Abstract
Introduction
The management of periprosthetic joint infections (PJI) of the lower limb is challenging, and evidence-based recommendations are lacking. The present clinical investigation characterized the pathogens diagnosed in patients who underwent revision surgery for PJI of total hip arthroplasty (THA) and total knee arthroplasty (TKA).
Methods
The present study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). The institutional databases of the RWTH University Medical Centre of Aachen, Germany, were accessed. The OPS (operation and procedure codes) 5–823 and 5–821 and the ICD (International Statistical Classification of Diseases and Related Health Problems) codes T84.5, T84.7 or T84.8 were used. All patients with PJI of a previous THA and TKA who underwent revision surgery were retrieved and included for analysis.
Results
Data from 346 patients were collected (181 THAs and 165 TKAs). 44% (152 of 346 patients) were women. Overall, the mean age at operation was 67.8 years, and the mean BMI was 29.2 kg/m2. The mean hospitalization length was 23.5 days. 38% (132 of 346) of patients presented a recurrent infection.
Conclusion
PJI remain a frequent cause for revisions after total hip and knee arthroplasty. Preoperative synovial fluid aspiration was positive in 37%, intraoperative microbiology was positive in 85%, and bacteraemia was present in 17% of patients. Septic shock was the major cause of in-hospital mortality. The most common cultured pathogens were Staph. epidermidis, Staph. aureus, Enterococcus faecalis, and Methicillin-resistant Staph aureus (MRSA). An improved understanding of PJI pathogens is important to plan treatment strategies and guide the choice of empirical antibiotic regimens in patients presenting with septic THAs and TKAs.
Level of Evidence: Level III, retrospective cohort study.
Similar content being viewed by others
Introduction
Arthroplasty aims to restore quality of life in patients with end-stage joint degeneration, fractures, or joint infections [1, 2]. According to the German Arthroplasty Register (EPRD), the number of arthroplasties performed increases yearly [3]. In Germany, 233,424 total hip arthroplasties (THAs) and 187,319 total knee arthroplasties (TKAs) were performed in 2016 [4, 5]. In 2019, the number of THAs and TKAs increased to 243,477 and 193,759, respectively [4, 5]. This indicates an increase of THAs and TKAs of 4% and 3%, respectively. As a direct consequence, the number of revision arthroplasties increased. In Germany, revision arthroplasties of the hip increased by 1% from 2016 to 2019 (35,464 to 35,859) [4, 5]. Revision arthroplasties of the knee increased by 4% from 2016 to 2019 (24,940–25,841) [4, 5]. The most important reasons for revision are implant periprosthetic joint infections (PJI), aseptic loosening, and wearing [3]. Almost one-third of all revisions are performed because of PJIs [3, 6, 7]. According to the time elapsed from implantation to symptom manifestation, PJI can be divided into early (< 4 weeks) and late (> 4 weeks) [8]. Intraoperative direct colonization, hematogenous spread and contamination are the most common modality for infection. A few hours after adhesion to the foreign body surface, bacteria and fungi form a multi-layered structure (immature biofilm), which is then transformed into a stable matrix (mature biofilm) [9]. When such biofilm is mature, small colony variants more resistant to antibiotics and the immune system are formed [10]. Proper treatment may achieve success rates of over 90% [11]. In 2020, Rimke et al. conducted a survey on in-hospital management algorithms for PJI [12]. Early infections were treated in 97.6% of cases following the “DAIR principle” (Debridement, Antibiotics, and Implant Retention). Mobile components of the implant should be replaced. The prerequisites for DAIR are intact soft tissues, stable implant, and the absence of multiple resistant bacteria [9]. For late infections, a one- or two-stage implant replacement is recommended [13]. The degree of maturity of the biofilm plays a decisive role in the recommendation [14]. Whether a one-stage procedure promotes greater outcomes than a two-staged procedure has not yet been fully clarified. The two-stage replacement is the most frequently used procedure in the USA [15]. One-step replacement should only be performed if no multiple resistant bacteria are detected, there are intact soft tissues, and patients who have not undergone multiple revisions [9, 16]. A 12-week course of antibiotic therapy is recommended and should start after intraoperative tissue sampling and debridement [17]. A two-stage replacement either with short (< 3 weeks) or long (> 6 weeks) intervals between implant replacement can be performed depending on pathogens and the quality of soft tissues and bones. If two-stage replacement does not yield a satisfactory result, a three-stage replacement or long-term antibiotic therapy in case of resistant pathogens should be applied. Concomitant antibiotic administration is mandatory. Antibiotic therapy aims to eradicate the infection, avoid microorganism resistance, and prevent biofilm formation [17]. International guidelines and high-level recommendations on the management algorithm for PJI are lacking. Therefore, the present study was conducted to characterise the pathogens identified in patients who underwent revision surgery for PJI of THA or TKA.
