Abstract
Objective
The traditional treatment of rheumatoid arthritis (RA) has some side effects. We aimed to explore the effect of metformin treatment on the expression of HMGB1, cytokines, T cell subtypes and the clinical outcomes in RA patients.
Methods
The present prospective cohort study recruited 124 RA patients (metformin group) who were treated with metformin and conventional therapy (methotrexate, hydroxychloroquine sulfate and sulfasalazine) and 98 RA patients (conventional therapy group) who were only treated with conventional therapy. All subjects were admitted from December 2018 to December 2021 and continuous medication for 90 days. The serum high mobility group box 1 (HMGB1), tumor necrosis factor α (TNF-α), interleukin (IL)-6, IL-1β and C-reactive protein (CRP) levels were measured by enzyme-linked immunosorbent assay (ELISA). Flow cytometric were used to analyze the expression of CD4+ and CD8+. Demographic and clinical statistics including age, body mass index (BMI), sex, course of disease, erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), visual analogue score (VAS)and disease activity score (DAS)-28 were collected.
Results
The serum levels of HMGB1, CRP, IL-6, CD4+ expression and CD4+/CD8+ ratio were significantly increased in patients with DAS-28 score ≥ 2.6. The serum HMGB1 and cytokines levels of metformin group declined more quickly during the study time. Pearson’s analysis supported that a positive correlation existed between the HMGB1 and IL-6, TNF-α, CRP, CD4+, CD4+/CD8+ ratio, and VAS scores. HMGB1 could be a potential diagnostic biomarker for RA patients in active phase. Serum HMGB1 (95% CI 1.133–1.397, P < 0.001) was a factor associated with active RA.
Conclusion
The serum HMGB1 levels were significantly increased in RA patients in active phase. The serum levels of HMGB1 and inflammatory factors and VAS scores were decreased gradually with metformin treatment. HMGB1 might act as a novel therapeutic target for RA.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic, systemic autoimmune disease characterized by aggressive arthritis [1]. The global prevalence of rheumatoid arthritis is approximately 5 per 1000 adults, which affects women two to three times more often than men [2]. There are many risk factors for RA, including environmental influences [3], lifestyle [4], cigarette smoking [5], hereditary [6], etc. [7]. The conventional treatment regimens (disease-modifying anti-rheumatic drugs (DMARDs) for RA are gradually being standardized [8, 9]. However, more and more RA patients are drug intolerant, and immunosuppressants such as methotrexate and leflunomide have serious side effects (hair loss, liver impairment, bone marrow suppression, etc.), which have limited the use of traditional DMARDs [10,11,12]. In recent years, metformin has been confirmed to be an effective drug for the treatment of RA [13, 14], but its treatment mechanism needs to be further clarified.
Inflammation in RA patients mainly affects small joints and is characterized by pain and swelling. Inflammatory pathways in the patient's body are activated and the secretion of inflammatory factors increases, exacerbating the patient's symptoms and pain, leading to a decrease in quality of life, physical function and work capacity [15]. Metformin not only improves metabolic indicators to alleviate chronic inflammation, but also possesses direct anti-inflammatory effects [16]. Animal studies have shown that metformin improves various diseases, such as polycystic ovary syndrome (PCOS) and ankylosing spondylitis, through its anti-inflammatory mechanisms [17, 18]. Furthermore, there is evidence indicating that metformin can inhibit the inflammatory response induced by collagen in rat arthritis [19]. High mobility group box 1 (HMGB1) is a highly conservative nucleoprotein in all cell types [20]. Recently, HMGB1 has been found to play multiple roles in the regulation of inflammation and responses to cells and tissues [21]. In addition, HMGB1 mediates inflammatory responses in a variety of diseases and physiological processes by mediating the formation of inflammatory corpuscle and thus activates inflammation-mediated cell death pathways [22, 23]. Previous studies suggested that HMGB1 can activate inflammatory pathways in RA [24], and inhibition of HMGB1 can suppress inflammatory responses in RA animal models [25]. Metformin has been confirmed to inhibit inflammatory response by inhibiting HMGB1 in animal models and cells of RA. However, at present, there is no clinical study to support the above results.
