Abstract
Alzheimer’s disease (AD) is a prominent form of dementia, characterized by aggregation of the amyloid β-peptide (Aβ) plaques and neurofibrillary tangles, loss of synapses and neurons, and degeneration of cognitive functions. Currently, although a variety of medications can relieve some of the symptoms, there is no cure for AD. Recent breakthroughs in the stem cell field provide promising strategies for AD treatment. Stem cells including embryonic stem cells (ESCs), neural stem cells (NSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) are potentials for AD treatment. However, the limitation of cell sources, safety issues, and ethical issues restrict their applications in AD. Recently, the direct reprogramming of induced neural progenitor cells (iNPCs) has shed light on the treatment of AD. In this review, we will discuss the latest progress, challenges, and potential applications of direct reprogramming in AD treatment.
Similar content being viewed by others
Introduction
Alzheimer disease (AD) is an aging-associated disorder with an incidence of 13% in people over 65 years of age [1]. In most countries, people with AD are a heavy burden to their families and the society. In China, the number of patients with AD and other dementias will reach an estimated 18 million by 2030 [2]. Thus, it is urgent to seek effective therapeutic strategies to cure this intractable disease. Although the neuropathogenesis of AD remains largely unknown, increasing evidence suggests that the accumulation and deposition of β-amyloid protein (Aβ), caspase activation, mitochondrial dysfunction, and neuronal loss contribute to the neuropathogenesis of AD. Specifically, the accumulation of Aβ in the brain is always believed to be the primary factor that triggers local inflammatory response and the extent of synaptic and forebrain cholinergic neuron loss [3-7], which cause direct decline in cognitive function. Currently, the chemical treatments of AD mainly include: (i) NMDA receptor channel blocker, such as Memantine [8,9] (antagonist to glutamate NMDA receptors). (ii) Enhancing the function of cholinergic neurons [10], such as Donepezil [11], Tacrine [12], Galanthamine [13], Rivastigmine [14], Huperzine A [15] (inhibitors of acetylcholinesterase, AChEI). (iii) Blocking Aβ’s production and decreasing its aggregation [16], such as Solanezumab [17] (humanized anti-Aβ monoclonal antibody), Bapineuzumab [18] (humanized anti-Aβ monoclonal antibody), Semagacestat [19] (small-molecule γ-secretase inhibitor). Unfortunately, these drugs have failed clinical trials, because they did not improve cognitive function. E.g., Semagacestat presented side effect, such as skin cancers and infections [17-19]. (iv) Scavenging free radical [20,21] such as N-acetyl-L-cysteine [22,23]. (v) Immune modulating [24], such as nonsteroidalanti-inflammatory drugs (NSAIDs) [25]. Although these treatments can alleviate symptoms to a certain extent (see Table 1) [26], they are incapable of preventing the degeneration of neurons and replacing the impaired ones in AD brains [27]. Stem-cell based therapy will provide a potential strategy for AD treatment, which is different from the chemical treatments.
Current situation of stem cell-based therapies for AD
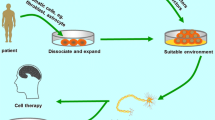
Increasing evidence suggests that embryonic stem cells (ESCs), neural stem cells (NSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) have potential for AD treatment. These cells can improve the ability of spatial learning and memory for animals [28-37] by cell replacement [28,29], Aβ reduction [30-33,38], neurotrophic action [31] and immune modulation [34,39-41] (see Table 2) (Figure 1).
Current situation of stem cell-based therapies for AD. Stem cell-based therapies for AD can be achieved by cell replacement, Aβ reduction, neurotrophic action and immune modulation. ESCs, NSCs, MSCs, iPSCs, and iNPCs have the capacity to differentiate into cholinergic neurons to replace the apoptotic ones after transplanted. NSCs and MSCs are able to reduce Aβ or tau’s level. MSCs can play a positive role in neuroprotection and immune modulation.
