Abstract
Background
Studies have investigated the association between serum lipids level or apolipoprotein levels and breast cancer (BC) risk. However, the relationship between serum lipids level and apolipoprotein levels and histological type of breast cancer remains unclear. This study was aimed to explore the association between serum lipids level and the histological type of BC, particularly to estrogen receptor (ER) and progesterone receptor (PR) positive BC.
Materials and methods
220 cases of pathology-confirmed BC were retrospectively collected in this study. Patients’ demographic information, clinical data, and pathological features were obtained from medical records. Serum levels including high-density lipoprotein-cholesterol (HDL-c), low-density lipoprotein-cholesterol (LDL-c), total cholesterol (TC), triglyceride (TG), apolipoprotein A (ApoA), ApoB, ApoE and lipoprotein a(LP(a)) were collected before treatment. Logistic regression analyses were used to show the association between serum lipids and subtypes of BC. Receiver operating characteristic (ROC) curves were generated to analyze the predictive performance.
Results
There were 70 ER-negative and 73 PR-negative BC. Patients with ER-negative BC had higher HDL-c, higher LDL-c, and higher LP(a) than those in ER-positive one (p < 0.05). Patients with PR-negative BC were more likely to have high LDL-c and high LP(a) levels than patients with PR-positive one (p < 0.05). Multivariate logistic regression analysis showed that serum HDL-c (odds ratio (OR): 0.27, 95% confidence interval (CI) 0.10–0.76), LDL-c (OR: 0.19, 95%CI 0.04–0.93) and LP(a) (OR: 0.23,95%CI 0.07–0.80) levels were negatively associated with ER-positive BC, and serum HDL-c and LDL-c levels were significantly negatively associated with PR-positive BC (OR: 0.32, 95%CI 0.12–0.82; OR: 0.14, 95%CI 0.03–0.77). In addition, ER and PR positive BC was negatively associated with serum HDL-c and LDL-c levels (OR = 0.39, 95% CI 0.17–0.91; OR = 0.22, 95% CI 0.06–0.85) after adjusting with confounders. Serum HDL-c level (OR = 0.13, 95% CI 0.02–0.87) was still independently associated with ER and PR positive BC in postmenopausal women. The area under the curves (AUCs) of HDL-c to identify ER-positive BC, PR-positive BC, and ER and PR positive BC were 0.65 (95%CI 0.58–0.73, P < 0.01), 0.62 (95%CI 0.54–0.69, P < 0.01) and 0.64 (95%CI 0.56–0.72, P < 0.01), respectively.
Conclusions
Serum HDL-c and LDL-c levels were related to ER or PR positive BC. Lipid levels may also have acceptable performance in identifying BC histological type.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the second common cause of death in women. In 2021, 281,550 new cases of BC occurred, which corresponds to 30% of all new cancer cases in women worldwide [1]. It’s important to determine the expression status of estrogen receptor (ER), progesterone receptor (PR), and HER2 in BC, because they are associated with prognosis, endocrine therapies, and adjuvant treatment decisions [2, 3]. ER and/or PR negative BC are more aggressive and have worse prognosis than ER and/or PR positive one. Comprehensive strategies are urgently needed to change the profiles of the BC burden.
Lipid metabolism is reprogrammed in tumors [4]. Reprogramming cholesterol metabolism in endocrine-related cancers is related to cell proliferation, migration and invasion, which could be potentially available for future hormonal therapy [5, 6]. Serum lipids and lipoproteins may have direct effects on tumor proliferation and migration [7,8,9]. The associations between lipids levels and cancer risk were also reported by several epidemiological studies [10, 11]. Cholesterol level is associated with lung cancer risk and outcomes [12, 13]. A recent study showed that triglycerides (TG)/high-density lipoprotein (HDL) ratio may have potential in identifying bladder cancer [4]. Low serum HDL levels are also associated with malignant behavior of pancreatic neuroendocrine neoplasms [14]. In addition, studies also showed that oxidized low-density lipoprotein-cholesterol (LDL-c), or high total serum cholesterol (TC) and HDL-cholesterol (HDL-c) was associated with more aggressive cancer [15, 16].
