Abstract
Objective
To assess the association between Parkinson’s disease (PD) and melanoma via systematic review and meta-analysis.
Methods
Comprehensive search in PubMed, Web of Science, Embase and four China databases (SinoMed, WanFang data, CNKI and VIP database) of epidemiologic evidences on PD and melanoma published before April 30, 2015. Studies which reported risk estimates of melanoma among PD patients or risk estimates of PD in patients with melanoma were included. Pooled odds ratios (ORs) with 95 % confidence intervals (CIs) were calculated by random-effects models. Heterogeneity across studies was assessed using Cochran Q and I2 statistics. Subgroup analyses and sensitivity analyses were conducted to evaluate sources of heterogeneity. Subgroup analyses were done according to temporal relationship, geographic region and gender respectively. We assessed publication bias using the Begg and Egger test. In addition, study appraisal was done using a scale for observational studies to ensure the quality of evidence.
Results
We identified 24 eligible studies on PD and melanoma with a total number of 292,275 PD patients: the pooled OR was 1.83 (95 % CI 1.46–2.30) overall, subgroup analyses by temporal relationship showed that risk of melanoma after PD diagnosis was significantly higher (OR 2.43, 95 % CI 1.77–3.32), but not before the diagnosis of PD (OR 1.09, 95 % CI 0.78–1.54). Subgroup analysis by geographic region showed that increased risk of melanoma in PD was found both in Europe (OR 1.44, 95 % CI 1.22–1.70) and in North America (OR 2.64, 95 % CI 1.63–4.28). Gender-specific subgroup analyses did not show difference between men (OR 1.64, 95 % CI 1.27–2.13) and women (OR 1.38, 95 % CI 1.04–1.82) in the risk of melanoma. In addition, we found the risk of non-melanoma skin cancers in PD was slightly higher (OR 1.20, 95 % CI 1.11–1.29) than general population. It was impossible to evaluate the association between PD and melanoma according to use of levodopa or gene polymorphism via meta-analysis since few observational or cohort studies have focused on it.
Conclusions
An association between PD and melanoma was confirmed. Most of the evidences were of high quality, and the conclusion was robust. Further research is needed to explore the mechanisms underlying this relationship.
Similar content being viewed by others
Introduction
A growing number of evidences suggest that people with Parkinson’s disease (PD) have a decreased risk of almost all cancers [1–3]. However, the incidence of melanoma is strikingly higher in patients with PD than that in general population [4–6]. Some case reports have linked the use of levodopa (L-dopa) with the occurrence of malignant melanoma [7, 8], while some reviews reported that there was apparently no effect of L-dopa on the risk for malignant melanoma [9, 10]. The underlying mechanism that link PD with melanoma is not clear, but it has aroused lots of interests.
The epidemiology of melanoma focus on well-known risk factors, such as skin color, hair color, gender and eye pigmentation, moreover, ultraviolet (UV) exposure is also a risk factor for melanoma [11]. White population is considered to have higher prevalence of melanoma than the dark population and the prevalence is higher in men than in women [11, 12]. Considering the varieties of the reported studies in study design, patient characteristics and geographical region, the discrepancy in the results may be related to insufficient statistical power of individual studies. Here we conducted a systematic review and meta-analysis to evaluate the relationship between PD and melanoma. We also evaluated the non-melanoma skin cancer risk ratio among patients with PD. Subgroup analyses with regard to temporal relationship between PD and melanoma diagnosis, geographic region and gender were also done.
Methods
Search strategy
We conducted a systematica search of the published original studies that reported the association between skin cancers and PD. We searched PubMed, Web of Science, Embase and four China databases (SinoMed, WanFang data, CNKI and VIP database) for literature published before April 30, 2015. We combined medical subject heading (MeSH) terms including “PD, Melanoma, Skin Neoplasms”, “Carcinoma, Basal Cell” “Carcinoma, Squamous Cell” and “Keratosis, Actinic” and text terms including “Parkinson disease, Parkinsons disease, Parkinson’s disease, melanoma*, skin neoplasm*, basal cell carcinoma, squamous cell carcinoma, actinic keratosis and skin cancer*” as search strategy. Restrictions were made to observational epidemiologic studies involving humans.
