Abstract
Melosira nummuloides is a marine diatom with potential use as food, fuel, and a dietary supplement. However, the efficacy of its extraction and drying techniques have not been explored. Here, M. nummuloides powders were prepared by two drying methods—hot-air drying (HAD) and freeze-drying (FD)—and extracted with hot water, ethanol, methanol, and chloroform:methanol (CM) at a ratio of 2:1 v/v. The antioxidant and anti-inflammatory activity of each extract was investigated. The CM extract had the greatest 2,2-diphenyl-1-picrylhydrazyl and 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity among the solvent extracts, and a slight difference in antioxidant activity was observed across the various drying methods. Compared to other extracts, both the FD-CM and HAD-CM extracts showed stronger anti-inflammatory effects by inhibiting nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 cells. Furthermore, the FD-CM extract contained a wide range of lipophilic compounds. Notably, myristic acid (29.08 ± 0.45 mg/g dry weight powder extract (DW)), oleic acid (25.20 ± 0.92 mg/g DW), palmitoleic acid (10.77 ± 0.41 mg/g DW), eicosapentaenoic acid (12.53 ± 1.00 mg/g DW), neophytadiene (8.42 ± 0.51 mg/g DW), and α-linolenic acid (1.27 ± 0.005 mg/g DW) were among the prominent compounds identified. It is plausible to suggest that the abundance of these lipophilic compounds contributes to the remarkable antioxidative and anti-inflammatory potential exhibited by the FD-CM extract. Our results provide insights into the preferential drying methods and extraction solvents for producing M. nummuloides-based products with enhanced antioxidant and anti-inflammatory activity.
Similar content being viewed by others
Introduction
Melosira nummuloides is a cold-water diatom species that belongs to the Melosiraceae family, mainly found during cold winters in temperate regions of the northern hemisphere. Owing to their high biocompatibility and ease of use, diatoms are suitable for various industrial and biomedical applications [1]. They possess biologically active compounds, including fatty acids, carotenoids, polysaccharides, antioxidants, enzymes, peptides, polymers, pigments, and sterols [2]. M. nummuloides, specifically, possesses nutritionally important unsaturated fatty acids—such as omega-3 and omega-6 fatty acids—and xanthophyll fucoxanthin, which exert promising pharmacological effects, including anticancer, anti-inflammatory, antioxidant, and anti-obesity functions [3,4,5]. Although these nutritional components are well described in the scientific literature, there has been a limited evaluation of the effects of the drying method and extraction solvent on the antioxidant and anti-inflammatory capacity of M. nummuloides bioproducts.
The drying technique and parameters of the extraction process have a great influence on the stability of biologically active compounds [6]. Hot-air drying (HAD) and freeze-drying (FD) are the most common methods proposed for drying microalgae [7]. FD removes water from raw microalgal material by sublimating ice, which helps to preserve its physical and biological properties. However, this method is costly and not energy-efficient [7, 8]. The HAD technique is also widely applied for drying various microalgal strains [9, 10], but it can alter the structural integrity, natural color, and major bioactive constituents of resultant food products [11]. The chemical processing of diatomic material is essential to optimize the concentration of known constituents and maintain their bioactive properties.
Extraction is a key step in the recovery of bioactive compounds from diatoms; it comprises the removal of unwanted components and retention of desired soluble constituents with the aid of a solvent. Selecting a suitable extraction method is paramount for the standardization of extracting bioactive compounds [12]. Several factors (such as solubility, selectivity, safety, and cost) are weighed in the selection of an extraction solvent. Water, ethanol, and methanol are considered universal solvents for extracting bioactive compounds without exerting toxic effects, these solvents have been used for food and/or drug extraction [12, 13]. These solvents have been used to extract bioactive compounds in some diatom species, for example, Chaetoceros calcitrans [14], naviculoids [15], and Phaeodactylum tricornutum [16]. In addition, chloroform/methanol is one of the most commonly used solvent mixtures for extracting lipids and fatty acids from diatoms, such as Fistulifera spp. [17], P. tricornutum [18], and Amphora spp. [19].
The present study aimed to compare the effects of drying methods (HAD and FD) and various organic solvents/combinations [hot water (HW), ethanol (EtOH), methanol (MeOH), and chloroform:methanol (CM) at a ratio of 2:1 v/v] on the antioxidant and anti-inflammatory properties of M. nummuloides bioproducts. To the best of our knowledge, this study is the first to investigate the effects of these factors on M. nummuloides product properties. Our findings provide guidance for the application of appropriate drying and extraction techniques to generate M. nummuloides products with enhanced bioactivity.
