Abstract
In this study, the reaction of pentafluoropyridine with hydroxylated naphtoquinones and hydroxylated antraquinones was investigated under basic conditions in DMF. One or two hydroxyl group in naphtoquinones and antraquinones react with pentafluoropyridine to give mono and di-tetrafluoropyridyl naphtoquinones/antraquinones. All the compounds were characterized using 1H, 13C and 19F-NMR spectroscopy.
Similar content being viewed by others
Background
Recently, organofluorine compounds have been used as building blocks in the pharmaceutical industry and in material science due to their unique properties (Matthew et al. 2010). In pharmacology and medicinal researches, it is common to substitute hydrogen with fluorine atom for increasing the lipophilicity and the biological activity of drugs (Anatoliy et al. 2009). Various multi-functional pyridine derivatives and the construction of new heterocyclic drug systems could be accessed from the high electron efficiency of pentafluoropyridine and appropriate nucleophile in simple reaction conditions (Satoru et al. 2006; Cindy et al. 2010). The reactions of pentafluoropyridine with nucleophiles occurs in the most activated 4-position of pentafluoropyridine to give products arising from the substitution of fluorine, located para to ring nitrogen to give a range of 4-substituted tetrafluoropyridine systems (Mark et al. 2012). Previously, we reported the synthesis of 4-substituted 2,3,5,6-tetrafluoropyridine derivatives by the reaction of pentafluoropyridine with malononitrile, 1-tetrazole-5-thiol, and piperazine (Beyki et al. 2015). In this paper, we have recently reported the reaction of pentafluoropyridine with a very important class of biologically active compounds (Elias and Alexandros 2002), hydroxylated naphtoquinones and hydroxylated antraquinones. This allows the synthesis of a wide range of 4-substituted tetrafluoropyridine.
Results and discussion
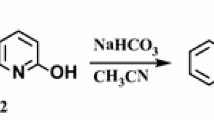
The reaction of pentafluoropyridine 1 with an equivalent of 5,8-dihydroxynaphthalene-1,4-dione 2 in the presence of potassium carbonate in DMF gave a mixture of two products, 2a and 2b arising from the displacement of the 4-fluor pyridine ring with naphtoquinones.
In hydroxylated naphtoquinones 2, the hydroxyl group deprotonates by potassium carbonate and attacks the para position of pentafluoropyridine to give 2a (Fig. 1). In 2b, both hydroxyl groups deprotonate and attack the para position of the pentafluoropyridine. The purification of 2a and 2b was achieved by column chromatography using ethyl acetate/n-hexane (1:10). The identification of 2a was done by 19F NMR analysis, in which the resonance attributed to the fluorine located in ortho ring nitrogen has a chemical shift of −87.6 ppm and the fluorine located in meta to ring nitrogen occurs at −162.0 ppm. The two resonances by 19F NMR and their chemical shift indicate the displacement of fluorine atoms attached to the para position of pyridine ring. In 1H NMR of 2a, the protons of the phenyl ring were observed at δ = 7.2–8.1 and hydroxyl group (OH) at 6.1 ppm. The mass spectrum of 2a displayed the molecular ion peak (M+) at m/z = 339, which is consistent with the proposed structure.
In 2b, 19F NMR spectroscopy shows two resonances at −83.5 and −84.3 ppm attributed to the fluorine located in the ortho position of the two ring nitrogen and the two resonances at −135.4 and −139.4 ppm attributed to the fluorine located in the meta position of two tetrafluoropyridine. In 2b, the four resonances by 19F NMR indicate the two rings of pentafluoropyridine displacement of fluorine atoms at the para positions of pyridine rings. The 1H, 13C NMR confirmed the structure of 3b. The protons of the phenyl ring were observed at 7.2 ppm. In MS spectrum, the molecular ion M+ +1 at m/z = 490 was observed.