Eligibility criteria
Studies were included if they reported an estimate of association between PD and melanoma, e.g. relative risk (RR), odds ratio (OR), standardized incidence ratio/event ratio (SIR/SER) with 95 % confidence intervals (CIs) or data sufficient to calculate them. Then studies in the form of case report, review, meta-analysis and meeting abstract were excluded. Next we evaluated the potential studies by screening titles and abstracts and the reference lists of all the articles to find other relevant articles and to further exclude unqualified studies. In details, two of the coauthors PH and XDY independently evaluated the study titles and abstracts, and disagreements in the study selection were discussed among the coauthors until consensus was reached. Also duplicate counting of events was carefully avoided.
Data extraction and classification
Two of the coauthors PH and XDY independently searched the literature and extracted the relevant information [see Additional file 1] including: name of first author, study data, study design, sample size, patient characteristics, geographical region, adjusted risk estimates with corresponding 95 % CIs and other relevant study characteristics. Attempts were made to contact authors to obtain gender-specific ORs and 95 % CIs if they were not provided in the original publications. Disagreements were resolved by consensus.
Subgroup analyses were performed according to temporal relationship between PD and melanoma diagnosis (study design), geographical region and gender. We also evaluated the relationship between non-melanoma skin cancers and PD.
Assessment of study quality
Two of the coauthors PH and XDY independently scored each study [see Additional file 2] according to the following five criteria items [13]: 1) definition of PD diagnosis (clearly defined using generally accepted criteria; less well-defined with partial use of generally accepted criteria; other or no criteria applied; with scores of 2, 1, 0, respectively); 2) validation of PD diagnosis (confirmed by neurological exam; confirmed by chart review; no attempt to validate; with scores of 2, 1, 0, respectively); 3) adjustment for confounding factors (multiple factors including age and smoking; age alone or along with factors other than smoking; none; with scores of 2, 1, 0, respectively); 4) source and definition of cancer diagnoses (based on chart or pathology review; based on ICD code/death certificate or derived from population register; none of the above; with scores of 2, 1, 0, respectively); and 5) representativeness of cases (includes the great majority of cases in a defined population or a representative population-based sample; includes only some cases in a population leading to an under-representative sample, with scores of 1, 0, respectively). Potential total scores ranged from 0 to 9. Studies scored 0–3, 4–6 and 7–9 were of low, medium and high quality. Final consistency of scores was achieved through consensus.
Statistical analysis
The STATA, version 12.0 (StataCorp, College Station, TX) was used to perform the analyses. We made no distinction between varying measures of association (SIR/SER, OR or RR) reported in different studies and used OR for all the studies. The pooled ORs with corresponding 95 % CIs were calculated with Der Simonian and Laird random-effects model [14]. Heterogeneity was assessed using Cochran’s Q and I2 statistics, the percentages of I2 around 25, 50 and 75 % mean low, medium and high heterogeneity, respectively [15]. Subgroup analyses and sensitivity analyses were conducted to evaluate sources of heterogeneity. Subgroup analyses were done according to temporal relationship, gender and geographic region, respectively. Publication bias was evaluated using Begg rank correlation test and the Egger regression asymmetry test [16, 17].
Results
Search results and study characteristics
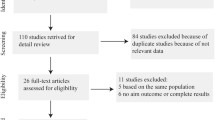
Twenty-four articles fulfilled our inclusion criteria and were included in our meta-analysis with a total number of 292,275 PD patients (Fig. 1). Among the 24 studies there were 16 cohort studies [1–6, 10, 18–26], six case control studies [27–32] and two cross-section studies [33, 34]. Nine of the studies also provided information on non-melanoma skin cancers and PD [3, 5, 10, 18, 19, 23, 27–29], and one study [33] only provided the risk of skin cancers in PD patients. Most of the studies were conducted in Europe and North America, only two studies were conducted in Asia [4, 26], and one study in Oceania [31].
