Abstract
Centella asiatica L. Urban (CA) is a valuable medicinal herb that contains various bioactive secondary metabolites. In the present study, the harvested CA was divided into whole plant and leaf parts, and were heated-air-dried or freeze-dried. The dried CA was extracted under various extraction conditions to obtain 16 extracts, and their antioxidant activities were examined. Twelve types of secondary metabolites (five polyphenolic acids, four triterpenoids, and three flavonoids) were quantified in each extract. Finally, the intercorrelations between secondary metabolites and antioxidant activities were analyzed through statistical analyses, such as Pearson correlation coefficient, scatter plotting, and principal component analysis. The antioxidant capacities of CA might be primarily influenced by major triterpenoids such as madecassoside and asiaticoside, which showed high content in the ethanol extracts of freeze-dried leaf parts. The present study provides a valuable groundwork for the development of optimal extraction processes for C. asiatica L. as an antioxidant material.
Similar content being viewed by others
Introduction
Centella asiatica L. Urban (CA), a topical herbaceous plant belonging to the family Umbelliferae (Apiaceae), is a valuable medicinal herb mainly grown in swampy areas in countries including India, Sri Lanka, Indonesia, Malaysia, Madagascar, and parts of Africa [1]. According to the Indian pharmacopeia, CA exerts various protective and therapeutic effects against leprosy, lupus, varicose ulcer, eczema, psoriasis, diarrhea, fever, and amenorrhea [2]. In addition, recent studies have discussed various pharmacological effects of CA, such as antioxidant [3], antimicrobial [4], anti-inflammatory [5], neuroprotective [6], memory improvement [7], and antidepressant compounds [8]. Owing to its proven medicinal properties, it has been gradually incorporated in Pharmacopoeias of other countries, such as England, France, Italy, the Netherlands, Germany, Poland, and China [9].
The major biologically active ingredients of CA, known as secondary metabolites, are isolated mainly from the aerial parts. Previous studies have reported a total of 57 secondary metabolites in CA, which are classified into four major clusters: triterpenoids (madecassoside, asiaticoside, madecassic acid, and asiatic acid), volatile mono and sesquiterpenes (caryophyllene, farnesol, and elemene), flavonoids (quercetin, kaempherol, catechin, rutin, apigenin, naringin, castiliferol, and castilicetin), and other compounds (vellarine and hydrocotyline) [10,11,12]. Among them, asiaticoside and madecassoside, which are the most abundant pentacyclic triterpenoids, and their corresponding aglycones, asiatic acid and madecassic acid have been extensively studied and accepted as the primary active components of CA [1].
Since herbaceous plants, including CA, have a high moisture content, a postharvest drying process is essential to control the moisture content to prevent nutrition loss and to encourage their supply during the off-season [13]. However, all forms of triterpene glycosides and phenolic compounds in natural materials can easily degrade due to several conditions, such as thermal treatment and oxidation reactions during processing [14]. Meanwhile, because heated-air drying usually induces deterioration of the nutrients despite its cost-effective advantage, high-cost freeze drying is largely being accepted as the most effective method to protect various nutrients [15]. Moreover, Niamnuy et al. [14] investigated the effect of various drying methods and temperatures on drying characteristics, phenolic compounds, triterpene compounds, antioxidant and antibacterial activities of CA extracts. Nevertheless, correlation studies between the deteriorated ingredients of CA induced by the drying process and changes in physiological activities have not been investigated.
Therefore, the present study aimed to optimize the extraction conditions by identifying the relationship between various extracts of CA and their antioxidant activities. To achieve this goal, the harvested CA was extracted according to different plant parts, drying method, and extraction conditions. Various statistical analyses, including scatter plot, and bivariate and multivariate correlations, were performed to determine the interrelationship between the secondary metabolites and antioxidant capacities of each extract.
Materials and methods
Cultivation, harvest, and drying of C. asiatica
C. asiatica was cultivated in the Centella Farm in Chungbuk Province (Chungju, South Korea), and harvested in July 2020. The harvested CA was immediately dried using two drying methods: freeze drying (FD) using a freeze drier (Ilshin Biobase Co., Ltd, Seoul, Korea) and heated-air drying (heated-air drying; HD) in a greenhouse facility at 70 °C without a forced convection system.