Obesity and hypercholesterolemia are both potentially associated with breast cancer risk [17]. The association between serum lipids levels and BC risk had been studied. Some clinical studies showed an inverse association between blood levels of TC, HDL-c and BC risk [18]. A positive association between blood levels of LDL and BC risk were also reported [19]. Moreover, some studies showed that the use of statin might be associated with decrease mortality of BC patients [20, 21]. Recently, a study showed that HDL-c levels were associated with malignant intraductal papillary mucinous neoplasms [22] which indicated that HDL may be related to more malignant histological type of neoplasms. The biological behavior of BC was also related to its histological type. However, the relationship between serum levels of lipid and apolipoprotein and histological type of BC has not been thoroughly investigated. In the present study, the association between serum lipid level and the histological type of BC, particularly to ER and PR positive BC, was observed.
Materials and methods
Patients
220 patients with biopsy proven unilateral primary BC during 2016–2019 in Affiliated Hospital of Nanjing University of Chinese Medicine were included in our study after excluding by these criteria:(1) patients below 20 years; (2) receiving hormone replacement therapy; (3) without complete blood lipids data; and (4) administration of hypolipidemic drugs within 1 month. Patients’ demographic information, clinical data, and pathological features were obtained from medical records. The laboratory data including serum HDL-c, LDL-c, TC, TG, apolipoprotein A (ApoA), ApoB, ApoE and lipoprotein(a) (LP(a)) were collected within 1 month of diagnosis. This retrospective study was approved by the Institutional Ethic Review Board of the Jiangsu Province Hospital of Chinese Medicine. Informed consent was waived because of the retrospective design. Declaration of Helsinki were adhered during the study.
Serum lipid determination
Blood samples were collected from each patients after at least 8 h of fasting in coagulant-coated tubes. HDL-c, LDL-c, TC, TG, ApoA, ApoB, ApoE and LP(a) were determined in a fully automatic biochemical analyzer. We also calculated the ratio of HDL/TC, LDL/TC, ApoA/ApoB, HDL/ApoA and LDL/ApoB.
Histological examinations
Immunohistochemical markers of estrogen receptor (ER) (n = 220), progesterone receptor (PR) (n = 217) were also collected from pathological records. Positive ER and PR expression were considered if greater than 1% of the tumor cells exhibited nuclear staining. Three cases did not have information of PR expression. The BC were classified into molecular subtypes: ER positive, PR positive, and ER + PR positive. WHO grade for BC was also evaluated: Grade I, well-differentiated; Grade II, moderately differentiated; Grade III, poorly differentiated.
Statistical analysis
Receiver-operating curve (ROC) were generated and the cutoff values for serum HDL-c, LDL, TC, TG, ApoA, ApoB, ApoE, LP(a), HDL/TC, LDL/TC, ApoA/ApoB, HDL/ApoA and LDL/ApoB were calculated using the Youden index (sensitivity + specificity − 1). The two-tailed t test (data with normal distribution) or Mann–Whitney U test (data with abnormal distribution) was used to compare the continuous variables, while the chi-square test was used to compare the categorical variables. Spearman correlation analysis was used to show the correlation among variables. Univariate and multivariate logistic regression analyses were used to show the association between the blood lipids levels and the histological subtypes. ROC curves were generated to analyze predictive performance of lipid levels. All statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY, USA). Sample sizes were estimated using PASS (version 2021). Test for two proportions was used (power = 0.80, α = 0.05). The estimated difference in the prevalence of low HDL-c between ER positive and ER negative BC was 17%. The estimated sample size was 224. Our sample size was close to the estimated one. P values less than 0.05 were considered statistically significant.
Results
The characteristics of subjects
A total of 220 women were included in this study, and the characteristics of subjects are listed in Table 1. There were 70 ER-negative and 73 PR-negative BC. The long diameters of ER or PR negative BC were larger than ER or PR positive BC, respectively (P < 0.05). Low grade of lesion (WHO I&II) were more common in ER or PR positive BC (P < 0.001). History of miscarriage was more common in women with ER-positive BC (P = 0.03). However, no significant differences were found in other characteristics, such as age, body mass index and BC family history.
Serum lipid levels
Significance differences were observed in the levels of serum HDL-c, LDL-c and LP between ER-positive and ER-negative BC (P < 0.05). The similar results were found in PR-positive and PR-negative BC (Table2). In addition, LDL/TC and LDL/ApoB were significantly associated with PR-positive BC (P < 0.05), but no significant differences were found in HDL/TC, ApoA/ApoB and HDL/ApoA.