Materials and methods
Chemicals
Sigma-Aldrich (St. Louis, MO, USA) provided the standard compounds for gas chromatography coupled with flame ionization detection (GC-FID) analysis, i.e., oleic acid (CAS No. 112-80-1; purity ≥ 99%), palmitic acid (CAS No. 57-10-3; purity ≥ 99%), α-linolenic acid (CAS No. 463-40-1; purity ≥ 99%), pentadecanoic acid (CAS No. 1002-84-2; purity ≥ 99%), heptadecanoic acid (CAS No. 506-12-7; purity ≥ 99%), stearic acid (CAS No. 57-11-4; purity ≥ 99%), myristic acid (CAS No. 544-63-8; purity ≥ 99%), eicosapentaenoic acid (CAS No. 10417-94-4; purity ≥ 99%) and palmitoleic acid (CAS No. 373-49-9; purity ≥ 98.5%), along with solvents, i.e., methanol (CAS No. 67-56-1; purity ≥ 99.9%), ethanol (CAS No. 64-17-5; purity ≥ 99.5%), water (CAS No. 7732-18-5; purity ≥ 99.9%), and chloroform (CAS No. 67-66-3; purity ≥ 99.5%). Neophytadiene (CAS No. 504-96-1; purity ≥ 95%) was purchased from BOC Sciences Co. (Shirley, NY, USA)
Materials
M. nummuloides diatoms were cultivated and provided by JDK Biotech, Ltd. (Korea). Briefly, the diatoms were cultivated with fresh Jeju lava seawater (originating from a saline volcanic rock aquifer) at Jeju Island, Republic of Korea. After collection, freshwater was used to desalinate the diatom samples [5].
Drying experiments
M. nummuloides samples were frozen in a − 75 °C ultra-low temperature freezer (MDF-U54V-PK; Panasonic Corp., Osaka, Japan) and then freeze-dried for 96 h. Freezing was initially conducted under reduced pressure in a FDTE-8012 freeze dryer (Operon Co., Gimpo, Korea), and an ultra-fine particle crusher (DSCH-550 S; Duksan Co., Ltd., Ansan, Korea) was used to ensure a sample particle size of ≤ 100 μm. During HAD, M. nummuloides samples were dried at 65 °C for approximately 24–48 h in a forced convention drying oven (HB-502 L; Hanbaek Co., Ltd., Bucheon, Korea) to obtain a final moisture content of approximately 2–4%.
Sample extraction
M. nummuloides samples were extracted with HW, EtOH, MeOH, or CM (10 g with each method). For the extraction using HW, powdered M. nummuloides samples were extracted using 400 mL of water, then autoclaved at 125 °C for 15 min, cooled at 25 °C for 24 h, and filtered with 150 mm filter paper (Advantec Toyo Roshi Kaisha, Ltd., Tokyo, Japan). For extractions using EtOH or MeOH, 10 g of powdered M. nummuloides samples were mixed with 400 mL of 70% ethanol or 80% methanol, sonicated (for 45 min at 20 °C), rested at 25 °C for 24 h, and filtered with 150 mm filter paper. For CM extractions, 10 g of powdered M. nummuloides samples were mixed with 400 mL of a chloroform:methanol mixture (2:1 v/v) with constant sonication (45 min at 20 °C), rested at 25 °C for 24 h, and filtered with 150 mm filter paper. The extracts were then transferred to a separating funnel, to which 400 mL of water was added and mixed well, the water layer was discarded, and the extracts were collected by being passed through sodium sulfate. A rotary evaporator was used to evaporate liquid from the resulting extracts, and the final products were freeze-dried at − 55° C for 72 h.
Radical scavenging activity assay for 2,2-diphenyl-1-picrylhydrazyl (DPPH∙)
DPPH∙ radical scavenging activity was determined as previously described [11]. Prior to analysis, a fresh DPPH∙ solution (200 µM in ethanol solution) was prepared, of which 160 µL was mixed with 40 µL of each M. nummuloides extract and incubated at 37 °C for 30 min (in the dark). Absorbance was then measured at 517 nm. The DPPH∙ scavenging ability was calculated via the following equation: DPPH∙ scavenging activity (%) = [(absorbance of control – absorbance of sample extract or standard)] / (absorbance of control)] × 100, using catechin as a positive control. The half-maximal effective concentration (EC50) value of each sample was calculated using GraphPad Prism 9.3.1 software (La Jolla, CA, USA). Data (mean ± standard deviation) reflect results of triplicate independent experiments.