After transplanted, ESCs, NSCs and bone marrow derived-MSCs (BM-MSCs) can survive well and migrate to various brain regions [28], where they differentiate into cholinergic neurons, restore hippocampus synaptic density, and improve spatial learning and memory abilities for animals [28,29,33]. Moreover, NSCs and MSCs also reduce Aβ or tau pathology by phagocytic activity of astrocytes derived from transplanted NSCs [30-32] or microglia activation mediated by grafted MSCs to retard inflammatory processes [33,34,38-41]. Meanwhile, transplanted NSCs also secrete a series of neurotrophic factors, such as GDNF, BDNF and MANF [30-32], supporting the grafted cells to create more functional cholinergic neurons. Moreover, grafted human umbilical cord blood-derived MSCs (hUCB-MSCs) can also ameliorate the pathogenesis of AD by reducing the apoptosis and proinflammatory cytokines, increasing anti-inflammatory cytokines [39,40] and modulating oxidative stress [41]. Although the iPSCs technology has opened a new window for AD treatment, and newly generated neurons from iPSCs of familial AD patients also expressed MAP2 and β III-tubulin, formed functional synaptic contacts, and exhibited normal electrophysiological activity in vitro, these neurons showed similar cellular pathological feature with those in AD patients [42]. These studies suggest that iPSCs derived from AD patients may not be suitable for their own treatment.
Although ESCs, NSCs, MSCs, and iPSCs have some advantages in AD treatment, there are also problems that need to be solved before transplantation (also see Table 2). Currently, the ethical issues and immune rejection for ESCs and NSCs remain concerns, and also low differentiation efficiency for neurons due to lineage barriers and the limitation of cell source will be a challenge for MSCs [41]. Furthermore, the safety issue and low efficiency of iPSCs into subtype specific neurons will also limit its application in AD treatment.
Generation of induced neural progenitors (iNPCs) by direct lineage conversion
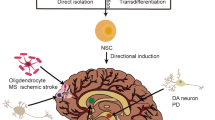
Although functional neurons have been successfully generated through direct reprogramming [43], the low yield and non-proliferative nature of neurons derived from direct reprogramming limit its broad application in cell transplantation therapy of AD. Recently, progress suggests that induced neural progenitors (iNPCs) that give rise to all types of neural cells hold promising therapeutic effects on AD [44-46]. In our laboratory, we have been one of the first groups in the world to successfully convert somatic cells into iNPCs by ectopic expression of defined transcription factors, which share high similarities with primary neural progenitors in proliferation, self-renewal, and differentiation abilities [47,48]. Meanwhile, Pei’s lab successfully achieved iNPCs from mouse embryonic fibroblasts by chemical cocktails under a physiological hypoxic condition, without introducing expression of exogenous genes. These chemical-induced NPCs (ciNPCs) resembled mouse brain-derived NPCs in both cell properties and gene expression profiles [49]. These strategies avoid the ethical issue and reduce the risk of tumor formation [50,51]. Recently, we have been working on the direct reprogramming of somatic cells into region-specific iNPCs and subtype-specific iNPCs by ectopic expression of defined transcription factors. Hopefully, these iNPCs will have high differentiation efficiency for region-specific or subtype -specific neurons, and significantly improve the therapeutic effects in AD (Figure 2). Although multipotent neural stem/progenitor cells (NSCs/NPCs), including iNPCs that give rise to all types of neural cells hold promising therapeutic effects on AD, the specificity and efficiency induction of homogeneous cholinergic neurons generation from NPCs/iNPCs remain a challenge. Studies have showed that NSCs/NPCs respond poorly to pre-patterning morphogens with low efficiency for specific neuronal subtypes, and are prone to more glial-restricted states under typical culture conditions in vitro [52]. Moreover, grafted NSCs/NPCs are more likely to terminally differentiate into astrocytes rather than functional neurons in response to injury [53,54]. Therefore, stem cell-based therapies for AD based on the regeneration of specific neuronal subtypes, such as forebrain cholinergic neurons, will be more attractive. Although the major pathogenesis of AD was characterized by the selective degeneration of basal forebrain cholinergic neurons, recent study has demonstrated that selective degeneration of septal and hippocampal GABAergic neurons in a mouse model of amyloidosis and tauopathy has also been detected [55]. Thus, the direct conversion of GABAergic neural progenitor can be used an alternative strategy for AD treatment. Recently, neural conversion from somatic cells can also be successfully achieved in vivo [56-59], suggesting that it may be feasible to convert activated astrocytes into region- or subtype-specific iNPCs in the AD patients’ brains in vivo. These studies provide a simpler, quicker, and safer therapeutic strategy, which will allow us to directly inject defined factors in AD brain to switch the active astrogliosis into neurogenesis in the future, such as forebrain cholinergic neurons, avoiding cell transplantation.