The reaction of pentafluoropyridine 1 with an equivalent of 1,4-dihydroxyanthracene-9,10-dione 3 in the presence of potassium carbonate as the base in DMF solvent gave 3c and 3d in 41 and 11 % yield respectively (Fig. 2). In 3c, a hydroxyl group of 3 attacks the para position of the pyridine ring, and in 3d, the two hydroxyl groups attack the most activated para position of pentafluoropyridine. The purification of 3c and 3d was achieved by column chromatography using ethyl acetate/n-hexane (1:10). The identification of 3c was done from the 19F NMR analysis in which the resonance attributed to the fluorine atom located in the ortho positions had a chemical shift of −88.2 ppm. The corresponding resonance for the meta to ring nitrogen in 3c occurs at −158.1 ppm. The 1H NMR spectra of compound 3c showed an H broad signal at 4.2 ppm for OH group, and the protons of the phenyl ring were observed at δ = 7.2–8.3 ppm. In the MS spectrum of 3c, the molecular ion M+ at m/z = 389 was observed.
Mass spectrometry and 19F NMR confirmed the product of 3d as a di-tetrafluoropyridine system. The 19F NMR 3d showed two resonances at −87.5 and −91.8 ppm attributed to the fluorine located in the ortho to ring nitrogen and the two resonances at −156.5 and −162.6 ppm attributed to the meta to ring nitrogen. Other spectroscopic techniques were consistent with the structures proposed. In 1H NMR, the spectra protons of the phenyl ring were observed at 7.5–8.7 ppm. The mass spectrum of 3d displayed the molecular ion peak (M+) at m/z = 538, which is consistent with the proposed structure.
Also, we observed that the reaction of pentafluoropyridine 1 with 1,8-dihydroxyanthracene-9,10-dione 4 in the presence of potassium carbonate in DMF solvent gave 4e. In basic conditions, the hydroxyl group of 4 deprotonation and attack on the para position of pentafluoropyridine gave 4e (Fig. 3). The structure of compounds 4e was confirmed by the NMR spectroscopic data and the MS analysis. In particular, the 19F NMR spectroscopy showed the chemical shift of fluorine atoms attached to the ortho and the meta position, observed respectively at −91.6 and −142.5 ppm. In 1H NMR, the protons of OH were observed at 4.2 ppm, and the protons of the phenyl ring were observed at 7.0–8.2 ppm. In the MS spectrum, the molecular ion M+ at m/z = 389 was observed.
Conclusion
We showed that one or two hydroxyl groups in hydroxylated naphtoquinones and hydroxylated antraquinones can react with the pentafluoropyridineto afford of mono and di-2,3,5,6-tetrafluoropyridine naphtoquinones/antraquinones.
Experimental
All materials and solvents were purchased from Merck and Aldrich and were used without any additional purification. The melting points of the products were determined in open capillary tubes using BAMSTEAB Electrothermal apparatus model 9002. Mass spectra were taken by a Micro mass Platform II: EI mode (70 eV). Silica plates (Merck) were used for TLC analysis.
Preparation of 5-hydroxy-8-(perfluoropyridin-4-yloxy) naphthalene-1,4-dione 2a and 5,8-bis(perfluoropyridin-4-yloxy)naphthalene-1,4-dione 2b
Pentafluoropyridine 1 (0.16 g, 1 mmol), 5,8-dihydroxynaphthalene-1,4-dione 2 (0.19 g, 1 mmol) and potassium carbonate (0.11 g, 1.0 mmol) were stirred together in DMF (5 mL) at reflux temperature for 8 h. After completion of the reaction (indicated by TLC), reaction mixture was evaporated to dryness and water (10 mL) was added and extracted with dichloromethane (2 × 10 mL) and ethylacetate (2 × 10 mL). The mixture was filtered, volatiles evaporated and the residue purified by column chromatography on silica gel using ethyl acetate/n-hexane (1:10).
5-hydroxy-8-(perfluoropyridin-4-yloxy)naphthalene-1,4-dione 2a
(0.1 g, 29 %) as an orange solid; mp 240 °C dec; 1H NMR (CDCl3): δ (ppm) 4.1 (1H, s, OH), 7.2–8.3 (4H, m, Ar–H), 19F NMR (DMSO): δ (ppm) −87.6 (2F, s, F-2,6), −162.0 (2F, s, F-3,5), 13C NMR (CDCl3): δ (ppm) 115.3, 117.2, 119.7, 120.3, 125.4, 128.3, 131.0, 137.5, 150.3, 159.2, 169.2, 170.1, 171.8, MS (EI), m/z (%) = 341 (M+) (32 %) 318, 309, 301, 291, 272, 255, 246, 224, 198, 180, 152, 126, 106, 86, 58, 44.