Radical scavenging activity assay for 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS∙)
The ABTS∙ radical scavenging assay was conducted as previously described [11]. Prior to analysis, a stock ABTS solution was prepared by mixing 7 mM ABTS into 2.45 mM potassium persulfate, then diluting the mixture with distilled water until an absorbance (734 nm) optical density (OD) value of 0.700 ± 0.005 was obtained. Next, 900 µL of the ABTS solution was mixed with 100 µL of each M. nummuloides extract and incubated at 25 °C for 3 min, whereafter absorbance was measured at OD 734 nm. The ABTS∙ scavenging ability was calculated via the following equation: ABTS∙ scavenging activity (%) = [(absorbance of control – absorbance of sample extract or standard)]/(absorbance of control)] × 100, with α-tocopherol as a positive control. The EC50 value of each sample was calculated using GraphPad Prism 9.3.1 software. Data (mean ± standard deviation) again represent triplicate independent experiments.
Nitric oxide (NO) production assay
RAW 264.7 monocyte/macrophage-like cells were purchased from the American Type Culture Collection (ATCC) and cultured according to the manufacturer’s instructions. First, RAW 264.7 cells were seeded (105 cells/well) into 24-well cell culture plates and incubated for 24 h in Dulbecco’s modified Eagle medium (DMEM; Roswell Park Memorial Institute, Buffalo, NY, USA) supplemented with 1% antibiotic (100 µg/mL of penicillin, 100 µg/mL streptomycin) and 10% fetal bovine serum (FBS) (v/v). Thereafter, the cells were starved for 24 h in DMEM medium supplemented with only 1% FBS (v/v) and 1% antibiotic. Following starvation, the RAW 264.7 cells were pretreated with M. nummuloides extracts (at 6, 12, and 24 µg/mL) for 2 h, then exposed to lipopolysaccharides (LPSs) (1 µg/mL) for 24 h. Next, 100 µL of culture media or sodium nitrite standard solutions were mixed well with 100 µL of Griess reagent and incubated at 25 °C for 10 min. In the final step, a microplate reader was used to measure the OD of samples at 550 nm. The half-maximal inhibitory concentration (IC50) value of each sample was calculated with GraphPad Prism 9.3.1 software. Data (mean ± standard deviation) comprise triplicate independent experiments.
Cell viability assays
The MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) cell viability assay was conducted as previously described by us [20]. Prior to cell viability assays, RAW 264.7 cells were exposed to different concentrations of M. nummuloides extracts and incubated for 24 h, then exposed to 100 µL MTT (1 mg/mL) at 37 °C for 2 h. Next, add 150 µL/ well of DMSO and shaken for 30 min in the dark. using a microplate reader to evaluated absorbance at 570 nm. All experiments were performed in triplicate.
Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS analysis was conducted as previously described [20] using a GCMS-QP-2010 Plus spectrometer with a DB-5MS GC column (30 m length, 0.25 μm film thickness, 0.25 mm internal diameter) (Shimadzu Co., Nakagyo-ku, Kyoto, Japan). Helium acted as the carrier gas at a constant flow rate of 1 mL/min, and the injection volume of each sample was 1 µL, delivered in splitless mode. The total run time was 67 min, and the temperature gradient exposure was controlled from 80 to 300 °C as follows: an initial 80 °C for 5 min; an increase from 80 to 280 °C at 5 °C/min for 10 min; and an increase from 280 to 300 °C at 10 °C/min for 10 min. Mass spectra were detected using the W9N08 Wiley library 9.0.
GC-FID analysis
Quantification of the lipophilic compounds in the different M. nummuloides extracts was performed using GC-FID analysis. An HP-5 column (30 m length, 0.320 mm internal diameter, 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA) was used for all GC-FID separations. All samples were analyzed in triplicate. The injection temperature was 230 °C, and the injection volume of each sample (1 µL) was delivered in splitless mode. Helium was used as the carrier gas at a constant flow rate of 1 mL/ min, and the detector temperature was set at 310 °C. The total run time was 30 min, with a temperature range gradient from 150 to 250 °C set as follows: an initial 150 °C for 5 min; an increase from 150 to 250 °C at 10 °C/min for 10 min; and holding 250 °C for 15 min. Lipophilic compounds were determined using a standard curve.
Statistical analysis
Data (mean ± standard deviation) represent triplicate independent experiments and were analyzed using one-way analysis (ANOVA) of variance with Tukey’s multiple comparison tests; statistical significance was set at P < 0.05 in GraphPad Prism 9.3.1 software.