Methods
Study design
The present study was conducted according to the principles of the Declaration of Helsinki and was approved by the ethics committee of the RWTH Aachen University (project ID EK 121/22). The present study follows the Strengthening the Reporting of Observational Studies in Epidemiology: the STROBE Statement [18]. The present investigation was conducted at the Department of Orthopaedics, Trauma and Reconstructive Surgery, of the University Hospital RWTH Aachen, Germany, and the Department of Orthopaedics of the Eifelklinik St. Brigida of Simmerath, Germany. In August 2022, the clinical databases of the institutions were accessed. For the databases of the German institutions the OPS (operation and procedure codes) 5–823 and 5–821 were used in combination with the ICD (International Statistical Classification of Diseases and Related Health Problems) codes T84.5, T84.7 or T84.8 (Table 1). Patients’ data were included in a Microsoft Excel spreadsheet (version 16.6).
All patients with a PJI of THA or TKA who had undergone THA or TKA revision surgery were retrieved. The inclusion criteria were: arthroplasty of knee or hip; microbiological evidence of pathogen using joint aspiration and/ or intraoperative histologic examination and/ or of blood cultures; the presence of at least one of these signs of inflammation at the joint: heat (calor), pain (dolor), redness (rubor), and swelling (tumor). The exclusion criteria were: any other non-infective ailment in a previously implanted arthroplasty; arthroplasty performed in joints other than knee and hip.
Data collection
The following data were recorded: gender, age at admission, height, weight and BMI, side, joint and the year of implantation. Data concerning the number and length of hospitalisation and the number of revisions were collected. Information on the type of pathogen was collected. Data on mortality were also retrieved. The perioperative risk was assessed using the American Society of Anaesthesiologists (ASA) score [19].
Statistical analysis
All analyses were conducted by the main author (FM) using the IBM SPSS Statistics software package, version 25. For descriptive statistics, frequency (amount of events/ number of observations) was used for binary variables. Arithmetic mean and standard deviation were adopted for continuous variables.
Results
Patient recruitment
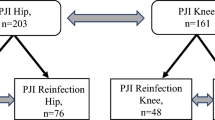
The database search resulted in 1331 procedures. Of them, 985 procedures were excluded with reason: procedure other than revision arthroplasty (N = 474), not performed at the knee or hip (N = 231), no evidence of infection (N = 209), no data of patients available (N = 64), uncertain data (N = 7). Finally, 346 patients were considered in the present study (Fig. 1).
Patient demographic
Data from 346 patients were collected (181 THAs and 165 TKAs). 44% (152 of 346 patients) were women. Overall, the mean age was 67.8 years, the mean BMI 29.2 kg/m2. The mean hospitalization length was 23.5 days. 38% (132 of 346) of patients presented a recurrent infection. Demographic information of the patients is shown in Table 2.
Results
In the 346 patients, a maximum of 18 operations were performed per in-hospital stay. In three patients, no operation was performed. On average, 2.6 ± 0.79 revisions per patient were performed. Overall, patients' surgical procedures lasted a mean of 213 ± 183.8 min. The shortest surgical duration was 36 min (one surgical session). The longest surgical duration was 1112 min (13 surgical sessions). Liners were changed in 171 patients; the implants were removed in 155 cases. Amputation was necessary for two patients. Pathogens were detected in 37% (128 of 346) of joint aspirations, 85% (294 of 346) of intraoperative microbiologic examinations, and 17% (59 of 346) of blood cultures. 312 (90%) patients survived, and 34 (10%) patients died during inpatient stay. 31 of these 34 (90%) died from septic shock. One patient died of kidney failure, one of ventricular fibrillation, and one of small cell lung cancer progression (Table 3).
Discussion
PJI are the second most common cause of surgical interventions after arthroplasty of the hip or knee joint, accounting for approximately 15% of all THA and TKA revisions [3]. In 2019, a total of 287 patients died in Germany as a result of a periprosthetic infection [20].