In this prospective observational cohort research, we aimed to explore the serum levels of HMGB1 in RA patients and its correlation with cytokines, T cell subtypes and the clinical outcomes of the patients’. This study may reveal the clinical significance of HMGB1 in RA patients, as well as provide novel research targets for RA treatment.
Methods
Subjects
The present prospective cohort study recruited 124 RA patients (metformin group) who were treated with metformin (orally at 0.25 g, twice a day) and conventional therapy (methotrexate, orally at 10 mg, once a week, hydroxychloroquine sulfate, orally at 0.2 g, twice a day, sulfasalazine, orally at 0.5 g, three times a day). We also enrolled 98 RA patients (conventional therapy group) who were only treated with conventional therapy. All subjects were admitted from December 2018 to December 2021 and continuous medication for 90 days. Patients were diagnosed with RA according to the 2010 rheumatoid arthritis classification criteria by the American College of Rheumatology/European League Against Rheumatism [26]. The exclusion criteria included: (1) patients with other autoimmune diseases; (2) patients with diabetes; (3) patients with serious infection, severe liver or renal dysfunctions, malignancy, or cardiovascular dysfunctions; (4) patients who dropped out of the study due to drug intolerance, hypoglycemia, or other reasons in the metformin treatment group. The disease activity score (DAS) 28 was used to evaluate patient's disease activity, including patients in the active phase (DAS-28 score < 2.6) and patients in remission (DAS-28 score ≥ 2.6).
All patients were followed up for 90 days. Written informed consent was obtained from all participants. This research has obtained approval from the ethics committee of Hunan Provincial People’s Hospital.
Blood sampling measurement
The serum HMGB1, tumor necrosis factor α (TNF-α), interleukin (IL)-6, IL-1β and C-reactive protein (CRP) levels were measured by enzyme-linked immunosorbent assay (ELISA). Blood samples of fasting cubital venous (5 mL) were collected within 24 h after admission for all cases. Samples were centrifuged at 2000g for 15 min, followed with ELISA tested using commercially available kits (HMGB1 MBS7722054 MyBioSource, IL-6 MBS175877 MyBioSource, CRP MBS8123937 MyBioSource, TNF-α MBS824943 MyBioSource). All these inflammatory factors were measured when the RA patients were just hospitalized and the 7, 14, 28, and 90 days during the treatment.
Flow cytometric analysis
5 ml of peripheral elbow vein blood was collected from all subjects at admission and 90 days of admission, and peripheral blood mononuclear cells were isolated as described elsewhere [27, 28]. CD4+ and CD8+ expression was measured using flow cytometry. After centrifugation (1500 rpm × 5 min), 200 μL of the above cell suspension was added to pre-chilled PBS, washed 2–3 times, and then the cells were resuspended in 100 μL of PBS solution. Add 5 μL of PE-Anti-Human CD4 (MBS2569691, MyBioSource) and FITC-Anti-CD8 (MBS9461514, MyBioSource) to the centrifuge tube. Then, incubated for 2 h at 4 °C and centrifuged again (3000 rpm × 10 min). Using FACS Calibur flow cytometer (BD Biosciences, USA) with Diva software (version 6.1, BD Pharmingen USA) to measure the expression of CD4+ as well as CD8+.
Data collection and scale scoring
Demographic and clinical statistics including age, body mass index (BMI), sex, course of disease, etc., were collected. Using an automatic biochemical analyzer to perform whole blood test by Hitachi 7600 of Hitachi Corporation, and erythrocyte sedimentation rate (ESR) was recorded. Rheumatoid factor (RF) was detected by immune turbidimetry. The visual analogue score (VAS) was recorded to assess the severity of the patient’s condition and recovery.
