–vascular invasion, neural invasion and intraoperative blood loss were risk factors for the prognosis of Siewert type II AEJ. However, only lymph node metastasis were independent prognostic predictors (Table 3). The prognostic factors for Siewert type III AEJ were summarized in Table 4. The results showed that tumor size, lymph node metastasis, TNM stage, lymphatic–vascular invasion, neural invasion and intraoperative blood loss were risk factors for the prognosis of Siewert type II AEJ. However, only tumor size and lymph node metastasis were independent prognostic predictors (Table 5). It was worth mentioning that the proximal margin length was not a prognostic predictor for Siewert type II/III AEJ.
Discussion
The optimal length of proximal margin for Siewert type II/III AEJ remains controversial, especially for transhiatal approach. Thus, our present study mainly focused on the influence of proximal margin length on the prognosis of patients with Siewert type II/III AEJ via transhiatal approach. We found that the proximal margin length does not influence the prognosis of patients with Siewert type II/III AEJ.
The optimal surgical approach for Siewert type II AEJ has not yet been agreed upon, although both transthoracic and transhiatal approach for Siewert type II has been attempted in the past few years (Mariette et al. 2011). The transthoracic approach is based on the principle that the proximal margin length has a critical impact on the prognosis, and the transhiatal approach is based on evidence that the abdominal lymph node metastasis is common and also has a great impact on the survival (Yamashita et al. 2011). The main goal of either approach remains complete tumor remove. A Dutch group has performed a prospective randomized controlled trial to compare the right transthoracic and transhiatal approaches for Siewert I/II tumors. As no significant difference was noted for prognosis between the two approaches, transthoracic approach is not recommended for Siewert type II tumors (Hulscher et al. 2002). A Japanese group performed another prospective randomized controlled trial to compare the effect of left thoracoabdominal and transhiatal approaches for Siewert II/III tumors with esophageal invasion of 3 cm or less (Sasako et al. 2006). The result showed that left thoracoabdominal approach could not improve the survival and will result in increased morbidity after operation, although this approach enabled complete dissection of the lower mediastinal lymph nodes. A meta-analysis conducted by Wei et al. showed that there was no difference in overall survival for Siewert type II AEJ between transthoracic and transhiatal approach, but transhiatal approach could decrease pulmonary complications and hospital stay (Wei et al. 2014). Therefore, the transhiatal approach was recommended for the treatment of Siewert type II/III AEJ with esophageal invasion of 3 cm or less (Hosoda et al. 2015). It was reported that the length of esophageal invasion was associated with lower mediastinal lymph node metastasis (Nakamura et al. 2012). Thus, for Siewert type II tumors with esophageal invasion more than 3 cm, mediastinal lymph node dissection via a transthoracic approach may provide a therapeutic benefit (Kurokawa et al. 2015). Given this situation, only Siewert type II/III AEJ patients with esophageal invasion of 3 cm or less were enrolled in our present study.
Previously, only two studies investigate the impact of proximal margin length on the prognosis of AEJ. Barbour et al. demonstrated that proximal margin greater than 3.8 cm is associated with improved prognosis for patients with Siewert types I/II/III AEJ that have undergone R0 resection with more than 15 lymph nodes examined (Barbour et al. 2007). However, Mine et al. reported that gross proximal margin length of more than 2.0 cm seem satisfactory for patients with Siewert type II/III AEJ treated by transhiatal approach (Mine et al. 2013). In our present study, we found that the proximal margin length does not influence the survival of patients with Siewert type II/III AEJ with transhiatal approach and R0 resection. This indicated that negative proximal margin may be suficient during the surgical resection of Siewert type II/III tumors. The conflict result between our present study and previous reports may contribute to several reasons. The most likely reason was that the sample size in our present study was significant larger than that in the studies reported by Mine et al. (100 cases) and Barbour et al. (275 cases with more than 15 lymph nodes examined). Second, all the three studies were based on single center data, which will result in bias. Third, they were all retrospective analysis. Thus, multicenter perspective randomized controlled trial should be carried out.
In our present study, the incidence of positive proximal margin was approximately 2.0 % (data not shown). The incidence was similar to 3.0 % described by Barbour et al. (Barbour et al. 2007) and 1.4 % reported by Mine et al. (2013). It was reported that positive margin was associated with deeper invasion and tumor size (Shen et al. 2006). Patients with stage III and IV disease may have a higher risk of sub-clinical intramural tumor infiltration to the esophagus (Bozzetti et al. 2000). Thus, in high risk patients, intraoperative frozen section analysis is always used to evaluate the tumor involvement of the proximal margins.
The influence of positive margin on the survival of patients with AEJ remains controversial (Migliore et al. 2014). Marriette et al. have shown that the median survival time of patients with positive proximal margin was significantly lower than that with negative proximal margin (Mariette et al. 2003). However, DiMusto et al. found that 80 % of patients with positive proximal margin died with distant metastasis, which would not be influenced by more extensive resection (DiMusto and Orringer 2007). They also found that adjuvant therapy for a positive margin neither improves prognosis nor reduces local recurrence. Shen et al. also reported that positive margin was not an independent prognostic factor for survival (Shen et al. 2006). Thus, the necessity of extensive resection during operation when positive margin was demonstrated by frozen section evaluation and the necessity of reoperation when positive margin was found by routine pathological examination need further investigation.