Strategies for direct reprogramming of iNPCs from somatic cells. iNPCs generated from different strategies. (A) Direct reprogramming of iNPCs by ectopic expression of defined transcription factors. (B) Direct reprogramming of region-specific iNPCs by expression of lineage-specific transcription factors. (C) Direct reprogramming of neuronal subtypes-specific iNPCs by using sets of defined transcription factors. (D) Generation of neuronal subtypes through direct reprogramming in vitro and in vivo.
In AD brain, the disease-related microenvironment, including aggregation of Aβ and inflammatory reaction, may decrease the proliferation and neurogenesis of transplanted cells, which will affect the treatment efficiency of AD. It is possible to improve the efficiency of iNPCs-based therapy by modulating the microenvironment via the use of a neurotrophic factor, Aβ-clear cells, and gene-engineered cells.
Conclusion and prospective
Progresses in the stem cell field have opened new windows to generate region-specific and subtypes-specific neural progenitors through direct reprogramming from somatic cells, which will set up a new concept for AD treatment. Moreover, instead of cell transplantation, directly reprogramming activated astrocytes in the pathological site of AD brain into region- or subtype-specific iNPCs by the direct injection of defined factors in vivo, will be a promising strategy for AD treatment in the future. Furthermore, the therapeutic efficacy of stem cells can also be improved by modulating the disease-related microenvironment by improving the proliferation, differentiation, and self-renew of the transplanted cells. Although the transplanted iNPC will face pathological situation and many potential problems,the experience gained would set up a great foundation for our future in vivo reprogramming work. For further studies, we should try a more specific, more efficient and virus free delivering method for in vivo reprogramming. Taken together, the direct reprogramming of region-specific and neuronal subtype-specific neural progenitors in vitro and in vivo will be a potential strategy for the effective treatment of AD in the future.
Abbreviations
- AD:
-
Alzheimer’s disease
- Aβ:
-
amyloid β-peptide, ESCs, embryonic stem cells
- NSCs:
-
Neural stem cells
- MSCs:
-
Mesenchymal stem cells
- iPSCs:
-
Induced pluripotent stem cells
- iNPCs:
-
Induced neural progenitor cells
- BM-MSCs:
-
Bone marrow-derived MSCs
- hUCB-MSCs:
-
Human umbilical cord blood-derived MSCs
References
William T, Laura B. 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8:131–68.
Ding D, Zhao Q, Guo Q, Meng H, Wang B, Yu P, et al. The shanghai aging study: study design, baseline characteristics, and prevalence of dementia. Neuroepidemiology. 2014;43(2):114–22.
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, et al. Soluble pool of abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46(6):860–6.
Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155(3):853–62.
Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68(1):1–14.
Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, et al. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA J Am Med Assoc. 2000;283(12):1571–7.
Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999;158(2):328–37.
Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–41.
Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev. 2005;3:CD003154.
Yan Z, Feng J. Alzheimer’s disease: interactions between cholinergic functions and beta-amyloid. Curr Alzheimer Res. 2004;1(4):241–8.