Statistical analysis
Data were expressed by mean ± SD or median (range) according to distribution, which was confirmed by Kolmogorov–Smirnov analysis. Mann–Whitney test or Student’s t test was used for comparison between two groups. Kruskal–Wallis test or one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was used for comparison among three or more groups. Chi-square test was used for rates. Pearson’s rank correlation was used for correlation analysis. Logistic regression was performed for risk factors of patients in active phase. P < 0.05 regarded a significant difference. All data used SPSS 18.0 for statistical analyses.
Results
Clinical characteristics of all participants
This study enrolled 222 RA patients. All subjects were divided into two groups including metformin group (n = 124) and conventional therapy group (n = 98). The clinical characteristics of all participants are shown in Table 1.
Expression of HMGB1, inflammatory factors and T cell subtypes in RA patients
Then, we measured the expression of HMGB1, inflammatory factors, CD4+ and CD8+ in RA patients. As shown in Fig. 1, the serum levels of HMGB1, CRP, IL-6, CD4+ expression and CD4+/ CD8+ ratio were significantly increased in patients with DAS-28 score ≥ 2.6 (p < 0.05).
Effect of metformin treatment on serum HMGB1, inflammatory factors levels and T cell subtypes in RA patients
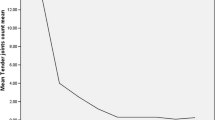
To further investigate the relationship between HMGB1 and inflammation in RA patients, we draw line graphs of all subjects to show the dynamic variations of all cytokines. It was shown that the HMGB1 and inflammatory factors levels were decreased gradually with the treatment in both groups (Fig. 2). We found no obvious differences in the serum HMGB1, TNF-α, CRP and IL-6 levels between two groups when the patients just hospitalized. However, the serum HMGB1 and cytokines levels of metformin group declined more quickly during the study time (p < 0.05).
In addition, we detected CD4+, CD8+ expression and VAS scores of patients after 90 days of the treatment. Compared with conventional therapy group, we found that the expression of CD4+, CD4+/CD8+ ratio and VAS scores were remarkably decreased in metformin group (Fig. 3, p < 0.05). Pearson’s analysis supported that a positive correlation existed between the HMGB1 and IL-6, TNF-α, CRP, CD4+, CD4+/ CD8+ ratio, VAS scores (Table 2).
Diagnostic value of HMGB1 for RA patients in active phase
Furthermore, we draw ROC curves to assess the diagnostic value of HMGB1 for RA patients in active phase. The result showed that HMGB1 could be a potential diagnostic biomarker for RA patients in active phase (Fig. 4), the AUC of HMGB1 was 0.932, cutoff value 80.21 pg/ml, sensitivity 83.8%, specificity 85.9%.
Risk factors of RA patients in active phase by logistic regression analysis
The risk variables for RA patients in active phase were calculated using binary regression analysis. Both univariate and multivariate logistic regression analyses were conducted to calculate the risk variables in patients with active RA. The results of the univariate analysis showed that ESR, RF, CRP, IL-6, CD4 +, CD4+/CD8+, and HMGB1 were factors associated with active RA (Table 3). Multivariate analysis established two models: Model 1 (age, BMI, disease duration, ESR, RF) and Model 2 (CRP, IL-6, TNF-α, CD4+, CD8+, CD4+/CD8+, HMGB1) and the analysis results indicated that ESR, RF, IL-6, CD4+, CD4+/CD8+, and HMGB1 were factors associated with active RA.
Discussion
Irregular treatment leads to joint deformity and loss of function can lead to a decrease in the patient's body, quality of life and social participation, bringing a huge economic burden to the patient's family and society [29]. Therefore, it is urgent to develop new biomarkers and comprehensive approaches to evaluate the patient's condition, as well as explore new therapeutic targets. In this research, our finding indicated that metformin treatment effectively reduced serum HMGB1 levels, and serum HMGB1 was a factor associated with active RA.