Correlation analysis
Based on receiver operating curve (ROC) analysis, the recommended cutoff values were 1.36 mmol/L for HDL-c, 2.08 mmol/L for LDL-c, 5.12 mmol/L for TC, 1.52 mmol/L for TG, 1.34 mmol/L for ApoA, 0.76 mmol/L for ApoB, 4.7 mmol/L for ApoE, 33 mmol/L for LP(a), 0.29 for HDL/TC,0.64 for LDL/TC, 1.22 for ApoA/ApoB, 1.09 mmol/g for HDL/ApoA and 2.66 mmol/g for LDL/ApoB. Then, we divided lipid levels into categorical variables based on the above cutoff value.
The Spearman correlation analysis are shown in Fig. 1. The categorical data was used in the analysis. The HDL-c was positively associated with the ApoA (r = 0.51, P < 0.001). In addition, statistical significance was found the levels of TC, LDL-c and ApoB (P < 0.001). LDL-c and Lp(a) were correlated to ER or PR expression (P < 0.05). HDL-c was correlated with ER or ER + PR expression (P < 0.05).
Univariate and multivariate logistic regression
Univariate and multivariate logistic regression analyses was used to identify the associated factors for ER-positive BC and PR-positive BC (Table 3). For ER-positive BC, the odds ratio (OR) value was 0.45 (95%CI 0.21–0.95) for serum HDL-c level, 0.43 (95% CI 0.14–1.29) for serum LDL-c level, and 0.57 (95% CI 0.25–1.31) for serum LP(a) level. After adjusting with age, body mass index (BMI) and miscarriage history, these factors were still independently associated with ER positive BC (OR = 0.27, 95% CI 0.10–0.76; OR = 0.19, 95% CI 0.04–0.93; OR = 0.23, 95% CI 0.07–0.80). For PR positive BC, the levels of serum HDL-c and LDL shown an independent association after additionally adjusting with confounding factors (OR = 0.32, 95% CI 0.12–0.82; OR = 0.14, 95% CI 0.03–0.77). Moreover, WHO grade was significant associated with positive ER (OR = 0.11, 95%CI: 0.04–0.30), PR (OR = 0.15, 95%CI 0.06–0.38) and ER + PR expression (OR = 0.23, 95%CI 0.11–0.47).
Subsequently, we showed the association between ER and PR positive BC and the levels of serum HDL-c and LDL-c (Table 4). After additionally adjusting with age and BMI, HDL-c and LDL-c were associated with the presence of ER and PR positive BC (OR = 0.39, 95% CI 0.17–0.91; OR = 0.22, 95% CI 0.06–0.85). Furthermore, we found that serum HDL-c (OR = 0.13, 95% CI 0.02–0.87) was still independently associated with ER and PR positive BC in postmenopausal women (Table 5).
ROC analysis
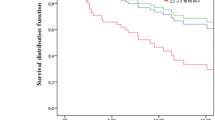
The performance of serum HDL-c, LDL-C and Lp(a) in identifying ER or PR expression is shown in Fig. 2. The area under the ROC curve (AUC) of serum HDL-c + LDL-c + LP(a) in identifying ER positive BC was 0.65 (95%CI 0.58–0.73, P < 0.01). The positive likelihood ratio (PLR) and negative likelihood ratio (NLR) were 1.78 and 0.54, respectively. Moreover, the AUCs of serum HDL-c plus LDL-c in identifying PR positive BC and ER/PR positive BC were 0.62 (95%CI 0.54–0.69, P < 0.01) (PLR and NLR were 1.63 and 0.71, respectively) and 0.64 (95%CI 0.56–0.72, P < 0.01) (PLR and NLR were 2.01 and 0.65, respectively), respectively.