Meguro K, Ouchi Y, Akanuma K, Meguro M, Kasai M. Donepezil can improve daily activities and promote rehabilitation for severe Alzheimer inverted question marks patients in long-term care health facilities. BMC Neurol. 2014;14(1):243.
Chen Y, Sun J, Peng S, Liao H, Zhang Y, Lehmann J. Tacrine-flurbiprofen hybrids as multifunctional drug candidates for the treatment of Alzheimer’s disease. Arch Pharm (Weinheim). 2013;346(12):865–71.
Hager K, Baseman AS, Nye JS, Brashear HR, Han J, Sano M, et al. Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer’s disease. Neuropsychiatr Dis Treat. 2014;10:391–401.
D'onofrio G, Sancarlo D, Addante F, Ciccone F, Cascavilla L, Paris F, Elia AC, Nuzzaci C, Picoco M, Greco A, Panza F, and Pilotto A: A pilot randomized controlled trial evaluating an integrated treatment of rivastigmine transdermal patch and cognitive stimulation in patients with Alzheimer's disease. Int J Geriatr Psychiatry 2014. doi:10.1002/gps.4247.
Wang BS, Wang H, Wei ZH, Song YY, Zhang L, Chen HZ. Efficacy and safety of natural acetylcholinesterase inhibitor huperzine A in the treatment of Alzheimer’s disease: an updated meta-analysis. J Neural Transm. 2009;116(4):457–65.
Brogi S, Butini S, Maramai S, Colombo R, Verga L, Lanni C, et al. Disease-modifying anti-Alzheimer’s drugs: inhibitors of human cholinesterases interfering with beta-amyloid aggregation. CNS Neurosci Ther. 2014;20(7):624–32.
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):311–21.
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370(4):322–33.
Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369(4):341–50.
Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of ginkgo biloba for dementia, North American EGb study group. JAMA. 1997;278(16):1327–32.
Sozio P, Cerasa LS, Laserra S, Cacciatore I, Cornacchia C, Di Filippo ES, et al. Memantine-sulfur containing antioxidant conjugates as potential prodrugs to improve the treatment of Alzheimer’s disease. Eur J Pharm Sci. 2013;49(2):187–98.
Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84(5):1173–83.
Koppal T, Drake J, Butterfield DA. In vivo modulation of rodent glutathione and its role in peroxynitrite-induced neocortical synaptosomal membrane protein damage. Biochim Biophys Acta. 1999;1453(3):407–11.
Gong B, Pan Y, Zhao W, Knable L, Vempati P, Begum S, et al. IVIG immunotherapy protects against synaptic dysfunction in Alzheimer’s disease through complement anaphylatoxin C5a-mediated AMPA-CREB-C/EBP signaling pathway. Mol Immunol. 2013;56(4):619–29.
Shie FS, Nivison M, Hsu PC, Montine TJ. Modulation of microglial innate immunity in Alzheimer’s disease by activation of peroxisome proliferator-activated receptor gamma. Curr Med Chem. 2009;16(6):643–51.
Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–84.
Haas C. Strategies, development, and pitfalls of therapeutic options for Alzheimer’s disease. J Alzheimers Dis. 2012;28(2):241–81.
Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594–9.
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Esfahani NMH, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78(2–3):59–68.
Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006;11(12):1116–25.
Xuan AG, Long DH, Gu HG, Yang DD, Hong LP, Leng SL. BDNF improves the effects of neural stem cells on the rat model of Alzheimer’s disease with unilateral lesion of fimbria-fornix. Neurosci Lett. 2008;440(3):331–5.
Xuan AG, Luo M, Ji WD, Long DH. Effects of engrafted neural stem cells in Alzheimer’s disease rats. Neurosci Lett. 2009;450(2):167–71.
Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci Lett. 2009;450(2):136–41.
Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28(2):329–43.
Park D, Lee HJ, Joo SS, Bae DK, Yang G, Yang YH, et al. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp Neurol. 2012;234(2):521–6.
Park D, Joo SS, Kim TK, Lee SH, Kang H, Lee HJ, et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012;21(1):365–71.