In the present analysis, the spectrum of pathogens in periprosthetic infections was examined in detail. Overall, 175 examined cases showed 66 distinct pathogen combinations with a total of 47 different pathogens. In more than two-thirds of the patients, a single pathogen was detected, and polybacterial infections were less common. This work focused only on the ten most common pathogens with statistical significance on patients’ survival and clinical outcome. An analysis of the remaining pathogens was not reasonable because of their low frequency. Considering Staphylococcus aureus and MRSA as one group, this was the most frequent microorganism, followed by Staphylococcus epidermidis and Enterococcus faecalis. To analyze the respective relevance of Staphylococcus aureus and methicillin-resistant Staphylococcus aureus, these were examined separately. Regarding the prevalence of the detected pathogens, the present study provides similar results compared to those found in the literature [21,22,23,24,25]. However, in the available data, specific pathogens were not further differentiated for either causing TKA or THA infections, which have high mortality rates in revision surgeries [26, 27].
Compared to aseptic revision surgeries, the postoperative mortality risk is markedly increased in septic revisions during the hospitalization period and in the following year [28,29,30]. Shahi et al. reported higher mortality compared to other major surgical procedures, such as cardiovascular surgery, cholecystectomies, kidney transplants, and carotid surgery. In addition, mortality risk accumulates with each additional revision procedure. Flurin et al. studied 373 cases in which Staphylococcus epidermidis was the most frequently detected pathogen. However, in 60% of patients, there was a mixed infection, while an isolated infection accounted for 35% [31].
In the present work, Staphylococcus epidermidis was detected in more than half of the patients with mixed infections. Contamination with pathogens of the transient skin flora is possible [32], and its involvement in periprosthetic infections should be clarified. Generally, a distinction between infection and contamination, for example, by molecular genetic testing of the pathogen, is only partially feasible [33]. On the other hand, collecting multiple samples in larger quantities obtained from different sites can be helpful [34]. A negative result for Staphylococcus epidermidis in several samples is unlikely for contamination. When interpreting the results, mixed infections from sample contamination with Staphylococcus epidermidis must be considered. A significant association between the detection of Staphylococcus aureus and a poor clinical outcome could be demonstrated in this work, in accordance with the previous studies. Patients in whom Staphylococcus aureus was isolated or detected as part of a mixed infection died significantly more frequently from septic shock than patients with other pathogens. Escherichia coli showed a statistically significant association with poor clinical outcomes only when the pathogen was part of a mixed infection. Compared to Staphylococcus aureus, which was detected in 39 cases, Escherichia coli was found in 12 cases, of which only three were in isolation. Three patients with mixed infections had a lethal septic shock during hospitalization. The limited available data do not allow clarification about the role of Escherichia coli in mixed infections as a determinant of poor clinical outcomes. Significantly higher mortality and the increased prevalence of MRSA compared to MSSA could be demonstrated in the elderly [35]. On the other hand, however, Senneville et al. did not find any difference between MRSA and MSSA regarding clinical outcomes [36]. In addition to MRSA, Fischbacher and Borens described a significantly increased risk of mortality associated with enterococcal infection. Similar results were obtained by Gundtoft et al., where THA infected by enterococci showed higher mortality compared to all other bacteria [37].
Enterococci cause 2–11% of all periprosthetic infections [38], while Enterococcus faecalis is more common than Enterococcus faecium [38,39,40]. This distribution is also shown in the present work. In addition, enterococci are detected more frequently in mixed infections and less frequently alone [39,40,41]. Periprosthetic infections caused by enterococci are difficult to treat and have high therapeutic failure rates from increasing antibiotic resistance [42]. Also, the formation of bacterial biofilms makes them more challenging to treat [43]. Therefore, often several revision surgeries are required [44]. Abdelaziz et al. [45] examined 121 patients after a second revision surgery following PJI. Enterococcal infections were associated with a significantly increased probability of revision after a one-stage procedure [45]. Revision surgery leads to more extended surgery and prolonged hospitalization and consequent poorer outcomes [38, 42]. The present study obtained similar results.
However, the influence of enterococcal infections on mortality was not significant compared to other pathogens. In addition, Enterococcus faecium and Streptococcus agalactiae were detected frequently in patients with diabetes mellitus, while other pathogens could not be detected significantly more frequently in diabetic patients. However, we acknowledge that our results are limited by the quantitative difference between the individual groups (diabetics = 47 vs. non-diabetics = 128). Considering the high prevalence of diabetics in the present study, diabetes mellitus appears to be a relevant comorbidity for the clinical course [46].