The receiver operating characteristic (ROC) curves of lipids in predicting breast cancer (BC) histological type. A: high-density lipoprotein-cholesterol (HDL-c), low-density lipoprotein-cholesterol (LDL-c) and lipoprotein (a) (LP(a)) in predicting estrogen receptor (ER)-positive breast cancer (ER + BC). B: HDL-c and LDL-c in predicting progesterone receptor (PR)-positive breast cancer (PR + BC). C: HDL-c and LDL-c in predicting ER and/or PR positive breast cancer (ER/PR + BC)
Discussion
ER and PR expression status in BC are associated with therapy and clinical prognosis. In the present study, we also observed that both ER-positive and PR-positive BC are negatively associated with worse prognostic characteristics, such as larger lesion and higher WHO grade. Clinical evidence for the association between the lipids level and the ER/PR expression status of BC has not been thoroughly investigated. The present study showed that low HDL-c and LDL-c were independently associated with ER-positive and PR-positive BC. Lipid levels also have potential to predict ER/PR expression in BC.
HDL-c and breast cancer risk have been investigated in different countries or regions. Most of the studies demonstrated an inverse correlation between HDL-c and breast cancer risk, disease free survival (DFS) or overall survival (OS) [23]. A meta-analysis also supported an association between HDL-c and BC risk [18]. Studies also showed the mechanism of HDL on cancer development or progression [24]. HDL-associated proteins may enhance anti-tumorigenesis effects by exerting their biological activities, such as antioxidant, anti-inflammatory, anti-angiogenesis, and immunomodulatory [25, 26]. In addition, high level of scavenger receptor type B-I (SR-BI) expression was involved in lipid internalization and lipoprotein consumption, which results in reducing HDL-c levels during cancer [24, 27]. We inferred that low HDL-c may positively associated with aggressive BC type. However, we found that the HDL level was negatively associated with ER or PR positive BC in the present study. The mechanisms are not clear. The cholesterol metabolism should be considered during the growth of BC. We speculated that ER or PR negative BC shows high proliferation which need more cholesterol. Then HDL is increased to transfer more cholesterol to BC cells besides liver cells. Moreover, some studies supported our results. An in vitro study showed that HDL stimulated proliferation in both ER-positive and ER-negative BC cell lines in a dose-dependent manner, but ER-negative cells showed a higher response [19]. A prospective study investigated the relationship between HDL-c and ER/PR positive BC, and showed a significant inverse association (HR = 0.45, 95% CI 0.21–0.97) [28]. However, a Mendelian randomization study showed that genetically raised HDL-c is associated with higher risk of ER-positive BC(OR = 1.13,95%CI 1.01–1.26) [29]. HDL-c levels was not only generically determined, but also environmentally associated. BC cells may modify the progress of lipid metabolism.
Some studies showed that LDL-c is not associated with breast cancer risk, while serum LDL level might be a predictor of BC progression [30,31,32]. However, it was demonstrated that significant upregulation of LDL receptor increased LDL uptake in cancer cells because of the demand of rapid proliferation. Two Mendelian randomization studies found that genetically elevated plasma LDL level appeared to be associated with increased BC risk [27, 33]. In addition, LDL treatment promoted breast cells viability, and enhanced tumor progression and migration [26]. LDL promoted ER-negative cells proliferation such as MDA-MB-231 and MDA-MB-436 faster than ER-positive cells such as MCF-7 [34]. This study found that serum LDL level was reverse associated with ER or PR positive BC. It seemed that increased LDL might be associated with more malignant breast cancer phenotype. Interestingly, a study also indicated that higher levels of LDL at diagnosis was associated with high proliferative tumors, with higher grade, and with advanced stages [30]. However, the underlying mechanism has not been clarified. Few studies have shown the association between LDL-c levels and BC histological types. This association may be also related to cholesterol metabolism. LDL promotes transport of cholesterol from liver to the tumor cells [35]. ER or PR negative BC may need more cholesterol. Therefore, the LDL-c levels increased in ER or PR negative BC. LDL receptor was found to be upregulated in triple negative BC [34, 36], which also suggested that the more malignant the BC is, the more their need for LDL.
ApoA was a major protein component of HDL-c. Few studies have identified the role of ApoA in breast cancer. An analyses performed on 7,557 subjects in France showed that ApoA was inversely associated with the breast cancer risk (HR = 0.36, 95% CI: 0.18–0.73) [9]. One study showed that serum ApoA level was an independent prognostic factor in invasive ductal breast cancer [37]. A recent study showed that ApoA or ApoE stimulated tumor growth in MCF-7 cells (ER-positive cells) and inhibit tumor ability in MDA-MB-231 cells (ER-negative cells) [38]. The present study found no significant association between ApoA or ApoE levels with BC histological type. In addition, ApoB is a major protein component of LDL-c. Few studies showed the association between ApoB and BC. One study found that breast cancer risk was inversely associated with ApoB (HR = 0.92, 95%CI 0.86–0.99) [39]. To the best of our knowledge, no study showed the association between serum ApoB levels and BC histological type. The present study showed a weak association between ApoB or LDL/ApoB and PR expression. Those data supported that BC histological type was also associated to cholesterol in HDL-c or LDL-c. More studies are needed to evidence such correlation.