Preparation of various C. asiatica extracts
After post-harvest drying, the separated leaf tissues and the whole tissue of CA were individually extracted under various conditions. First, Water extracts were prepared with cold-water (CW) and hot-water (HW). Briefly, 20 volumes (w/v) of water was added to the dried C. asiatica, and then extracted either by stirring at room temperature for 48 h (CW) or by decoction at 95 ℃ (HW). Ethanolic (EtOH) extracts were prepared with 50% EtOH (50E) or 70% EtOH (70E) by stirring at room temperature for 48 h. All extracts were individually filtered through filter paper (Whatman, Brentford, UK) followed by centrifugation (Gyrogen Co., Ltd., Daejeon, South Korea) to remove insoluble materials, and evaporated using a rotary vacuum evaporator (Eyela, Tokyo, Japan) to remove excess solvent from the final product (Eyela, Tokyo, Japan). Finally, a total of 16 extracts were lyophilized by freeze-drying (Ilshin Biobase, Seoul, Korea), and the extraction yields were calculated against each raw material.
Antioxidant activity
To measure the antioxidant power of each extract, two types of free radicals, 2,2′-azino-bis (3-ethylbenzothiaziline-6-sulfuric acid) (ABTS; Sigma-Aldrich, St. Louis, MO, USA) and 2–2-diphenyl-1-picrylhydrazyl (DPPH Sigma-Aldrich) were used in the present study. The ABTS and DPPH radical scavenging activities were determined according to the method reported by Kim et al. [16]. The radical scavenging activities of the extracts were calculated against the mg ascorbic acid equivalent antioxidant capacity (AEAC)/g extract. The reducing power of each extract was measured by ferric reducing ability of plasma (FRAP) activity according to the method reported by Kim et al. [16]. The FRAP activity of extract was expressed as ascorbic acid equivalent (AAE)/g extract.
Measurement of total polyphenol and flavonoid contents
The total polyphenol content (TPC) of each extract was determined using Folin-Ciocalteu’s method [17] with slight modifications. Briefly, the extract (10 μL) was mixed with 10 μL of 2% sodium carbonate (Na2CO3; Sigma-Aldrich) and incubated at room temperature for 10 min, and 200 μL of 1 M Folin-Ciocalteu’s reagent (Sigma-Aldrich) was added and incubated for 30 min to generate the blue color, and the absorbance was measured at 750 nm. The total polyphenol content of each extract was calculated as mg gallic acid equivalent (GAE)/g extract. Total flavonoid content (TFC) of each extract was measured according to the method described previously [18]. Briefly, 20 μL extract was mixed with 80% ethanol (40 μL) and 10% aluminum nitrite [Al(NO3)3; Sigma-Aldrich] (40 μL), potassium acetate (CH3COOK; 20 μL), and 80% ethanol (150 μL), and reacted at room temperature for 10 min. The generated yellow color was measured at 415 nm, and the total flavonoid content was expressed as mg quercetin equivalent (QE)/g extract.
Determination of secondary metabolites in C. asiatica extracts by HPLC–DAD
Terpenoids
Four terpenoids, including madecassic acid (CAS No. 18449-41-7), madecassoside (CAS No. 34540-22-2), asiatic acid (CAS No. 464-92-6), and asiaticoside (CAS No. 16830-15-2) were obtained from Sigma-Aldrich, and analyzed using an HPLC system (Agilent 1200 series HPLC system; Agilent Technologies, Palo Alto, CA, USA). A YMC-Triart C18 column (250 × 4.6 mm, 5 μm; YMC Co., Ltd., Kyoto, Japan) was used as the stationary phase, and the column temperature was set at 30 °C. The mobile phase containing acetonitrile (A) and water (B) was set in gradient mode as follows: 0 min → 15 min (22% A), 15 min → 20 min (22% → 24% A), 20 min → 28 min (24% A), 28 min → 32 min (24% → 40% A), 32 min → 45 min (40% A), 45 min → 48 min (40% → 46% A), 48 min → 50 min (46% → 70% A), 50 min → 55 min (70% A), and 55 min → 60 min (70% → 22% A). The column was equilibrated for 15 min with initial conditions of the mobile phase to ensure reproducibility of the analysis. The injection volume was 20 μL, and the flow rate of the mobile phase was set to 1 mL/min. Before analysis, all standard references and each extract were filtered through a syringe filter (0.2 μm pore size; Pall Life Science, Ann Arbor, MI, USA) containing polyvinyl difluoride (PVDF; Pall Life Science). A diode array detector (DAD) was used at a wavelength of 257 nm.