Results and discussion
Extraction yield
Comparing the yield extract (%) of M. nummuloides samples processed by FD (Additional file 1: Table S1), it was found that the HAD samples resulted in higher yields. Among the HAD samples, HAD-HW exhibited the highest extract yield (1.096%), followed by HAD-EtOH (0.898%), HAD-MeOH (0.811%), and HAD-CM (0.723%). In comparison, the extract yield of the FD samples was lower, with FD-CM at 0.487%, FD-HW at 0.475%, FD-EtOH at 0.413%, and FD-MeOH at 0.350%. The Pearson’s correlation analysis showed a negative relationship between extract yield and antioxidant activities (DPPH• and ABTS• radical scavenging; with R = – 0.244, and R= – 0.609, respectively) and anti-inflammatory activities (No assay, R = – 0.429) of M. nummuloides extracts (Additional file 1: Table S2).
Antioxidant activity
M. nummuloides is a potential alternative source of food, fuel, and dietary supplements, containing valuable components such as omega-3, omega-6 fatty acids, polyphenols, and fucoxanthin [3, 5]. However, it remains unclear what the effects of the drying method and the extraction solvents are on the antioxidant capacity, and anti-inflammatory capacity of M. nummuloides products.
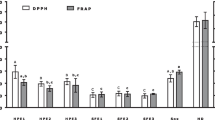
To evaluate antioxidant activity, the DPPH∙ and ABTS∙ scavenging assays on the following extracts: HAD-HW, FD-HW, HAD-EtOH, FD-EtOH, HAD-MeOH, FD-MeOH, HAD-CM, and FD-CM were used. The EC50 values obtained for each assay are listed in Table 1. The DPPH∙ radical scavenging activities of the different solvent extracts increased during both drying methods as the extract concentration increased from 2 to 8 mg/mL (Fig. 1a; Table 1). Similarly, all extracts demonstrated ABTS∙ radical scavenging activity in a dose-dependent manner (Fig. 1b). In both antioxidant assays, extracts obtained using a CM solvent displayed significantly higher radical scavenging activity than those extracted using other solvents. The highest antioxidant activity was observed in FD-CM (2.396 and 2.902 mg/mL for the DPPH∙ and ABTS∙ assays, respectively), followed by FD-EtOH, FD-MeOH, and FD-HW. The highest DPPH∙ and ABTS∙ antioxidant activity in samples subjected to HAD were observed in HAD-CM (with EC50 values of 2.816 and 2.791 mg/mL, respectively), followed by HAD-EtOH, HAD-MeOH, and HAD-HW. No significant difference was detected between any extracts processed by HAD and FD in terms of DPPH∙ and ABTS∙ radical scavenging activity.
Antioxidant activity of different Melosira nummuloides extracts. DPPH∙ (a) and ABTS∙ (b) radical scavenging activity assays were used to assess the antioxidant activity of extracts. Data (mean ± standard deviation) represent three triplicate independent experiments. HAD-HW hot-air drying and hot water extract, FD-HW freeze-drying and hot water extract, HAD-EtOH hot-air drying and ethanol extract, FD-EtOH freeze-drying and ethanol extract, HAD-MeOH hot-air drying and methanol extract, FD-MeOH freeze-drying and methanol extract, HAD-CM hot-air drying and chloroform:methanol (2:1 v/v) extract, FD-CM freeze-drying and chloroform:methanol (2:1 v/v) extract
Our findings cumulatively suggest that, regardless of the drying method, M. nummuloides extracted with CM solvent possessed the highest antioxidant activity. They also demonstrated that the antioxidant properties of M. nummuloides extracts depended on the extraction process. This is consistent with findings from a previous study that showed that the free radical scavenging activity of algal Haematococcus pluvialis varies with the extraction solvents used [21]. Moreover, López et al. [22] reported the effects of water, water/methanol (1/1), methanol, and ethanol solvents on the antioxidant activity of Stypocaulon scoparium algal extracts [22]. As shown in Fig. 1; Table 1, FD-CM extracts showed highest antioxidant potential compared to other extracts. Moreover, they had a high content of compounds that reportedly have excellent antioxidant effects, such as myristic acid [23], α-linolenic acid [24, 25], palmitoleic acid [26], and eicosapentaenoic acid [27] which may contribute to the high antioxidant potential observed. Indeed, the supplementation of polyunsaturated fatty acids in the diet has shown to decrease oxidative stress-induced mitochondrial dysfunction and apoptosis in endothelial cells. This effect is achieved through an enhancement of the activity of endogenous antioxidant enzymes [24, 27].