Association between melanoma and PD according to temporal relationship
A total of 22 studies were included in the analysis, we divided all the 22 studies into three subgroups according to temporal relationship (study design), including “PD diagnosis preceding melanoma (cohort studies)” with 14 studies [1–6, 10, 19–22, 24–26], “melanoma preceding PD diagnosis (case–control studies)” with nine studies [3, 23, 24, 27–32] and “co-occurrence of PD and melanoma (cross-sectional studies)” with only one study [34]. Two studies provided data separately for melanoma before and after PD diagnosis [3, 24]. As shown in Fig. 2, the overall pooled OR was 1.83 (95 % CI 1.46–2.30), however, there was a significant heterogeneity across studies (I2 = 82.4 %, PQ < 0.001). Subgroup analysis showed that the pooled OR for “melanoma preceding PD diagnosis” group was 1.09 (95 % CI 0.78–1.54), with evidence of moderate heterogeneity (I2 = 58.1 %, PQ = 0.014). People with PD had increased risks (OR 2.43, 95 % CI 1.77–3.22) of melanoma compared with those without PD as shown in the “PD diagnosis preceding melanoma” group, with a significant heterogeneity across studies (I2 = 87.8 %, PQ < 0.001). Only one study reported the co-occurrence of PD and melanoma and the OR was 1.83 (95 % CI 0.98–3.40). We examined the source of heterogeneity by excluding the study of poor quality [23]. After excluding this study from the analysis, the pooled OR appeared significantly stronger (OR 1.93, 95 % CI 1.54–2.42), but the heterogeneity was still high (I2 = 82.0 %, PQ < 0.001). When we further exclude the study that reported the highest OR (OR 20.90) [25] and the study that reported the lowest OR (OR 0.48) [31], the pooled OR was 1.80 (95 % CI 1.52–2.14), and the heterogeneity was reduced by nearly 22 % (I2 = 64.5 %, PQ < 0.001). In detail, the heterogeneity in “melanoma preceding PD diagnosis” group was completely eliminated (I2 < 0.1 %, PQ = 0.999), while the heterogeneity in “PD diagnosis preceding melanoma” group was significant (I2 = 78.4 %, PQ < 0.001). To further examine the source of heterogeneity across studies in “PD diagnosis preceding melanoma” group, we did subgroup analysis by geographic region (see the following).
Association between melanoma and PD according to temporal relationship. Subtotal = pooled odds ratios (ORs) within each subcategory. Overall = pooled OR for all studies. Squares indicate study specific ORs; error bars indicate 95 % confidence intervals (CIs); diamonds indicate ORs and 95 % CIs from pooled analyses
Geographic region-specific analysis
In the subgroup analysis by geographic region (Fig. 3), the overall pooled OR were 2.01 (95 % CI 1.57–2.57), with high heterogeneity (I2 = 78.4 %, PQ < 0.001). Most of studies were done in Europe and North America, the pooled OR for Europe was 1.51 (95 % CI 1.24–1.85) with medium heterogeneity (I2 = 63.3 %, PQ = 0.018) and 2.69 for North America (95 % CI 1.77–4.08) with relatively lower heterogeneity (I2 = 50.3 %, PQ = 0.09). Both Israel (OR 4.40, 95 % CI 2.57–7.52) and Taiwan (OR 2.11, 95 % CI 0.21–21.25) only had one study.
Association between melanoma and PD according to geographic region. Subtotal = pooled odds ratios (ORs) within each subcategory. Overall = pooled OR for all studies. Squares indicate study specific ORs; error bars indicate 95 % confidence intervals (CIs); diamonds indicate ORs and 95 % CIs from pooled analyses
Gender-specific analysis
In the subgroup analysis by gender, we included 9 studies for men [2, 5, 19, 21, 22, 24, 28–30] and 7 studies for women [2, 5, 19, 22, 24, 28, 29]. In men, one study provided data separately for melanoma before and after PD diagnosis [24]. Our results (Fig. 4) showed that the association between melanoma and PD was similar in both men (OR 1.64, 95 % CI 1.27–2.13) and women (OR 1.38, 95 % CI 1.04–1.82), with evidence of moderate heterogeneity (I2 = 40.0 %, PQ = 0.045).
Association between melanoma and PD according to gender. Subtotal = pooled odds ratios (ORs) within each subcategory. Overall = pooled OR for all studies. Squares indicate study specific ORs; error bars indicate 95 % confidence intervals (CIs); diamonds indicate ORs and 95 % CIs from pooled analyses
Non-melanoma skin cancers and PD
We included 10 studies [3, 5, 10, 18, 19, 23, 27–29, 33] that provided information on non-melanoma skin cancers and PD (Fig. 5). Two studies provided the ORs both before and after PD [3, 23]. One study provided only gender-specific ORs [29]. The cumulative estimated risk associated with non-melanoma skin cancers was 1.20 (95 % CI 1.11–1.29) with evidence of moderate heterogeneity (I2 = 41.9 %, PQ = 0.056). After excluding the study of low quality [23], the overall pooled OR was 1.25 (95 % CI 1.19–1.32) and the heterogeneity was completely eliminated (I2 < 0.1 %, PQ = 0.713).
Evaluation for publication bias
The publication bias was detected from results of Begg test and showed no significant evidence for bias (P = 0.503). The Egger test also did not show significant publication bias (P = 0.125).