There were several limitations in our present study. First, it was a retrospective analysis. To clarify the influence of proximal margin length on the prognosis of patients, a well-designed randomized clinical trial should be carried out. Second, the present analysis was limited to proximal margins ranging from 0 to 5.0 cm, the influence of proximal margins more than 5.0 cm were not evaluated. Third, the sample size was not large enough. This may result in bias during analysis. Fourth, the association between proximal margin length and anastomotic recurrence was not investigated. Fifth, the risk factors for positive proximal margin were not evaluated in our present study.
Conclusions
For Siewert type II/III AEJ with esophageal invasion of 3 cm or less, proximal margin length does not influence the prognosis of patients after transhiatal curative gastrectomy.
References
Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, Rusch VW et al (2007) Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg 246:1–8
Bozzetti F, Bignami P, Bertario L, Fissi S, Eboli M (2000) Surgical treatment of gastric cancer invading the oesophagus. Eur J Surg Oncol 26:810–814
DiMusto PD, Orringer MB (2007) Transhiatal esophagectomy for distal and cardia cancers: implications of a positive gastric margin. Ann Thorac Surg 831993–831998 (discussion 1998–1999)
Gertler R, Stein HJ, Langer R, Nettelmann M, Schuster T, Hoefler H et al (2011) Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann Surg 253:689–698
Hasegawa S, Yoshikawa T (2010) Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric Cancer 13:63–73
Hosoda K, Yamashita K, Katada N, Watanabe M (2015) Overview of multimodal therapy for adenocarcinoma of the esophagogastric junction. Gen Thorac Cardiovasc Surg 63:549–556
Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P et al (2002) Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347:1662–1669
Ito H, Clancy TE, Osteen RT, Swanson RS, Bueno R, Sugarbaker DJ et al (2004) Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg 199:880–886
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011(14):113–123
Keeney S, Bauer TL (2006) Epidemiology of adenocarcinoma of the esophagogastric junction. Surg Oncol Clin N Am 15:687–696
Kurokawa Y, Hiki N, Yoshikawa T, Kishi K, Ito Y, Ohi M et al (2015) Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery 157:551–555
Mariette C, Castel B, Balon JM, Van Seuningen I, Triboulet JP (2003) Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol 29:588–593
Mariette C, Piessen G, Briez N, Gronnier C, Triboulet JP (2011) Oesophagogastric junction adenocarcinoma: which therapeutic approach? Lancet Oncol 12:296–305
Migliore M, Rassl D, Criscione A (2014) Longitudinal and circumferential resection margin in adenocarcinoma of distal esophagus and cardia. Future Oncol 10:891–901
Mine S, Sano T, Hiki N, Yamada K, Kosuga T, Nunobe S et al (2013) Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg 100:1050–1054
Nakamura M, Iwahashi M, Nakamori M, Naka T, Ojima T, Iida T et al (2012) Lower mediastinal lymph node metastasis is an independent survival factor of Siewert type II and III adenocarcinomas in the gastroesophageal junction. Am Surg 78:567–573
Parry K, Haverkamp L, Bruijnen RC, Siersema PD, Ruurda JP, van Hillegersberg R (2015) Surgical treatment of adenocarcinomas of the gastro-esophageal junction. Ann Surg Oncol 22:597–603
Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T et al (2006) Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 7:644–651
Shen JG, Cheong JH, Hyung WJ, Kim J, Choi SH, Noh SH (2006) Influence of a microscopic positive proximal margin in the treatment of gastric adenocarcinoma of the cardia. World J Gastroenterol 12:3883–3886
Siewert JR, Stein HJ (1998) Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85:1457–1459
Wei MT, Zhang YC, Deng XB, Yang TH, He YZ, Wang ZQ (2014) Transthoracic vs transhiatal surgery for cancer of the esophagogastric junction: a meta-analysis. World J Gastroenterol 20:10183–10192
Yamashita H, Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T (2011) Optimal extent of lymph node dissection for Siewert type II esophagogastric junction carcinoma. Ann Surg 254:274–280
Authors’ contributions
HWZ and FF designed and instructed this study. FF drafted manuscript. YZT, GHX and SSL searched literatures. YZT, GHX and ZL input data; ZL, MG and XL analyzed the data. DMF revised manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was supported in part by grants from the National Natural Scientific Foundation of China [Nos. 31100643, 31570907, 81572306, 81502403, XJZT12Z03].
Competing interests
The authors declare that they have no competing interests.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (The Ethics Committee of Xijing Hospital, China) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fan Feng, Yangzi Tian and Guanghui Xu contributed equally to this work
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Feng, F., Tian, Y., Xu, G. et al. The length of proximal margin does not influence the prognosis of Siewert type II/III adenocarcinoma of esophagogastric junction after transhiatal curative gastrectomy. SpringerPlus 5, 588 (2016). https://doi.org/10.1186/s40064-016-2240-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-2240-3