M. nummuloides extract-related inhibition of NO production in LPS-stimulated RAW 264.7 cells
The equilibrium between reactive oxygen species (ROS) and NO levels plays a key role in cell signaling, maintaining homeostasis, and regulating physiological functions [28, 29]. However, excessive ROS or NO levels can disrupt cellular homeostasis, oxidize and impair cellular components, cause irreversible damage to DNA, and hinder their original functions, which can lead to various diseases, including cancer. Therefore, regulating inflammatory responses by maintaining a balance between ROS and NO levels is a promising therapeutic strategy. LPS, a common macrophage activator, binds to specific receptors present on host effector cells, leading to the secretion of proinflammatory cytokines [30, 31]. The Griess assay, which measures NO production levels in LPS-stimulated RAW 264.7 cells, is frequently used to evaluate the anti-inflammatory effects of plant extracts or secondary metabolites [30, 32, 33]. In the present study, the inhibitory effects of M. nummuloides extracts on LPS-induced NO accumulation in RAW 264.7 cells were investigated. As shown in Fig. 2, NO production increased in these cells following LPS exposure. All M. nummuloides extracts, except for the HAD-HW and FD-HW ones, significantly reduced NO levels in LPS-stimulated RAW 264.7 cells. The IC50 values of suggest that, compared to other extracts, FD-CM had a higher inhibitory effect on NO production in LPS-stimulated RAW 264.7 cells—suggesting that it exhibited the most potent anti-inflammatory activity—followed by HAD-CM and FD-MeOH. The MTT assay (Additional file 1: Fig S1, Table 2) results indicated that the observed inhibitory effects of these extracts were not attributed to cytotoxic activity. This suggests that M. nummuloides extracts may modulate anti-inflammatory responses by suppressing LPS-induced NO accumulation in RAW 264.7 cells.
Anti-inflammatory effects of M. nummuloides extracts on nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 cells. RAW 264.7 cells were exposed to lipopolysaccharides for 24 h. Data (mean ± standard deviation) represent triplicate independent experiments. Letters a–e indicate significant differences (P < 0.05). HAD-HW hot-air drying and hot water extract, FD-HW freeze-drying and hot water extract, HAD-EtOH hot-air drying and ethanol extract, FD-EtOH freeze-drying and ethanol extract, HAD-MeOH hot-air drying and methanol extract, FD-MeOH freeze-drying and methanol extract, HAD-CM hot-air drying and chloroform:methanol (2:1 v/v) extract, FD-CM freeze-drying and chloroform:methanol (2:1 v/v) extract
Moreover, several lipophilic compounds were identified in the FD-CM extracts, including ɑ-linolenic acid, oleic acid, palmitoleic acid, eicosapentaenoic acid, and neophytadiene, again reinforcing our earlier observation that FD-CM extracts had the highest anti-inflammatory potential. Previous studies have similarly reported that the anti-inflammatory mechanisms of palmitoleic acid in macrophages that are exposed to LPS are mediated via inhibition of the inflammasome pathway, which inhibits nuclear factor kappa B independently of peroxisome proliferator-activated receptors [34]. In addition, oleic acid—the main component of olive oil extracts—attenuates the LPS-induced inflammatory response in murine RAW264.7 macrophages [35]. Oleic acid can also alleviate inflammation, oxidative stress, and LPS-induced acute kidney injury in mice [36]. Palmitoleic acid has demonstrated even greater anti-inflammatory potential than oleic and palmitic acids in human endothelial cells [37]. The anti-inflammatory and anti-oxidative effects of ɑ-linoleic acid have been described in mice with LPS-induced acute lung injury [38], and anti-inflammatory effects have also been recorded for α-linolenic acid isolated from Actinidia polygama fruits [39]. Lastly, neophytadiene from Turbinaria ornata has been shown to inhibit inflammatory responses in LPS-treated RAW 264.7 macrophages and Sprague–Dawley rats [40].
FD-CM extract composition
Fatty acid content can be analyzed using various techniques, such as GC-MS, GC-FID and LC-MS (liquid-chromatography mass spectrometry) [41,42,43]. However, using LC-MS to determine fatty acid content has some limitations, including lower selectivity and larger solvent consumption [41]. In contrast, GC-MS utilizes established databases for fatty acid identification, thereby resulting in higher efficiency and selectivity, which could provide more structural information than GC-FID [41, 44]. Therefore, GC-MS is a widely accepted, cost-effective, and commonly used technique to identify and quantify fatty acids in biological samples [41]. Because FD-CM exhibited the highest antioxidant and anti-inflammatory activity among extracts, we subjected it to a composition analysis via GC-MS. The major compounds in the FD-CM extract were fatty acids, comprising palmitic (45.69 ± 1.09% area), myristic (17.91 ± 0.37% area), palmitoleic (7.11 ± 0.09% area), pentadecanoic (2.01 ± 0.09% area), oleic (1.55 ± 0.17% area), and eicosapentaenoic (1.16 ± 0.10% area) acid, neophytadiene (1.15 ± 0.02% area), and alpha-linolenic acid (0.91 ± 0.12% area) (Table 3). This result is consistent with those of previous studies reporting that CM is one of the most commonly used solvents for extracting lipids and fatty acids from diatoms, such as Fistulifera spp. [17], P. tricornutum [18], and Amphora spp. [19].