Discussion
Parkinson’s disease (PD) is a neurodegenerative disorder, characterized by depletion of dopamine in the striatum and loss of melanin-positive, dopaminergic neurons in the substantia nigra pars compacta (SNpc) [35, 36]. On the other hand, cancer is a large group of diseases, characterized by uncontrolled growth of cells and the ability of metastasis. Although PD and cancer seem to drive the cells to different outcomes, that is either degeneration or overproliferation, the association between PD and cancer has been supported by plenty of epidemiologic studies, which have shown that the incidences of most cancers are lower in PD patients compared with controls [1–3]. However, the incidence of melanoma is strikingly higher in patients with PD than that in general population [4–6]. The underlying mechanism that link PD with cancer is not clear, but it has aroused lots of interests.
The results of our meta-analysis show a definite association between PD and melanoma. Since studies included were of different study design, the heterogeneity across studies was high. Subgroup analysis by study design reduced some part of heterogeneity and the heterogeneity further decreased after subgroup analysis by geographic region. The result that risk of melanoma in PD patients is significantly higher than that in controls is in agreement with the result of previous meta-analysis [37]. We involved more eligible evidences and carefully assessed the quality of evidences which made the results much more confirmed and we also added comparison between different regions in the risk of melanoma. Possible mechanisms of the close association between PD and melanoma are as follows.
Melanin is the primary determinant of skin and hair color in human and more melanin pigment means darker in skin color. Melanin exists in neurons within SN is termed as “neuromelanin”; it is a protective factor that can save neurons from oxidative stress. Abnormalities in melanin can cause various skin cancers, including melanoma, while PD is correlated with abnormalities in neuromelanin, it suggests that PD closely links to melanoma through melanin [38]. Previous studies show melanin can reduce the susceptibility of skin to develop into melanoma [39, 40]. However, decreased level of neuromelanin in SN is associated with increased susceptibility of the neurons to oxidative stress and impaired motor functions [41, 42]. Thus, melanin acts as a key connection between PD and melanoma.
As the synthesis of dopamine and melanin share biomedical pathways and some case reports have found PD patients developed local melanoma after initiation of L-dopa therapy [7, 43-45], it is plausible that some scholars link the high prevalence of melanoma in PD to L-dopa therapy [46]. However, findings from recent clinical and epidemiologic studies suggest that the link between L-dopa therapy and melanoma was coincidental rather than causal [10, 20]; it is PD that increases the risk of melanoma rather than L-dopa therapy. However, to get reliable results we need to do meta-analysis, but it was impossible yet since there were only two observational studies evaluating the association between use of L-dopa and the incidences of melanoma in PD [10, 20]. Opponents argue that melanocytes have not been shown to be able to incorporate external L-dopa. In addition, it has been shown that dopamine is toxic for melanocytes in vitro [47]. Furthermore, the increased rate of melanoma before the diagnosis of PD weakens the hypothesis that melanoma may be induced by the treatment of PD [28]. On the other hand, we found that PD may also increase the risk of non-melanoma skin cancers [18, 19, 28]. The association between PD and non-melanoma skin cancers also supports the conclusion of L-dopa as a causal factor, unless the biochemical pathway including L-dopa is common to all types of skin cancer. Other anti-Parkinsonian drugs, such as selegiline and CEP-1347, are also found to have no influence on the association between PD and melanoma [20, 25].
Another potential explanation is that PD and melanoma have genetic correlation. PD is characterized by genetic susceptibility to environmental toxins due to decreased enzymatic detoxification [48, 49]. Lack of expression of enzymes encoded by GSTM1 can lead to death of neurons related to PD [50-52]. In addition, CYP2D6 polymorphism and VDR polymorphism also play important roles in the pathogenesis of PD [51, 53, 54]. Considering that high frequency of polymorphisms of CYP2D6 or the polymorphism of VDR, and null for GSTM1 gene in melanoma patients [55-57], it is plausible that changes in GSTM1, CYP2D6 and VDR genes may increase the risk for both PD and melanoma. Furthermore, some PD-related genes, such as Parkin [58, 59], alpha-synuclein [60, 61], LRRK2 [62, 63] and DJ-1 [64, 65], were also found to play critical roles in the pathogenesis of melanoma. To sum up, mutations of those genes can increase the risk of both PD and melanoma. However, scholars have found recently that the LRRK2 G2019S mutation carriers had statistically significant increased risks for non-skin cancers and there was no association with melanoma [66]. Thus, to get reliable results we need more studies focusing on the association between gene polymorphism and the risk of melanoma in PD to allow for meta-analysis.