Wu QY, Li J, Feng ZT, Wang TH. Bone marrow stromal cells of transgenic mice can improve the cognitive ability of an Alzheimer’s disease rat model. Neurosci Lett. 2007;417(3):281–5.
Habisch HJ, Schmid B, von Arnim CA, Ludolph AC, Brenner R, Storch A. Efficient processing of Alzheimer’s disease amyloid-Beta peptides by neuroectodermally converted mesenchymal stem cells. Stem Cells Dev. 2010;19(5):629–33.
Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer’s disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33(3):588–602.
Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, et al. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17(3):423–39.
Lee HJ, Lee JK, Lee H, Shin JW, Carter JE, Sakamoto T, et al. The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer’s disease. Neurosci Lett. 2010;481(1):30–5.
Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482(7384):216–20.
Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9(4):374–82.
Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108(25):10343–8.
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–41.
Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–3.
Tian C, Ambroz RJ, Sun L, Wang Y, Ma K, Chen Q, et al. Direct conversion of dermal fibroblasts into neural progenitor cells by a novel cocktail of defined factors. Curr Mol Med. 2012;12(2):126–37.
Tian C, Liu Q, Ma K, Wang Y, Chen Q, Ambroz R, et al. Characterization of induced neural progenitors from skin fibroblasts by a novel combination of defined factors. Sci Rep. 2013;3:1345.
Cheng L, Hu W, Qiu B, Zhao J, Yu Y, Guan W, et al. Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 2014;24(6):665–79.
Xu XL, Yang JP, Fu LN, Ren RT, Yi F, Suzuki K, et al. Direct reprogramming of porcine fibroblasts to neural progenitor cells. Protein Cell. 2014;5(1):4–7.
Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11(1):100–9.
Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci U S A. 2011;108(20):8299–304.
Holmin S, Almqvist P, Lendahl U, Mathiesen T. Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci. 1997;9(1):65–75.
Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34.
Loreth D, Ozmen L, Revel FG, Knoflach F, Wetzel P, Frotscher M, et al. Selective degeneration of septal and hippocampal GABAergic neurons in a mouse model of amyloidosis and tauopathy. Neurobiol Dis. 2012;47(1):1–12.
Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, et al. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110(17):7038–43.
Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15(10):1164–75.
Grande A, Sumiyoshi K, Lopez-Juarez A, Howard J, Sakthivel B, Aronow B, et al. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013;4:2373.
Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In Vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14(2):188–202.
Acknowledgements
This work was partly supported by research grants by National Basic Research Program of China (973 Program Grant No. 2014CB965001), National Natural Science Foundation of China (#81271419), Innovative Research Groups of the National Natural Science Foundation of China (#81221001), and Joint Research Fund for Overseas Chinese, Hong Kong and Macao Young Scientists of the National Natural Science Foundation of China (#81329002); National Institutes of Health: R01 NS 41858–01, R01 NS 061642–01, P20 RR15635-01, the State of Nebraska, DHHS-LB606 Stem Cell 2009–10 (JZ), LB606 Stem Cell-2010-10 (CT). Julie Ditter, Lenal Bottoms, Johna Belling, Jaclyn Ostronic and Robin Taylor provided outstanding administrative and secretarial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors’ contribution
1Center for Translational Neurodegeneration and Regenerative Therapy, Shanghai Tenth People's Hospital affiliated to Tongji University School of Medicine, Shanghai 200072, China; 2Department of Pharmacology and Experimental Neurosciences, University of Nebraska Medical Center, 68198–5930, Omaha, Nebraska, United States. All authors contributed to the preparation of this manuscript and have provided final approval of the version to be published.
Siqiang Lai and Min Zhang contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lai, S., Zhang, M., Xu, D. et al. Direct reprogramming of induced neural progenitors: a new promising strategy for AD treatment. Transl Neurodegener 4, 7 (2015). https://doi.org/10.1186/s40035-015-0028-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40035-015-0028-y