Following the tentative identification of the major lipophilic compounds present in the FD-CM extract, the GC-FID was used to analyze the accumulation of the following lipophilic compounds (higher % peak area and a similarity cut-off of 85%): pentadecanoic, palmitic, heptadecanoic, stearic, oleic, α-linolenic, myristic, palmitoleic, and eicosapentaenoic acid and neophytadiene. The most abundant compound was palmitic acid, followed sequentially by myristic, oleic, pentadecanoic, stearic, heptadecanoic, eicosapentaenoic, and palmitoleic acid and then neophytadiene, with α-linolenic acids being the least abundant (Table 4, Additional file 1: Fig. S2–S4). Notably, FD-CM had a high content of compounds that are known for excellent antioxidant and/or anti-inflammatory effects, such as myristic acid [29.08 ± 0.45 mg/g dry weight powder extract (DW)], oleic acid (25.20 ± 0.92 mg/g DW), eicosapentaenoic acid (12.53 ± 1.00 mg/g DW), palmitoleic acid (10.77 ± 0.41 mg/g DW), neophytadiene (8.42 ± 0.51 mg/g DW) and α-linolenic acid (1.27 ± 0.005 mg/g DW), which may contribute to the antioxidant and anti-inflammatory potential of FD-CM. In this study, the antioxidant and anti-inflammatory activity of M. nummuloides samples obtained with different extraction solvents and via either HAD or FD were analyzed. The FD-CM extract (with CM at a ratio of 2:1 v/v) exhibited the highest antioxidant and anti-inflammatory capacity. This functional potential was supported with the identification of several lipophilic compounds in FD-CM, namely, ɑ- linolenic, oleic, palmitoleic, and eicosapentaenoic acid and neophytadiene. Our findings demonstrated that FD was the optimal drying technique and CM was the optimal extraction processes for obtaining M. nummuloides bioproducts with enhanced bioactivity.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic-acid)
- DPPH:
-
2,2-Diphenyl-1-picryhydrazyl
- EC50 :
-
The effective concentration that causes 50% of the maximum response
- EtOH:
-
Ethanol
- FD:
-
Freeze-drying
- FD-HW:
-
Freeze-drying and hot water extract
- FD-EtOH:
-
Freeze-drying and ethanol extract
- FD-MeOH:
-
Freeze-drying and methanol extract
- FD-CM:
-
Freeze-drying and chloroform:methanol (2:1 v/v) extract
- GAE:
-
Gallic acid equivalent
- GC-FID:
-
Gas chromatography with flame ionization detection
- GC-MS:
-
Gas chromatography mass spectrometry
- HAD:
-
Hot-air drying
- HAD-HW:
-
Hot-air drying and hot water extract
- HAD-EtOH:
-
Hot-air drying and ethanol extract
- HAD-MeOH:
-
Hot-air drying and methanol extract
- HAD-CM:
-
Hot-air drying and chloroform:methanol (2:1 v/v) extract
- HPLC:
-
High performance liquid chromatography
- IC50 :
-
The inhibitory concentration that causes 50% of the maximum inhibition
- MeOH:
-
Methanol
- MTT:
-
3-(4,5-Dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide
References
Khavari F et al (2021) Microalgae: therapeutic potentials and applications. Mol Biol Rep 48(5):4757–4765
Khaligh SF, Asoodeh A (2022) Recent advances in the bio-application of microalgae-derived biochemical metabolites and development trends of photobioreactor-based culture systems. 3 Biotech 12(10):1–19
Li Y et al (2016) Nutrient efficacy of microalgae as aquafeed additives for the adult black tiger prawn, P enaeus monodon. Aquac Res 47(11):3625–3635
Kim Y et al (2022) Compositional characteristics of the Microalga Melosira nummuloides Mass-cultured using Jeju lava seawater. Korean J Fisheries Aquat Sci 55(2):91–101
Yun I et al (2022) Anti-obesity effect of Microalga, Melosira nummuloieds ethanolic extract in high-fat-diet-induced obesity C57BL/6J mice. Funct Foods Health Disease 12(12):693–714
Chua LY et al (2019) Influence of drying methods on the antibacterial, antioxidant and essential oil volatile composition of herbs: a review. Food Bioprocess Technol 12:450–476
Hosseinizand H et al (2017) Economic analysis of drying microalgae Chlorella in a conveyor belt dryer with recycled heat from a power plant. Appl Therm Eng 124:525–532
Liapis AI, Bruttini R (2020) Freeze drying. In: Mujumdar Arun S (ed) Handbook of industrial drying. CRC Press, Boca Raton