The microenvironment of fluctuating lipid-metabolic conditions may affect BC phenotype [40]. Borgquist. et al. [41] found that cholesterol-lowering medication may have a role in preventing breast cancer recurrence in ER/PR positive early stage BC, which showed an interaction of serum lipids with estrogen-sensitive breast tissues. In fact, many cholesterol-derived metabolites such as 27-hydroxycholesterol (27HC) can promote cellular adaptation by altering cholesterol targets during BC development. As an endogenous selective ER modulator (SERM), 27HC exhibited sufficient estrogenic activity to support the proliferation of ER-positive BC cells [17, 19, 42, 43], which might contribute to the identification of drug-targets and the design of novel therapies in BC patients.
This study has several limitations. First, the sample size was relatively small, even though it was close to the estimated one. Second, it would be better to collect multiple lipid measures besides baseline. This study may fail to show the dynamic correlation during the tumor development. Third, this study only observed the phenomenon between lipid levels and BC histological type. The underlying mechanisms in cell/mouse model levels were not investigated. Fourth, the correlation between lipid serum levels with clinical parameters like overall and.
progression-free survival was not studied. In addition, this is a retrospective study, selection bias cannot be avoided. Finally, some confounding factors were not considered, such as lifestyle and diet habits.
In conclusion, this study shows that low HDL-c and LDL-c are associated with ER-negative and PR-negative BC. Lipid levels might be associated with BC phenotype. Intervention on lipid may be a potential strategy for the treatment of ER or PR-negative BC. Lipid levels may also have acceptable performance in identifying BC histological type. Further studies are needed to confirm this association and explore the possible mechanism.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- BC:
-
Breast cancer
- CI:
-
Confidence interval
- HDL-c:
-
High-density lipoprotein-cholesterol
- LDL-c:
-
Low-density lipoprotein-cholesterol
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- ApoA:
-
Apolipoprotein A
- ApoB:
-
Apolipoprotein B
- ApoE:
-
Apolipoprotein E
- LP(a):
-
Lipoprotein(a)
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic
- BMI:
-
Body mass index
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541–57.
Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021;32(10):1216–35.
Crocetto F, Pandolfo SD, Aveta A, Martino R, Trama F, Caputo VF, et al. A comparative study of the triglycerides/HDL ratio and pseudocholinesterase levels in patients with bladder cancer. Diagnostics (Basel). 2022;12(2):431.
Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2(2):132–41.
Revilla G, Cedó L, Tondo M, Moral A, Pérez JI, Corcoy R, et al. LDL, HDL and endocrine-related cancer: From pathogenic mechanisms to therapies. Semin Cancer Biol. 2021;73:134–57.
Ganjali S, Banach M, Pirro M, Fras Z, Sahebkar A. HDL and cancer—causality still needs to be confirmed? Update 2020. Semin Cancer Biol. 2021;73:169–77.
Zhou Y, Luo G. Apolipoproteins, as the carrier proteins for lipids, are involved in the development of breast cancer. Clin Transl Oncol. 2020;22(11):1952–62.
Zhang Y, Yang X. Prognostic Significance of Pretreatment Apolipoprotein A-I as a Noninvasive Biomarker in Cancer Survivors: A Meta-Analysis. Dis Markers. 2018;2018:1034037.
YuPeng L, YuXue Z, PengFei L, Cheng C, YaShuang Z, DaPeng L, et al. Cholesterol Levels in Blood and the Risk of Prostate Cancer: A Meta-analysis of 14 Prospective Studies. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1086–93.
Katzke VA, Sookthai D, Johnson T, Kühn T, Kaaks R. Blood lipids and lipoproteins in relation to incidence and mortality risks for CVD and cancer in the prospective EPIC-Heidelberg cohort. BMC Med. 2017;15(1):218–31.