Metformin acts on a variety of intracellular signaling pathways, exerting immunomodulatory and anti-inflammatory effects by controlling the inflammatory response and the activation and differentiation of T and B cells [30]. Metformin exerted anti-inflammatory effects by inhibiting catabolic products and blocking HMGB1 translocation [31]. Sun et al. suggested metformin improved LPS-inhibited cellular autophagy by targeting HMGB1 via the AMPK/mTOR pathway [32]. These results indicated that metformin could inhibit inflammatory responses in animals and cells by mediating HMGB1. In our clinical study, we also found that serum HMGB1 levels and VAS scores were remarkably decreased with metformin treatment. This suggested that HMGB1 might play a significant role in the progression of RA, prompting us to further investigate biomarkers for active RA. Several biomarkers have been used to assess or diagnose RA. Zhang et al. suggested that regulatory T cells (Tregs) and interleukin-35 (IL-35) were decreased in RA patients and negatively correlated with ESR and DAS28 [33]. A cross-sectional study by Nakhjavani et al. confirmed the serum YKL-40 level of RA patients was significantly elevated than that of healthy controls and YKL-40 could be used to evaluate the activity of RA [34]. Soleimani et al. supported the serum glucose-6-phosphate isomerase (G6PI) could be used as a diagnostic marker for RA [35]. In our study, we found that the serum HMGB1 levels were elevated in activity-phase RA patients and could be a potential diagnostic biomarker for RA patients in active phase.
HMGB1, as a new inflammation related factor, has been confirmed to produce a marked effect in multiple diseases. Several in vivo studies have found that HMGB1 mediates inflammatory responses by regulating the ratio of CD4+ and CD8+ T cells to activate inflammatory pathways [36, 37]. Our findings were similar to these studies, we also found a positive correlation existed between the HMGB1 and CD4+, CD4+/CD8+ ratio in this study. In addition, previous clinical studies have shown the clinical significance and prognostic role of HMGB1 in pneumonia, cholecystitis and pancreatitis [38,39,40]. Modulation of the HMGB1 signaling pathway can suppress cytokine expression, alleviate cardiomyocyte apoptosis, and reduce fibrosis in RA model [25]. Targeting HMGB1 can improve the prevalent destructive events in RA [41]. In the present study, we also observed that HMGB1 levels were associated with disease activity and clinical outcomes, which was consistent with the results of Pullerits et al. [42]. However, Ozturk et al. found that the serum HMGB1 levels were no difference in septic and non-septic arthritis, which might be related to different study populations and disease types [43]. More prospective clinical studies are still needed in the future to validate our conclusions.
This present research also has some limitations. First, we only included a small size of study population and it is a single-center study. Secondly, we only checked a small number of inflammatory factors and T cells subtypes. Finally, the molecular mechanism of HMGB1 affecting RA development is unclear.
Conclusion
This study showed that the serum HMGB1 levels were significantly increased in RA patients in active phase. The serum levels of HMGB1 and inflammatory factors and VAS scores were decreased gradually with metformin treatment. Pearson’s analysis supported that a positive correlation existed between the HMGB1 and IL-6, TNF-α, CRP, CD4+, CD4+/CD8+ ratio, VAS scores. HMGB1 could be a potential diagnostic biomarker for RA patients in active phase and might act as a therapeutic target for RA.
Availability of data and materials
All data can be requested from the authors.
Change history
16 December 2023
A Correction to this paper has been published: https://doi.org/10.1186/s40001-023-01573-x
References
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6(1):1–14.
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–72.
Liao KP, Alfredsson L, Karlson EW. Environmental influences on risk for rheumatoid arthritis. Curr Opin Rheumatol. 2009;21(3):279.
Hazes JM, Dijkmans BA, Vandenbroucke JP, De Vries RR, Cats A. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. Ann Rheum Dis. 1990;49(12):980–2.
Ishikawa Y, Terao C. The impact of cigarette smoking on risk of rheumatoid arthritis: a narrative review. Cells. 2020;9(2):475.
Frisell T, Saevarsdottir S, Askling J. Family history of rheumatoid arthritis: an old concept with new developments. Nat Rev Rheumatol. 2016;12(6):335–43.
Oliver JE, Silman AJ. Risk factors for the development of rheumatoid arthritis. Scand J Rheumatol. 2006;35(3):169–74.
Nell VPK, Machold KP, Eberl G, Stamm TA, Uffmann M, Smolen JS. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology. 2004;43(7):906–14.