Chen C-L, Chang J-S, Lee D-J (2015) Dewatering and drying methods for microalgae. Drying Technol 33(4):443–454
de Farias Neves F, Demarco M, Tribuzi G (2019) Drying and quality of microalgal powders for human alimentation. In: Vítová Milada (ed) Microalgae-from physiology to application. IntechOpen, London
Cuong DM et al (2022) Evaluation of phytochemical content and the antioxidant and antiproliferative potentials of leaf layers of cabbage subjected to hot air and freeze-drying. J Food Qual. https://doi.org/10.1155/2022/8040456
Zhang Q-W, Lin L-G, Ye W-C (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med 13:1–26
Mustafa A, Turner C (2011) Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal Chim Acta 703(1):8–18
Foo SC et al (2015) Efficient solvent extraction of antioxidant-rich extract from a tropical diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Asian Pac J Trop Biomed 5(10):834–840
Trentin R et al (2022) Total phenolic levels, in vitro antioxidant properties, and fatty acid profile of two microalgae, tetraselmis marina strain IMA043 and naviculoid diatom strain IMA053, isolated from the North Adriatic sea. Mar Drugs 20(3):207
Sun J et al (2022) A simple and efficient strategy for fucoxanthin extraction from the microalga Phaeodactylum tricornutum. Algal Res 61:102610
Liang Y et al (2014) Profiling of fatty acid methyl esters from the oleaginous diatom Fistulifera sp. strain JPCC DA0580 under nutrition-sufficient and-deficient conditions. J Appl Phycol 26:2295–2302
Hamilton ML et al (2016) Heterotrophic production of omega-3 long-chain polyunsaturated fatty acids by trophically converted marine diatom Phaeodactylum tricornutum. Mar Drugs 14(3):53
Hogan P et al (2021) Effect of biomass pre-treatment on supercritical CO2 extraction of lipids from marine diatom Amphora sp. and its biomass evaluation as bioethanol feedstock. Heliyon 7(1):e05995
Kim JS et al (2022) Antioxidant and antiproliferative activities of solvent fractions of broccoli (Brassica oleracea L.) sprout. Appl Biol Chem 65(1):1–11
Dong S et al (2014) Four different methods comparison for extraction of astaxanthin from green alga Haematococcus pluvialis. Sci World J. https://doi.org/10.1155/2014/694305
López A et al (2011) The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem 125(3):1104–1109
Liu C et al (2019) Antioxidant capacity and hepatoprotective activity of myristic acid acylated derivative of phloridzin. Heliyon 5(5):e01761
Oppedisano F et al (2020) The anti-inflammatory and antioxidant properties of n-3 PUFAs: their role in cardiovascular protection. Biomedicines 8(9):306
Oyalo J, Mburu M (2021) Health potential of Chia (Salvia hispanica L.) seeds-derived α-linoleic acid and α-linolenic acids: a review. Eur J Agric Food Sci 3(4):5–10
Wang Q et al (2019) Chemical profile, quality and antioxidant properties of palmitoleic acid rich oil from decaisnea insignis seeds by different extraction techniques. Food Sci Technol Res 25(6):755–763
Bettadahalli S et al (2020) Evidence on oleic acid and EPA + DHA role in retinal antioxidant defense, leukocyte adhesion, and vascular permeability: insight from hyperlipidemic rat model. J Funct Foods 67:103864
Ray PD, Huang B-W, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24(5):981–990
Swindle EJ, Metcalfe DD (2007) The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev 217(1):186–205
Yoon W-J, Lee NH, Hyun C-G (2010) Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J Oleo Sci 59(8):415–421
Bryant CE et al (2010) The molecular basis of the host response to lipopolysaccharide. Nat Rev Microbiol 8(1):8–14
Wadsworth TL, Koop DR (1999) Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem Pharmacol 57(8):941–949
Kim DK et al (2022) Antioxidant activity of banana flesh and antiproliferative effect on breast and pancreatic cancer cells. Food Sci Nutr 10(3):740–750
Souza CO et al (2017) Palmitoleic acid reduces the inflammation in LPS-stimulated macrophages by inhibition of NF κB, independently of PPAR s. Clin Exp Pharmacol Physiol 44(5):566–575
Müller AK et al (2021) Olive oil extracts and oleic acid attenuate the LPS-Induced Inflammatory response in murine RAW264. 7 macrophages but induce the release of prostaglandin E2. Nutrients 13(12):4437