In addition, autophagy deficits have been linked to neurodegenerative disease, such as PD, causing problems in the clearance of injured mitochondria and aggregated proteins [67, 68], whereas autophagy is also suppressed in melanoma [69, 70]. Defective autophagy can promote melanoma initiation and progression by causing impaired antigen presentation and melanoma immune escape. Thus, reduction of autophagy plays an important role both in PD and in melanoma. Whether PD links to melanoma through defective autophagy still needs much more direct evidences.
As to the risk factors of the development of melanoma, previous meta-analysis have shown family history of melanoma, high density of freckles, light skin color and fair hair color as major risk factors for melanoma [71]. White population is considered to have higher prevalence of melanoma than the dark population. However, since studies are mainly from Europe and North America, it is impossible to evaluate the correlation between race and the risk of developing melanoma in PD. More observational studies focusing on the association between PD and melanoma from other race are needed. In addition, with regard to geographic region, increased risk of melanoma in PD was found both in Europe (1.51 times) and in North America (2.69 times). The frequency of melanoma seems to be lower in Europe than in North America, it may be due to the following reasons. In Europe, melanoma incidence varies among north/south and east/west regions with Northern Europe gaining the most profoundly increase in melanoma incidence. Variations in Europe could be explained by differences in skin phenotype as well as in sun-exposure behaviors. Also it may be in part due to differences in case reporting and registration. Only half of European countries have good quality cancer registries, leading to the possibility of melanoma being under-reported in certain countries.
We also performed gender-specific meta-analysis since incidence of melanoma had been shown to be lower in women than men [12]. However, our results show that the connection between PD and melanoma is similar in both men and women, that is, increased risk of melanoma in PD was found both in males and females.
We found that PD patients had slightly increased risk of non-melanoma skin cancers than general population (OR 1.20, 95 % CI 1.11–1.29). After excluding the study of low quality [23], the heterogeneity was completely eliminated and no significant change was found in OR (1.25, 95 % CI 1.19–1.32). However, the results of a previous meta-analysis [37] indicated that there was no association between PD and non-melanoma skin cancers (OR 1.11, 95 % CI 0.94–1.30). Our results only indicate a possible association between non-melanoma skin cancers and PD. These conflicting results must be resolved by including more reliable studies into meta-analysis in the future. In addition, we found OR for non-melanoma in PD was only 1.2; there was no significant increase. One of the common causative factor for melanoma and non-melanoma skin cancers is long-term sun exposure, it may be hypothesized that PD patients are more sensitive to sun-exposure-induced skin lesions. The increased frequency of non-melanoma in PD may be due to a disease-specific susceptibility. However, melanoma occurs at a higher frequency in PD patients than non-melanoma skin cancers, it seemed that besides sun-exposure as a common risk factor, the shared biochemical pathways between the synthesis of both dopamine and melanin made PD patients more likely to develop melanoma. In the future, it is necessary to see the difference in the risk factors between PD patients who developed melanoma and those who developed non-melanoma skin cancers.
Limitations
There were several limitations in our meta-analysis. Firstly, most of the studies included were not focusing on the association between PD and melanoma; melanoma was mostly evaluated along with other cancers. Moreover, the number of cases with both PD and melanoma is usually small. Lastly, the majority of these studies did not collect enough data on risk factors for us to explore potential explanations.
Conclusion
In conclusion, melanoma occurs more frequently among patients with PD. Most of the evidences were of high quality, and the conclusion was robust. According to the references, most scholars consider that there are no strong evidences supporting the idea that dopaminergic therapy shall increase the risk of melanoma in PD. However, reliable conclusion will need more studies focusing on the association between L-dopa use and the development of melanoma in PD. The positive association between PD and melanoma may be explained by pigmentation changes in melanin and/or melanin synthesis enzyme, genetic correlations or autophagy deficits.
References
Kareus SA, Figueroa KP, Cannon-Albright LA, Pulst SM. Shared predispositions of parkinsonism and cancer: a population-based pedigree-linked study. Arch Neurol. 2012;69:1572–7.
Ong EL, Goldacre R, Goldacre M. Differential risks of cancer types in people with Parkinson’s disease: a national record-linkage study. Eur J Cancer. 2014;50:2456–62.
Wirdefeldt K, Weibull CE, Chen H, Kamel F, Lundholm C, Fang F, et al. Parkinson’s disease and cancer: A register-based family study. Am J Epidemiol. 2014;179:85–94.
Inzelberg R, Rabey JM, Melamed E, Djaldetti R, Reches A, Badarny S, et al. High prevalence of malignant melanoma in Israeli patients with Parkinson’s disease. J Neural Transm. 2011;118:1199–207.