Benjamin O, Bansal P, Goyal A, Lappin SL. Disease modifying anti-rheumatic drugs (DMARD). 2018.
Grove ML, Hassell AB, Hay EM, Shadforth MF. Adverse reactions to disease-modifying anti-rheumatic drugs in clinical practice. QJM. 2001;94(6):309–19.
Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–16.
Lin Y-J, Anzaghe M, Schülke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9(4):880.
Matsuoka Y, Morimoto S, Fujishiro M, Hayakawa K, Kataoka Y, Suzuki S, Ikeda K, Takamori K, Yamaji K, Tamura N. Metformin repositioning in rheumatoid arthritis. Clin Exp Rheumatol. 2020;39:763–8.
Salvatore T, Pafundi PC, Galiero R, Gjeloshi K, Masini F, Acierno C, Di Martino A, Albanese G, Alfano M, Rinaldi L. Metformin: a potential therapeutic tool for rheumatologists. Pharmaceuticals. 2020;13(9):234.
Rein P, Mueller RB. Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther. 2017;4(2):247–61.
Bharath LP, Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. 2021;320(5):C873–9.
Xue J, Li X, Liu P, Li K, Sha L, Yang X, Zhu L, Wang Z, Dong Y, Zhang L. Inulin and metformin ameliorate polycystic ovary syndrome via anti-inflammation and modulating gut microbiota in mice. Endocr J. 2019;66(10):859–70.
Yan N, Wang L, Li Y, Wang T, Yang L, Yan R, Wang H, Jia S. Metformin intervention ameliorates AS in ApoE-/- mice through restoring gut dysbiosis and anti-inflammation. PLoS ONE. 2021;16(7): e0254321.
Fan K-J, Wu J, Wang Q-S, Xu B-X, Zhao F-T, Wang T-Y. Metformin inhibits inflammation and bone destruction in collagen-induced arthritis in rats. Ann Transl Med. 2020;8(23):1565.
Yang H, Wang H, Andersson U. Targeting inflammation driven by HMGB1. Front Immunol. 2020;11:484.
Deng M, Scott MJ, Fan J, Billiar TR. Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J Leukoc Biol. 2019;106(1):161–9.
Zhang T, Sun L, Wang T, Liu C, Zhang H, Zhang C, Yu L. Gestational exposure to PM2.5 leads to cognitive dysfunction in mice offspring via promoting HMGB1-NLRP3 axis mediated hippocampal inflammation. Ecotoxicol Environ Safety. 2021;223:112617.
Zhao H, Gu Y, Chen H. Propofol ameliorates endotoxin-induced myocardial cell injury by inhibiting inflammation and apoptosis via the PPARγ/HMGB1/NLRP3 axis. Mol Med Rep. 2021;23(3):1–1.
Cecchinato V, D’Agostino G, Raeli L, Nerviani A, Schiraldi M, Danelon G, Manzo A, Thelen M, Ciurea A, Bianchi ME. Redox-mediated mechanisms fuel monocyte responses to CXCL12/HMGB1 in active rheumatoid arthritis. Front Immunol. 2018;9:2118.
Lu X, Gong S, Wang X, Hu N, Pu D, Zhang J, Wang Y, Luo J, An Q, Ju B. Celastrol exerts cardioprotective effect in rheumatoid arthritis by inhibiting TLR2/HMGB1 signaling pathway-mediated autophagy. Int Arch Allergy Immunol. 2021;182(12):1245–54.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham Iii CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Ganji A, Farahani I, Khansarinejad B, Ghazavi A, Mosayebi G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells Mol Dis. 2020;83: 102437.
Aly SS, Fayed HM, Ismail AM, Abdel Hakeem GL. Assessment of peripheral blood lymphocyte subsets in children with iron deficiency anemia. BMC Pediatr. 2018;18(1):1–6.
Otón T, Carmona L. The epidemiology of established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2019;33(5): 101477.