5,8-bis(perfluoropyridin-4-yloxy)naphthalene-1,4-dione 2b
(0.08 g, 16 %) as an orange/yellow solid; mp 330 °C dec; 1H NMR (CDCl3): δ (ppm) 7.2 (5H, m, Ar–H), 19F NMR (CDCl3): δ (ppm) −83.5 (2F, s, F-2,6), −84.2 (2F, s, F-2′,6′), −135.4 (2F, s, F-3,5), −139.4 (2F, s, F-3′,5′). 13C NMR (CDCl3): δ (ppm) 91.8, 128.8, 130.9, 178.0, MS (EI), m/z (%) = 490 (M+ +2) (25 %), 461, 443, 409, 384, 369, 350, 331, 318, 302, 268, 255, 239, 212, 181, 167, 149, 136, 104, 90, 77, 57, 43.
Preparation of 1-hydroxy-4-(perfluoropyridin-4-yloxy) anthracene-9,10-dione 3c and 1,4-bis(perfluoropyridin-4-yloxy)anthracene-9,10-dione 3d
Pentafluoropyridine 1 (0.16 g, 1 mmol), 1,4-dihydroxyanthracene-9,10-dione 3 (0.24 g, 1 mmol) and potassium carbonate (0.11 g, 1.0 mmol) were heated in DMF (5 mL) at reflux for 12 h (monitored by TLC). After completion of the reaction, the solvent was evaporated to dryness; water (10 mL) was added and extracted with dichloromethane (2 × 10 mL) and ethylacetate (2 × 10 mL). The mixture was filtered, volatiles evaporated and the residue purified by column chromatography on silica gel using ethyl acetate/n-hexane (1:10).
1-hydroxy-4-(perfluoropyridin-4-yloxy)anthracene-9,10-dione 3c
(0.16 g, 41 %) as a yellow solid; mp 355 °C dec; 1H NMR (CDCl3): δ (ppm) 4.1 (1H, s, OH), 7.2-8.3 (6H, m, Ar–H), 19F NMR (CDCl3): δ (ppm) −88.2 (2F, s, F-2,6), −158.1 (2F, s, F-3,5),13C NMR (CDCl3): δ (ppm) 126.5, 127.0, 133.6, 133.9, 138.8, 145.4, 148.5, 160.9, 188.9, MS (EI), m/z (%) = 389 (M), 371, 354, 340, 321, 307, 290, 263, 237, 212, 195, 181, 157, 143, 125, 112, 91, 77, 57, 43.
1,4-bis(perfluoropyridin-4-yloxy)anthracene-9,10-dione 3d
(0.06 g, 11 %) as a red/black solid; mp 315 °C dec; 1H NMR (CDCl3): δ (ppm) 4.2 (1H, s, OH), 7.2–8.7 (6H, m, Ar–H), 19F NMR (CDCl3): δ (ppm) −87.5 (2F, s, F-2,6), −91.8 (2F, s, F-2′,6′), −156.5 (2F, s, F-3,5), −162.6 (2F, s, F-3′,5′),13C NMR (CDCl3): δ (ppm) 120.9, 122.2, 123.4, 123.6, 124.5, 127.1, 128.5, 129.1, 130.1, 132.4, 136.5, 139.2, 140.4, 146.4, 147.4, 166.1, 170.2, MS (EI), m/z (%) = 538 (M), 496, 480, 439, 411, 398, 384, 362, 347, 331, 316, 302, 283, 270, 255, 239, 225, 209, 196, 181, 167, 154, 129, 105, 86, 57, 43.