Zhang B et al (2022) Oleic acid alleviates LPS-induced acute kidney injury by restraining inflammation and oxidative stress via the Ras/MAPKs/PPAR-γ signaling pathway. Phytomedicine 94:153818
de Souza CO et al (2018) Palmitoleic acid has stronger anti-inflammatory potential in human endothelial cells compared to oleic and palmitic acids. Mol Nutr Food Res 62(20):1800322
Zhu X et al (2020) Alpha-linolenic acid protects against lipopolysaccharide-induced acute lung injury through anti-inflammatory and anti-oxidative pathways. Microb Pathog. https://doi.org/10.1016/j.micpath.2020.104077
Ren J, Chung SH (2007) Anti-inflammatory effect of α-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-κB and mitogen-activated protein kinase pathways. J Agric Food Chem 55(13):5073–5080
Bhardwaj M et al (2020) Neophytadiene from Turbinaria ornata suppresses LPS-induced inflammatory response in RAW 264.7 macrophages and sprague dawley rats. Inflammation 43(3):937–950
Chiu H-H, Kuo C-H (2020) Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples. J Food Drug Anal 28(1):60–73
Zhang H, Wang Z, Liu O (2015) Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J Pharm Anal 5(4):223–230
Christinat N, Morin-Rivron D, Masoodi M (2017) High-throughput quantitative lipidomics analysis of nonesterified fatty acids in plasma by LCMS. In: Greening David W, Simpson Richard J (eds) Serum/Plasma proteomics. Springer, Berlin
Ecker J et al (2012) A rapid GC–MS method for quantification of positional and geometric isomers of fatty acid methyl esters. J Chromatogr B 897:98–104
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This research was supported by Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries, Korea (20220255) and by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education(2016R1A6A1A03012862).
Author information
Authors and Affiliations
Contributions
SKC conceived the study and revised the paper. MKE, JYM and J-EP revised the paper. Do Manh Cuong write the paper. The experiments were performed by DMC and DKK. All authors participated equally in reviewing and the finalizing manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig S1.
Effect of Melosira nummuloides extracts on the viability of RAW 264.7 cells. Cells were exposed to the extracts for 24 h, and the MTT assay was used to assess cell viability. Data (mean ± standard deviation) determined by triplicate independent experiments. HAD-HW, hot-air drying hot water extract; FD-HW, freeze-drying hot water extract; HAD-EtOH, hot-air drying ethanol extract; FD-EtOH, freeze-drying ethanol extract; HAD-MeOH, hot-air drying methanol extract; FD-MeOH, freeze-drying methanol extract; HAD-CM, hot-air drying chloroform:methanol in the ratio of 2:1 v/v extract; FD-CM, freeze-drying chloroform:methanol at a ratio of 2:1 v/v extract. Fig S2. Gas chromatography with flame ionization detectionchromatograms of fatty acid components in FD-CM (freeze-drying chloroform:methanol at a ratio of 2:1 v/v extract). Fig S3. Gas chromatography with flame ionization detectionchromatograms of neophytadiene standard compounds in FD-CMextracts. FD-CM, freeze-drying chloroform:methanol at a ratio of 2:1 v/v extract. Fig S4. Gas chromatography with flame ionization detectionchromatograms of eicosapentaenoic acid standard compounds in FD-CMextracts. FD-CM, freeze-drying chloroform:methanol at a ratio of 2:1 v/v extract. Table S1. Extractyield (%) of different solvent extracts of Melosira nummuloides following hot air drying and freeze drying. Table S2. Correlation analysis of yield extract and the antioxidant (DPPH· and ABTS· assay), and anti-inflammatory (NO assay) properties of Melosira nummuloides extracts.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cuong, D.M., Kim, D.K., Ediriweera, M.K. et al. Effects of the drying method and extraction solvent on antioxidant and anti-inflammatory activity of Melosira nummuloides bioproducts. Appl Biol Chem 66, 59 (2023). https://doi.org/10.1186/s13765-023-00817-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-023-00817-y