Kim J-W, Choe J-Y, Park S-H. Metformin and its therapeutic applications in autoimmune inflammatory rheumatic disease. Korean J Intern Med. 2022;37(1):13.
Han Y, Yuan F, Deng C, He F, Zhang Y, Shen H, Chen Z, Qian L. Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release. Aging (Albany NY). 2019;11(22):10252.
Sun B, Ying S, Ma Q, Li H, Li J, Song J. Metformin ameliorates HMGB1-mediated oxidative stress through mTOR pathway in experimental periodontitis. Genes Dis. 2021. https://doi.org/10.1016/j.gendis.2021.06.003.
Zhang X, Zhang X, Zhuang L, Xu C, Li T, Zhang G, Liu Y. Decreased regulatory T-cell frequency and interleukin-35 levels in patients with rheumatoid arthritis. Exp Ther Med. 2018;16(6):5366–72.
Jafari-Nakhjavani MR, Ghorbanihaghjo A, Bagherzadeh-Nobari B, Malek-Mahdavi A, Rashtchizadeh N. Serum YKL-40 levels and disease characteristics in patients with rheumatoid arthritis. Caspian J Intern Med. 2019;10(1):92.
Soleimani N, Hosseinzadeh M, Habibagahi Z. Value of serum glucose-6-phosphate isomerase in patients with rheumatoid arthritis and correlation with disease activity: a case–control study. J Educ Health Promot. 2019;8:125.
Wang R, Wang P. HMGB1 promotes myocardial ischemic injury and regulates the proportion of CD4+, CD8+ T cells and Th17 cells in spleen through TLR4. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018;34(9):794–9.
Xin K, Sun J, Liu P, Ge J, Leng C, Pang F. Expression and significance of HMGB1 in patients with sepsis and effects on prognosis. All Life. 2020;13(1):164–70.
Amini M, Pakdaman A, Shapoori S, Mosayebi G. High mobility group box-1 (HMGB1) protein as a biomarker for acute cholecystitis. Rep Biochem Mol Biol. 2019;7(2):204.
Chen L, Long X, Xu Q, Tan J, Wang G, Cao Y, Wei J, Luo H, Zhu H, Huang L. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol Immunol. 2020;17(9):992–4.
Gao N, Yan C, Zhang G. Changes of serum procalcitonin (PCT), C-reactive protein (CRP), interleukin-17 (IL-17), interleukin-6 (IL-6), high mobility group protein-B1 (HMGB1) and D-dimer in patients with severe acute pancreatitis treated with continuous renal replacement therapy (CRRT) and its clinical significance. Med Sci Monit. 2018;24:5881.
Kaur I, Behl T, Bungau S, Kumar A, Mehta V, Setia D, Uddin MS, Zengin G, Aleya L, Arora S. Exploring the therapeutic promise of targeting HMGB1 in rheumatoid arthritis. Life Sci. 2020;258: 118164.
Pullerits R, Urbonaviciute V, Voll RE, Forsblad-D’Elia H, Carlsten H. Serum levels of HMGB1 in postmenopausal patients with rheumatoid arthritis: associations with proinflammatory cytokines, acute-phase reactants, and clinical disease characteristics. J Rheumatol. 2011;38(7):1523–5.
Ozturk A, Bilgetekin YG, Atilla HA, Emlek M, Ersan O, Çetin E, Yalcindag A. High mobility group box-1 in patients with bacterial septic arthritis of the knee: a controlled prospective study. Erciyes Med J. 2020;42(4):463–8.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
LZ designed and wrote the main manuscript. YZ, SJ and YF collected the data. JH and BX analyzed the data. HR and LH finally reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All patients signed the informed consent. The present study obeyed the Declaration of Helsinki. Study approval was obtained by the ethical committee of Human Provincial People’s Hospital [2023]-59.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The “Ethics approval and consent to participate” statement which was incorrect in the original version has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Zhou, Y., Jiang, S. et al. Effects of metformin therapy on HMGB1 levels in rheumatoid arthritis patients. Eur J Med Res 28, 512 (2023). https://doi.org/10.1186/s40001-023-01476-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01476-x