Preparation of 1-hydroxy-8-(perfluoropyridin-4-yloxy)anthracene-9,10-dione 4e
Pentafluoropyridine 1 (0.16 g, 1 mmol), 1,8-dihydroxyanthracene-9,10-dione 4 (0.24 g, 1 mmol) and potassium carbonate (0.11 g, 1.0 mmol) were heated in DMF (5 mL) at reflux for 12 h (monitored by TLC). After completion of the reaction, the solvent was evaporated to dryness; water (10 mL) was added and extracted with dichloromethane (2 × 10 mL) and ethylacetate (2 × 10 mL). The mixture was filtered, volatiles evaporated and the residue purified by column chromatography on silica gel using ethyl acetate/n-hexane (1:8).
1-hydroxy-8-(perfluoropyridin-4-yloxy)anthracene-9,10-dione 4e
(0.09 g, 23 %) as a red solid; mp 340 °C dec; 1H NMR (CDCl3): δ (ppm) 4.3 (1H, s, OH), 7.1–8.0 (6H, m, Ar–H), 19F NMR (CDCl3): δ (ppm) −91.6 (2F, s, F-2,6), −162.5 (2F, s, F-3,5),13C NMR (CDCl3): δ (ppm) 120.9, 122.2, 123.2, 123.6, 124.5, 127.1, 128.8, 130.2, 130.9, 131.2, 132.4, 136.5, 139.2, 140.4, 146.4, 147.9, 161.0, 164.0, 167.8. MS (EI), m/z (%) = 389 (M+), 387, 370, 364, 360, 342, 316, 299, 281, 255, 240, 223, 202, 184, 169, 155, 141, 127, 101, 79, 57, 43.
References
Anatoliy MS, Ludmila AR, Alexander EF, Victor EK, Kirill GN, Alexandr AS, Andrei AG (2009) Synthesis of 3-cyano-2-fluoropyridines. J Fluor Chem 130:236–240
Beyki K, Haydari R, Maghsoodlou MT (2015) Synthesis of 2,3,5,6-tetrafluoro-pyridine derivatives from reaction of pentafluoropyridine with malononitrile, piperazine and tetrazole-5-thiol. SpringerPlus 4:757
Cindy C, Stéphane C, Alexandre R, Karine V, Jean-Dominique C, François M, Sylvette B (2010) Fluorination of 2-chloropyridine over metal oxide catalysts as new catalytic fluorination systems. Catal Commun 12:151–153
Elias AC, Alexandros TS (2002) Generation of libraries of pharmacophoric structures with increased complexity and diversity by employing polymorphic scaffolds. Eur J Org Chem 3341:3350
Mark AF, Graham S, Rachel S, Dmitrii SY, Judith AKH, Antonio V (2012) Reactions of 4-substituted tetrafluoropyridine derivatives with sulfur nucleophiles: SNAr and annelation processes. J Fluor Chem 143:148–154
Matthew WC, Emma LP, Graham P, Rachel S, Graham S, Ian W, Dmitrii SY, Judith AKH, John AC, David DM (2010) Annelation of perfluorinated heteroaromatic systems by 1,3-dicarbonyl derivatives. Tetrahedron 66:3222–3227
Satoru A, Tsutomu K, John TG, Takashi I, Hiroki Y (2006) Synthetic application of fluorinated vinamidinium salts: synthesis of fluorinated 1,3-butadienylphosphonates by the reaction with Horner–Wadsworth–Emmons reagents. J Fluor Chem 127:1235–1241
Authors’ contributions
Study conception and design: The reaction of pentafluoropyridine with5,8-dihydroxynaphthalene-1,4-dione, 1,4-dihydroxyanthracene-9,10-dione and 1,8-dihydroxyanthracene-9,10-dione to give highly fluorinated heterocyclic materials. The attractive of this protocol are cleaner reaction, non-toxic catalyst which makes it a useful and attractive process for the preparation of 4-substituted-tetra fluoropyridine. Acquisition of data: from articles and books. Analysis and interpretation of data: by RH and KB. Drafting of manuscript: KB. Critical revision: MTM. All authors read and approved the final manuscript.
Acknowledgements
Financial support from the Research Council of the University of Sistan and Baluchestan is gratefully acknowledged.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Beyki, K., Haydari, R. & Maghsoodlou, M.T. Reaction of hydroxylated naphtoquinones/antraquinones with pentafluoropyridine. SpringerPlus 5, 110 (2016). https://doi.org/10.1186/s40064-016-1708-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-1708-5