Conclusion
Periprosthetic joint infections remain a frequent cause for revisions after total hip and knee arthroplasty. Preoperative synovial fluid aspiration was positive in 37% of patients, intraoperative microbiology was positive in 85% and bacteraemia was present in 17%. Septic shock was the major cause of in-hospital mortality. The most common cultured pathogens were Staph. epidermidis, Staph. aureus, Enterococcus faecalis, and Methicillin-resistant Staph. aureus (MRSA). An improved understanding of PJI pathogens is important to determine treatment strategies and guide the choice of empirical antibiotic regimens in patients presenting with septic THAs and TKAs.
Availability of data and materials
The data underlying this article are available at reasonable request to the senior author AD (arne.driessen@luisenhospital.de).
References
Migliorini F, Driessen A, Oliva F, Maffulli GD, Tingart M, Maffulli N. Better outcomes and reduced failures for arthroplasty over osteotomy for advanced compartmental knee osteoarthritis in patients older than 50 years. J Orthop Surg Res. 2020;15(1):545. https://doi.org/10.1186/s13018-020-02079-6.
Migliorini F, Maffulli N, Trivellas M, Eschweiler J, Hildebrand F, Betsch M. Total hip arthroplasty compared to bipolar and unipolar hemiarthroplasty for displaced hip fractures in the elderly: a Bayesian network meta-analysis. Eur J Trauma Emerg Surg. 2022. https://doi.org/10.1007/s00068-022-01905-2.
Grimberg A, Jansson VWD, Lützner J, Melsheimer O, Morlock M, Steinbrück A. Endoprothesenregister Deutschland, Jahresbericht 2020. 2020.
Statistisches Bundesamt. Fallpauschalenbezogene Krankenhausstatistik (DRG-Statistik) Operationen und Prozeduren der vollstationären Patientinnen und Patienten in Krankenhäusern (4-Steller) für das Jahr 2019. 2020; 1–62
Statistisches Bundesamt. Fallpauschalenbezogene Krankenhausstatistik (DRG-Statistik) Operationen und Prozeduren der vollstationären Patientinnen und Patienten in Krankenhäusern (4-Steller) für das Jahr 2016. 2017
Zarringam D, Saris DBF, Bekkers JEJ. Identification of early prognostic factors for knee and hip arthroplasty; a long-term follow-up of the CHECK cohort. J Orthop. 2020;19:41–5. https://doi.org/10.1016/j.jor.2019.10.020.
Robinson MG, Greene N, Katakam A, Chen A, Bedair HS, Humphrey T, Melnic CM. The effect of the COVID-19 pandemic on revision total hip and knee arthroplasty at a large academic hospital network. J Orthop. 2021;28:117–20. https://doi.org/10.1016/j.jor.2021.11.012.
Mühlhofer H, Gollwitzer H, Lenze F, Feihl S, Pohlig F, von Eisenhart-Rothe R, Schauwecker J. Periprothetischer Infekt des Hüftgelenks. Der Orthopäde. 2015;44:357.
Preininger B, Trampuz A. Die Behandlung periprothetischer Infektionen. Universimed:Verfügbar unter. 2017. https://www.universimed.com/ch/article/orthopaedie-traumatologie/die-behandlung-periprothetischer-infektionen-2098399. Accessed on January 2022
Neut D, van der Mei HC, Bulstra SK, Busscher HJ. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop. 2007;78(3):299–308. https://doi.org/10.1080/17453670710013843.
Renz N, Trampuz A. Periprothetische Infektionen: aktueller Stand der Diagnostik und Therapie. Orthop Rheuma. 2015;18:20–8.
Rimke C, Enz A, Bail HJ, Heppt P, Kladny B, von Lewinski G, Lohmann CH, Osmanski-Zenk K, Haas H, Mittelmeier W. Evaluation of the standard procedure for the treatment of periprosthetic joint infections (PJI) in Germany - results of a survey within the EndoCert initiative. BMC Musculoskelet Disord. 2020;21(1):694. https://doi.org/10.1186/s12891-020-03670-y.
Kleber C, Schaser K, Trampuz A. Komplikationsmanagement bei infizierter Osteosynthese. Chirurg. 2015;86:925–34.
Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015;86(2):147–58. https://doi.org/10.3109/17453674.2014.966290.
Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–25. https://doi.org/10.1093/cid/cis803.