Rugbjerg K, Friis S, Lassen CF, Ritz B, Olsen JH. Malignant melanoma, breast cancer and other cancers in patients with Parkinson’s disease. Int J Cancer. 2012;131:1904–11.
Constantinescu R, Elm J, Auinger P, Sharma S, Augustine EF, Khadim L, et al. Malignant melanoma in early-treated Parkinson’s disease: the NET-PD trial. Mov Disord. 2014;29:263–5.
Skibba JL, Pinckley J, Gilbert EF, Johnson RO. Multiple primary melanoma following administration of levodopa. Arch Pathol. 1972;93:556–61.
Fiala KH, Whetteckey J, Manyam BV. Malignant melanoma and levodopa in Parkinson’s disease: causality or coincidence? Parkinsonism Relat Disord. 2003;9:321–7.
Zanetti R, Loria D, Rosso S. Melanoma, Parkinson’s disease and levodopa: causal or spurious link? A review of the literature. Melanoma Res. 2006;16:201–6.
Olsen JH, Tangerud K, Wermuth L, Frederiksen K, Friis S. Treatment with levodopa and risk for malignant melanoma. Mov Disord. 2007;22:1252–7.
Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170:11–9.
Dao Jr H, Kazin RA. Gender differences in skin: a review of the literature. Gend Med. 2007;4:308–28.
Bajaj A, Driver JA, Schernhammer ES. Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Cause Control. 2010;21:697–707.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Elbaz A, Peterson BJ, Bower JH, Yang P, Maraganore DM, McDonnell SK, et al. Risk of cancer after the diagnosis of Parkinson’s disease: a historical cohort study. Mov Disord. 2005;20:719–25.
Olsen JH, Friis S, Frederiksen K, McLaughlin JK, Mellemkjaer L, Moller H. Atypical cancer pattern in patients with Parkinson’s disease. Br J Cancer. 2005;92:201–5.
Constantinescu R, Romer M, Kieburtz K, Group DIotPS. Malignant melanoma in early Parkinson’s disease: the DATATOP trial. Mov Disord. 2007;22:720–2.
Driver JA, Logroscino G, Buring JE, Gaziano JM, Kurth T. A prospective cohort study of cancer incidence following the diagnosis of Parkinson’s disease. Cancer Epidemiol Biomarkers Prev. 2007;16:1260–5.
Becker C, Brobert GP, Johansson S, Jick SS, Meier CR. Cancer risk in association with Parkinson disease: a population-based study. Parkinsonism Relat Disord. 2010;16:186–90.
Fois AF, Wotton CJ, Yeates D, Turner MR, Goldacre MJ. Cancer in patients with motor neuron disease, multiple sclerosis and Parkinson’s disease: record linkage studies. J Neurol Neurosurg Psychiatry. 2010;81:215–21.
Lo RY, Tanner CM, Van Den Eeden SK, Albers KB, Leimpeter AD, Nelson LM. Comorbid cancer in Parkinson’s disease. Mov Disord. 2010;25:1809–17.
Schwid SR, Bausch J, Oakes D, Schuchter L, Tanner C, Forrest M, et al. Cancer incidence in a trial of an antiapoptotic agent for Parkinson’s disease. Mov Disord. 2010;25:1801–8.
Sun LM, Liang JA, Chang SN, Sung FC, Muo CH, Kao CH. Analysis of Parkinson’s disease and subsequent cancer risk in Taiwan: a nationwide population-based cohort study. Neuroepidemiology. 2011;37:114–9.
Elbaz A, Peterson BJ, Yang P, Van Gerpen JA, Bower JH, Maraganore DM, et al. Nonfatal cancer preceding Parkinson’s disease: a case–control study. Epidemiology. 2002;13:157–64.
Olsen JH, Friis S, Frederiksen K. Malignant melanoma and other types of cancer preceding Parkinson disease. Epidemiology. 2006;17:582–7.
Powers KM, Smith-Weller T, Franklin GM, Longstreth Jr WT, Swanson PD, Checkoway H. Diabetes, smoking, and other medical conditions in relation to Parkinson’s disease risk. Parkinsonism Relat Disord. 2006;12:185–9.
Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Prospective case–control study of nonfatal cancer preceding the diagnosis of Parkinson’s disease. Cancer Causes Control. 2007;18:705–11.
Lubomski M, Rushworth RL, Tisch S. Hospitalisation and comorbidities in Parkinson’s disease: a large Australian retrospective study. J Neurol Neurosurg Psychiatry. 2015;86:324–30.