Merino Salvador M, Gómez de Cedrón M, Moreno Rubio J, Falagán Martínez S, Sánchez Martínez R, Casado E, et al. Lipid metabolism and lung cancer. Crit Rev Oncol Hematol. 2017;112:31–40.
Ma C, Wang X, Guo J, Liu P. Prognostic significance of preoperative serum triglycerides and high-density lipoproteins cholesterol in patients with non-small cell lung cancer: a retrospective study. Lipids Health Dis. 2021;20(1):69.
Quan L, Liu Y, Cui W, Wang X, Zhang W, Wang Z, et al. The associations between serum high-density lipoprotein cholesterol levelsand malignant behavior in pancreatic neuroendocrine neoplasms. Lipids Health Dis. 2022;21(1):58.
Yang L, Sun J, Li M, Long Y, Zhang D, Guo H, et al. Oxidized low-density lipoprotein links hypercholesterolemia and bladder cancer aggressiveness by promoting cancer stemness. Cancer Res. 2021;81(22):5720–32.
Jamnagerwalla J, Howard LE, Allott EH, Vidal AC, Moreira DM, Castro-Santamaria R, et al. Serum cholesterol and risk of high-grade prostate cancer: results from the REDUCE study. Prostate Cancer Prostatic Dis. 2018;21(2):252–9.
Garcia-Estevez L, Moreno-Bueno G. Updating the role of obesity and cholesterol in breast cancer. Breast Cancer Res. 2019;21(1):35–43.
Touvier M, Fassier P, His M, Norat T, Chan DS, Blacher J, et al. Cholesterol and breast cancer risk: a systematic review and meta-analysis of prospective studies. Br J Nutr. 2015;114(3):347–57.
Cedó L, Reddy ST, Mato E, Blanco-Vaca F, Escolà-Gil JC. HDL and LDL: potential new players in breast cancer development. J Clin Med. 2019;8(6):853–74.
Harborg S, Heide-Jørgensen U, Ahern TP, Ewertz M, Cronin-Fenton D, Borgquist S. Statin use and breast cancer recurrence in postmenopausal women treated with adjuvant aromatase inhibitors: a Danish population-based cohort study. Breast Cancer Res Treat. 2020;183(1):153–60.
Wu QJ, Tu C, Li YY, Zhu J, Qian KQ, Li WJ, et al. Statin use and breast cancer survival and risk: a systematic review and meta-analysis. Oncotarget. 2015;6(40):42988–3004.
Wang C, Lin T, Wang X, Yu Z, Zhuge X, Cui W, et al. Low high-density lipoprotein cholesterol levels are associated with malignant intraductal papillary mucinous neoplasms: a multicenter study. Lipids Health Dis. 2021;20(1):94–102.
Li X, Tang H, Wang J, Xie X, Liu P, Kong Y, et al. The effect of preoperative serum triglycerides and high-density lipoprotein-cholesterol levels on the prognosis of breast cancer. Breast. 2017;32:1–6.
Pirro M, Ricciuti B, Rader DJ, Catapano AL, Sahebkar A, Banach M. High density lipoprotein cholesterol and cancer: Marker or causative? Prog Lipid Res. 2018;71:54–69.
Ridtitid W, DeWitt JM, Schmidt CM, Roch A, Stuart JS, Sherman S, et al. Management of branch-duct intraductal papillary mucinous neoplasms: a large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest Endosc. 2016;84(3):436–45.
Wei Y, Huang Y, Yang W, Huang Q, Chen Y, Zeng K, et al. The significances and clinical implications of cholesterol components in human breast cancer. Sci Prog. 2021;104(3):368504211028395.
Ganjali S, Ricciuti B, Pirro M, Butler AE, Atkin SL, Banach M, et al. High-density lipoprotein components and functionality in cancer: state-of-the-art. Trends Endocrinol Metab. 2019;30(1):12–24.
His M, Zelek L, Deschasaux M, Pouchieu C, Kesse-Guyot E, Hercberg S, et al. Prospective associations between serum biomarkers of lipid metabolism and overall, breast and prostate cancer risk. Eur J Epidemiol. 2014;29(2):119–32.
Nowak C, Ärnlöv J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat Commun. 2018;9(1):3957.