Ilchmann T, Zimmerli W, Ochsner PE, Kessler B, Zwicky L, Graber P, Clauss M. One-stage revision of infected hip arthroplasty: outcome of 39 consecutive hips. Int Orthop. 2016;40(5):913–8. https://doi.org/10.1007/s00264-015-2833-4.
Li C, Renz N, Trampuz A, Ojeda-Thies C. Twenty common errors in the diagnosis and treatment of periprosthetic joint infection. Int Orthop. 2020;44(1):3–14. https://doi.org/10.1007/s00264-019-04426-7.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008.
Nicholas JH, Gildasio SDO, Umang KJ, John YSK (2015) ASA class is a reliable independent predictor of medical complications and mortality following surgery, Int J Surg. 18:184–90. https://doi.org/10.1016/j.ijsu.2015.04.079
Statistisches Bundesamt. Ergebnisse der Todesursachestatistik für Deutschland, ausführliches 4-stellige ICD-Klassifikation, für das Jahr 2019. 2020.
Tsai JC, Sheng WH, Lo WY, Jiang CC, Chang SC. Clinical characteristics, microbiology, and outcomes of prosthetic joint infection in Taiwan. J Microbiol Immunol Infect. 2015;48(2):198–204. https://doi.org/10.1016/j.jmii.2013.08.007.
Gundtoft PH, Pedersen AB, Schønheyder HC, Møller JK, Overgaard S. One-year incidence of prosthetic joint infection in total hip arthroplasty: a cohort study with linkage of the Danish Hip Arthroplasty Register and Danish Microbiology Databases. Osteoarthritis Cartilage. 2017;25(5):685–93. https://doi.org/10.1016/j.joca.2016.12.010.
Rosteius T, Jansen O, Fehmer T, Baecker H, Citak M, Schildhauer TA, Geßmann J. Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. J Med Microbiol. 2018;67(11):1608–13. https://doi.org/10.1099/jmm.0.000835.
Peel TN, Cheng AC, Buising KL, Choong PF. Microbiological aetiology, epidemiology, and clinical profile of prosthetic joint infections: are current antibiotic prophylaxis guidelines effective? Antimicrob Agents Chemother. 2012;56(5):2386–91. https://doi.org/10.1128/aac.06246-11.
Vassallo C, Borg AA, Farrugia D, Mercieca C. The epidemiology and outcomes of septic arthritis in the maltese islands: a hospital-based retrospective cohort study. Mediterr J Rheumatol. 2020;31(2):195–205. https://doi.org/10.31138/mjr.31.2.195.
Natsuhara KM, Shelton TJ, Meehan JP, Lum ZC. Mortality during total hip periprosthetic joint infection. J Arthroplasty. 2019;34(7s):S337-s342. https://doi.org/10.1016/j.arth.2018.12.024.
Lum ZC, Natsuhara KM, Shelton TJ, Giordani M, Pereira GC, Meehan JP. Mortality during total knee periprosthetic joint infection. J Arthroplasty. 2018;33(12):3783–8. https://doi.org/10.1016/j.arth.2018.08.021.
Shahi A, Tan TL, Chen AF, Maltenfort MG, Parvizi J. In-hospital mortality in patients with periprosthetic joint infection. J Arthroplasty. 2017;32(3):948-952.e941. https://doi.org/10.1016/j.arth.2016.09.027.
Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. J Arthroplasty. 2018;33(2):521–6. https://doi.org/10.1016/j.arth.2017.09.021.
Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Joint Surg Am. 2013;95(24):2177–84. https://doi.org/10.2106/jbjs.L.00789.
Flurin L, Greenwood-Quaintance KE, Patel R. Microbiology of polymicrobial prosthetic joint infection. Diagn Microbiol Infect Dis. 2019;94(3):255–9. https://doi.org/10.1016/j.diagmicrobio.2019.01.006.
Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. Topographical and temporal diversity of the human skin microbiome. Science (New York, NY). 2009;324(5931):1190–2. https://doi.org/10.1126/science.1171700.
Tolo I, Thomas JC, Fischer RSB, Brown EL, Gray BM, Robinson DA. Do Staphylococcus epidermidis genetic clusters predict isolation sources? J Clin Microbiol. 2016;54(7):1711–9. https://doi.org/10.1128/jcm.03345-15.
Lee A, Mirrett S, Reller LB, Weinstein MP. Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol. 2007;45(11):3546–8. https://doi.org/10.1128/jcm.01555-07.