Dong J, Gao J, Nalls M, Gao X, Huang X, Han J, et al. Susceptibility loci for pigmentation and melanoma in relation to Parkinson’s disease. Neurobiol Aging. 2014;35:1512. e5-10.
Ferreira J, Silva JM, Freire R, Pignatelli J, Guedes LC, Feijo A, et al. Skin cancers and precancerous lesions in Parkinson’s disease patients. Mov Disord. 2007;22:1471–5.
Bertoni JM, Arlette JP, Fernandez HH, Fitzer-Attas C, Frei K, Hassan MN, et al. Increased melanoma risk in Parkinson disease: a prospective clinicopathological study. Arch Neurol. 2010;67:347–52.
Kempster PA, Gibb WR, Stern GM, Lees AJ. Asymmetry of substantia nigra neuronal loss in Parkinson’s disease and its relevance to the mechanism of levodopa related motor fluctuations. J Neurol Neurosurg Psychiatry. 1989;52:72–6.
Agid Y, Ruberg M, Javoy-Agid F, Hirsch E, Raisman-Vozari R, Vyas S, et al. Are dopaminergic neurons selectively vulnerable to Parkinson’s disease? Adv Neurol. 1993;60:148–64.
Rui L, Xiang G, Yi L, Honglei C. Meta-analysis of the relationship between Parkinson’s disease and melanoma. Neurology. 2011;76:2002–9.
Pan T, Li X, Jankovic J. The association between Parkinson’s disease and melanoma. Int J Cancer. 2011;128:2251–60.
Harbour JW, Brantley Jr MA, Hollingsworth H, Gordon M. Association between choroidal pigmentation and posterior uveal melanoma in a white population. Br J Ophthalmol. 2004;88:39–43.
Dwyer T, Blizzard L, Ashbolt R, Plumb J, Berwick M, Stankovich JM. Cutaneous melanin density of Caucasians measured by spectrophotometry and risk of malignant melanoma, basal cell carcinoma, and squamous cell carcinoma of the skin. Am J Epidemiol. 2002;155:614–21.
Enochs WS, Sarna T, Zecca L, Riley PA, Swartz HM. The roles of neuromelanin, binding of metal ions, and oxidative cytotoxicity in the pathogenesis of Parkinson’s disease: a hypothesis. J Neural Transm Park Dis Dement Sect. 1994;7:83–100.
De Marco F, Foppoli C, Coccia R, Blarzino C, Perluigi M, Cini C, et al. Ectopic deposition of melanin pigments as detoxifying mechanism: a paradigm for basal nuclei pigmentation. Biochem Biophys Res Commun. 2004;314:631–7.
Przybilla B, Schwab U, Landthaler M, Braun-Falco O. Development of two malignant melanomas during administration of levodopa. Acta Derm Venereol. 1985;65:556–7.
Sandyk R. Accelerated growth of malignant melanoma by levodopa in Parkinson’s disease and role of the pineal gland. Int J Neurosci. 1992;63:137–40.
Pfutzner W, Przybilla B. Malignant melanoma and levodopa: is there a relationship? Two new cases and a review of the literature. J Am Acad Dermatol. 1997;37:332–6.
Sober AJ, Wick MM. Levodopa therapy and malignant melanoma. JAMA. 1978;240:554–5.
Wick MM, Byers L, Frei 3rd E. L-dopa: selective toxicity for melanoma cells in vitro. Science. 1977;197:468–9.
Arriagada C, Paris I, Matas MJ S d l, Martinez-Alvarado P, Cardenas S, Castaneda P, et al. On the neurotoxicity mechanism of leukoaminochrome o-semiquinone radical derived from dopamine oxidation: mitochondria damage, necrosis, and hydroxyl radical formation. Neurobiol Dis. 2004;16:468–77.
Paris I, Dagnino-Subiabre A, Marcelain K, Bennett LB, Caviedes P, Caviedes R, et al. Copper neurotoxicity is dependent on dopamine-mediated copper uptake and one-electron reduction of aminochrome in a rat substantia nigra neuronal cell line. J Neurochem. 2001;77:519–29.
Ahmadi A, Fredrikson M, Jerregard H, Akerback A, Fall PA, Rannug A, et al. GSTM1 and mEPHX polymorphisms in Parkinson’s disease and age of onset. Biochem Biophys Res Commun. 2000;269:676–80.