Rodrigues Dos Santos C, Fonseca I, Dias S, Mendes de Almeida JC. Plasma level of LDL-cholesterol at diagnosis is a predictor factor of breast tumor progression. BMC Cancer. 2014;14:132–42.
Orho-Melander M, Hindy G, Borgquist S, Schulz CA, Manjer J, Melander O, et al. Blood lipid genetic scores, the HMGCR gene and cancer risk: a Mendelian randomization study. Int J Epidemiol. 2018;47(2):495–505.
Beeghly-Fadiel A, Khankari NK, Delahanty RJ, Shu XO, Lu Y, Schmidt MK, et al. A Mendelian randomization analysis of circulating lipid traits and breast cancer risk. Int J Epidemiol. 2020;49(4):1117–31.
Johnson KE, Siewert KM, Klarin D, Damrauer SM, Chang KM, Tsao PS, et al. The relationship between circulating lipids and breast cancer risk: a Mendelian randomization study. PLoS Med. 2020;17(9): e1003302.
Gallagher EJ, Zelenko Z, Neel BA, Antoniou IM, Rajan L, Kase N, et al. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene. 2017;36(46):6462–71.
Flote VG, Vettukattil R, Bathen TF, Egeland T, McTiernan A, Frydenberg H, et al. Lipoprotein subfractions by nuclear magnetic resonance are associated with tumor characteristics in breast cancer. Lipids Health Dis. 2016;15:56–68.
de Gonzalo-Calvo D, López-Vilaró L, Nasarre L, Perez-Olabarria M, Vázquez T, Escuin D, et al. Intratumor cholesteryl ester accumulation is associated with human breast cancer proliferation and aggressive potential: a molecular and clinicopathological study. BMC Cancer. 2015;15:460.
Lin X, Hong S, Huang J, Chen Y, Chen Y, Wu Z. Plasma apolipoprotein A1 levels at diagnosis are independent prognostic factors in invasive ductal breast cancer. Discov Med. 2017;23(127):247–58.
Ben Hassen C, Gutierrez-Pajares JL, Guimaraes C, Guibon R, Pinault M, Fromont G, et al. Apolipoprotein-mediated regulation of lipid metabolism induces distinctive effects in different types of breast cancer cells. Breast Cancer Res. 2020;22(1):38.
Borgquist S, Butt T, Almgren P, Shiffman D, Stocks T, Orho-Melander M, et al. Apolipoproteins, lipids and risk of cancer. Int J Cancer. 2016;138(11):2648–56.
González-Ortiz A, Galindo-Hernández O, Hernández-Acevedo GN, Hurtado-Ureta G, García-González V. Impact of cholesterol-pathways on breast cancer development, a metabolic landscape. J Cancer. 2021;12(14):4307–21.
Borgquist S, Giobbie-Hurder A, Ahern TP, Garber JE, Colleoni M, Láng I, et al. Cholesterol, cholesterol-lowering medication use, and breast cancer outcome in the BIG 1–98 study. J Clin Oncol. 2017;35(11):1179–88.
McDonnell DP, Park S, Goulet MT, Jasper J, Wardell SE, Chang CY, et al. Obesity, cholesterol metabolism, and breast cancer pathogenesis. Cancer Res. 2014;74(18):4976–82.
Blücher C, Stadler SC. Obesity and breast cancer: current insights on the role of fatty acids and lipid metabolism in promoting breast cancer growth and progression. Front Endocrinol (Lausanne). 2017;8:293.
Acknowledgements
None
Funding
This study was funded by National Natural Science Foundation of China (81773460) and Medical development and Medical Assistance Foundation of Jiangsu Province Hospital of Chinese Medicine.
Author information
Authors and Affiliations
Contributions
WC and XC participated in the design of the study. XW and YW wrote the manuscript. XW, YW, MW, XC and XC collected and analyzed the data. WC, XC, XW and YW contributed to interpretation of data and preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Affiliated Hospital of Nanjing University of Chinese Medicine. The study was in accordance with Declaration of Helsinki. The need for Informed Consent was waived by the Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, X., Wang, Y., Wang, M. et al. The association between serum lipid levels and histological type of breast cancer. Eur J Med Res 27, 154 (2022). https://doi.org/10.1186/s40001-022-00784-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00784-y