Morgenstern M, Erichsen C, von Rüden C, Metsemakers WJ, Kates SL, Moriarty TF, Hungerer S. Staphylococcal orthopaedic device-related infections in older patients. Injury. 2016;47(7):1427–34. https://doi.org/10.1016/j.injury.2016.04.027.
Senneville E, Joulie D, Legout L, Valette M, Dezèque H, Beltrand E, Roselé B, d’Escrivan T, Loïez C, Caillaux M, Yazdanpanah Y, Maynou C, Migaud H. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis. 2011;53(4):334–40. https://doi.org/10.1093/cid/cir402.
Gundtoft PH, Pedersen AB, Varnum C, Overgaard S. Increased mortality after prosthetic joint infection in primary THA. Clin Orthop Relat Res. 2017;475(11):2623–31. https://doi.org/10.1007/s11999-017-5289-6.
Thompson O, Rasmussen M, Stefansdottir A, Christensson B, Akesson P. A population-based study on the treatment and outcome of enterococcal prosthetic joint infections. A consecutive series of 55 cases. J Bone Joint Infect. 2019;4(6):285–91. https://doi.org/10.7150/jbji.35683.
Tornero E, Senneville E, Euba G, Petersdorf S, Rodriguez-Pardo D, Lakatos B, Ferrari MC, Pilares M, Bahamonde A, Trebse R, Benito N, Sorli L, del Toro MD, Baraiaetxaburu JM, Ramos A, Riera M, Jover-Sáenz A, Palomino J, Ariza J, Soriano A. Characteristics of prosthetic joint infections due to Enterococcus sp. and predictors of failure: a multi-national study. Clin Microbiol Infect. 2014;20(11):1219–24. https://doi.org/10.1111/1469-0691.12721.
Ascione T, Balato G, Mariconda M, Fantoni M, Giovannenze F, Pagliano P. Clinical and prognostic features of prosthetic joint infections caused by Enterococcus spp. Eur Rev Med Pharmacol Sci. 2019;23(2 Suppl):59–64. https://doi.org/10.26355/eurrev_201904_17475.
Renz N, Trebse R, Akgün D, Perka C, Trampuz A. Enterococcal periprosthetic joint infection: clinical and microbiological findings from an 8-year retrospective cohort study. BMC Infect Dis. 2019;19(1):1083. https://doi.org/10.1186/s12879-019-4691-y.
Kheir MM, Tan TL, Higuera C, George J, Della Valle CJ, Shen M, Parvizi J. Periprosthetic joint infections caused by enterococci have poor outcomes. J Arthroplasty. 2017;32(3):933–47. https://doi.org/10.1016/j.arth.2016.09.017.
Jacqueline C, Caillon J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J Antimicrob Chemother. 2014;69(Suppl 1):i37-40. https://doi.org/10.1093/jac/dku254.
Rasouli MR, Tripathi MS, Kenyon R, Wetters N, Della Valle CJ, Parvizi J. Low rate of infection control in enterococcal periprosthetic joint infections. Clin Orthop Relat Res. 2012;470(10):2708–16. https://doi.org/10.1007/s11999-012-2374-8.
Abdelaziz H, Grüber H, Gehrke T, Salber J, Citak M. What are the factors associated with re-revision after one-stage revision for periprosthetic joint infection of the hip? A case-control study. Clin Orthop Relat Res. 2019;477(10):2258–63. https://doi.org/10.1097/corr.0000000000000780.
Rathmann W, Scheidt-Nave C, Roden M, Herder C. Type 2 diabetes: prevalence and relevance of genetic and acquired factors for its prediction. Deutsches Arzteblatt international. 2013;110(19):331–7. https://doi.org/10.3238/arztebl.2013.0331.
Acknowledgements
None.
Funding
Open Access funding enabled and organized by Projekt DEAL. No external source of funding was used.
Author information
Authors and Affiliations
Contributions
FM: data extraction, writing; VP: data extraction; AD: data extraction; MB: project administration; FH: supervision; AB: supervision; UKH: visualisation; CDW: writing; NM: writing. FM and CDW equally contributed to the final version of the manuscript and share the first authorship. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the ethics committee of the RWTH Aachen University (project ID EK 121/22). All patients signed informed consent to participate to the present study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Migliorini, F., Weber, C.D., Bell, A. et al. Bacterial pathogens and in-hospital mortality in revision surgery for periprosthetic joint infection of the hip and knee: analysis of 346 patients. Eur J Med Res 28, 177 (2023). https://doi.org/10.1186/s40001-023-01138-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01138-y