Santt O, Baranova H, Albuisson E, Bignon YJ, Lucotte G. Interaction between GSTM1-null and CYP2D6-deficient alleles in the pathogenesis of Parkinson’s disease. Eur J Neurol. 2004;11:247–51.
Perez-Pastene C, Graumann R, Diaz-Grez F, Miranda M, Venegas P, Godoy OT, et al. Association of GST M1 null polymorphism with Parkinson’s disease in a Chilean population with a strong Amerindian genetic component. Neurosci Lett. 2007;418:181–5.
Payami H, Lee N, Zareparsi S, Gonzales mcNeal M, Camicioli R, Bird TD, et al. Parkinson’s disease, CYP2D6 polymorphism, and age. Neurology. 2001;56:1363–70.
Kim JS, Kim YI, Song C, Yoon I, park JW, Choi YB, et al. Association of vitamin D receptor gene polymorphism and Parkinson’s disease in Koreans. J Korean Med Sci. 2005;20:495–8.
Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053–62.
Stahl S, Bar-Meir E, Friedman E, Regev E, Orenstein A, Winkler E. Genetics in melanoma. Isr Med Assoc J. 2004;6:774–7.
Meyle KD, Guldberg P. Genetic risk factors for melanoma. Hum Genet. 2009;126:499–510.
Cesari R, Martin ES, Calin GA, Pentimalli F, Bichi R, McAdams H, et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc Natl Acad Sci U S A. 2003;100:5956–61.
Veeriah S, Taylor BS, Meng S, Fang F, Yilmaz E, Vivanco I, et al. Somatic mutations of the Parkinson’s disease-associated gene PARK2 in glioblastoma and other human malignancies. Nat Genet. 2010;42:77–82.
Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K. The role of alpha-synuclein in Parkinson’s disease: insights from animal models. Nat Rev Neurosci. 2003;4:727–38.
Matsuo Y, Kamitani T. Parkinson’s disease-related protein, alpha-synuclein, in malignant melanoma. PLoS ONE. 2010;5:e10481.
Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–3.
Paisan-Ruiz C, Houlden H. Common pathogenic pathways in melanoma and Parkinson disease. Neurology. 2010;75:1653–5.
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–9.
Maita C, Tsuji S, Yabe I, Hamadas S, Ogata A, Iguchi-Ariga SM, et al. Secretion of DJ-1 into the serum of patients with Parkinson’s disease. Neurosci Lett. 2008;431:86–9.
Agalliu I, San Luciano M, Mirelman A, Giladi N, Waro B, Aasly J, et al. Higher frequency of certain cancers in LRRK2 G2019S mutation carriers with Parkinson disease: a pooled analysis. JAMA Neurol. 2015;72(1):58–65.
Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, et al. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–72.
Pan T, Kondo S, Le W, Jankovic J. The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain. 2008;131:1969–78.
Ren H, Fu K, Mu C, Li B, Wang D, Wang G. DJ-1, a cancer and Parkinson’s disease associated protein, regulates autophagy through JNK pathway in cancer cells. Cancer Lett. 2010;297:101–8.
Miracco C, Cevenini G, Franchi A, Luzi P, Cosci E, Mourmouras V, et al. Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Hum Pathol. 2010;41:503–12.
Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040–59.
Acknowledgments
We thank all the authors of the included studies, and especially thank Ri-Hui Liu who helped us with the STATA software problems. This work was supported by grants from the National Program of Basic Research of China (2011CB504104), the Natural Science Fund (30872728, 81371407) and the Natural Science Foundation of Shanghai (14ZR1425700).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SDC and QX designed the whole study and gave suggestions on revising the article. PH and XDY searched and selected the studies, analyzed the data, drafted and revised the article. PH prepared figures. All authors read and approved the final manuscript.
Pei Huang and Xiao-Dong Yang contributed equally to this work.
Additional files
Additional file 1:
Characteristics of studies included in the Meta-analysis. Including information of first author, year of publication, country, study design, number of patients and controls, duration of follow-up. (PDF 74 kb)
Additional file 2:
Quality assessment of studies included in the Meta-analysis. Criterion include five parts: 1) definition of PD diagnosis; 2) validation of PD diagnosis; 3) adjustment for confounding factors; 4) source and definition of cancer diagnoses; 5) representativeness of cases. Potential total scores ranged from 0 to 9. (PDF 127 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Huang, P., Yang, XD., Chen, SD. et al. The association between Parkinson’s disease and melanoma: a systematic review and meta-analysis. Transl Neurodegener 4, 21 (2015). https://doi.org/10.1186/s40035-015-0044-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40035-015-0044-y