Polyphenolic acids and flavonoids
Five polyphenolic acids including chlorogenic acid (CAS No. 327-97-9), caffeic acid (CAS NO. 331-39-5), 3,4-dicaffeoylquinic acid (CAS No. 14534-61-3), 1,5-dicaffeoylquinic acid (CAS No. 30964-13-7), and ellagic acid (CAS No. 476-66-4), and three flavonoids including luteolin 7-glucoside (cynaroside; CAS No. 68321-11-9), scutellarein 7-glucuronide (scutellarin; CAS No. 27740-01-8), and quercetin 3-glucuronide (miquelianin; CAN No. 22688-79-5) were obtained from the Natural product Institute of Science and Technology (NIST; Anseong, Korea). Analysis was performed using an HPLD system with a YMC-Triart C18 column (150 × 4.6 mm, 3 μm; YMC Co., Ltd., Kyoto, Japan) and DAD. The mobile phase composed of methanol containing 0.1% trifluoroacetic acid (TFA) (A) and water containing 0.1% TFA (B), was programmed as follows: 0 min → 6 min (30% → 37% A), 6 min → 12 min (37% A), 12 min → 16 min (37% → 41% A), 16 min → 25 min (41% A), 25 min → 38 min (41% → 56% A), 38% → 42 min (56% → 100% A), 42 min → 45 min (100% → 30% A), and 45 min → 60 min (30% A). The injection volume was 10 μL and the flow rate of the mobile phase was set at 0.4 mL/min. Before analysis, all standard references and each extract were filtered through a syringe filter (0.2 μm pore size; Pall Life Science, Ann Arbor, MI, USA) containing polyvinyl difluoride (PVDF; Pall Life Science). A diode array detector (DAD) was used at a wavelength of 325 nm.
Statistical analysis
Pearson correlation coefficient
Pearson correlation coefficients were determined using PASW Statistics 18 (IBM Co., Armonk, NY, USA) to confirm the bivariate correlation between each secondary metabolite and their antioxidant capacity.
Principal component analysis
Principal component analysis (PCA) was performed using R software (Ver 3.5.3 and 4.0.5; The R Foundation for Statistical Computing) and RStudio software (Ver. 1.4.1106; The R Foundation for Statistical Computing) to determine the multivariate correlation between secondary metabolites and antioxidant capacities depending on the preparation conditions. The results displayed as a biplot graph, was developed to visualize the trends among different groups, and the loading and contribution plots were employed to reveal the involvement of different components in each group. Each principal component represents a certain amount of variability in the data and the first two principal components (PC1 and PC2) typically account for most of the variation within the whole dataset [19].
Results and discussion
Yields of various C. asiatica extracts
In the present study, 16 extracts were prepared from C. asiatica, depending on the postharvest drying methods, plant parts, and extraction conditions. The detailed characteristics of each extract are listed in Additional file 1: Table S1. The mean yield of the 16 extracts was 29.4%. The highest yield was observed in 50% EtOH extract (HD-L-50E; 37.3%) prepared from the heated-air-dried leaf parts, whereas the lowest yields were observed in cold-water extracts (FD-L-CW, 23.3% and FD-W-CW, 22.7%) prepared from freeze-dried leaf parts and whole plant. Overall, 50% EtOH resulted in the highest extraction yield (mean yield 32.3%), followed by hot-water extracts (30.2%), 70% EtOH extracts (29.1%), and cold-water extracts (26.1%). This suggests that 50% EtOH could be an invaluable solvent in extracting additional ingredients from C. asiatica than the other solvents used. In addition, the mean extraction yield was slightly, but not significantly, higher in the extracts from heated-air-dried CA (mean yield 30.1%) compared with those from freeze-dried CA (mean yield 28.8%), implying that heated-air drying might effectively isolate more ingredients from CA than freeze-drying. To date, comparative studies on the effects of drying methods on the physiological activities and physicochemical properties have been demonstrated for Cucurbita moschata Duch [15], Citrus limon [20], and Dioscorea spp. [13]; to our knowledge, our study is the first to demonstrate the relationship between drying methods, extraction solvents, and extraction yields in C. asiatica.
Radical scavenging activity and reducing capacity
In general, the antioxidant activity of CA is attributed to its secondary metabolites, such as anthocyanins, flavonoids, and phenolic compounds [21]. The free radical scavenging activities and reducing power abilities of various CA extracts are provided in Table 1. In the results of ABTS radical scavenging activity, among the extracts considered, higher activities were observed in FD-L-70E (78.7 mg AEAC/g), FD-W-70E (77.8 mg AEAC/g), and FD-L-50E (77.1 mg AEAC/g). Another free radical, DPPH, was strongly inhibited in FD-W-70E (55.4 mg AEAC/g), FD-L-70E (50.6 mg AEAC/g), and FD-L-50E (47.8 mg AEAC/g). In addition, the highest FRAP activity was observed in FD-L-70E (74.9 mg AAE/g), FD-W-70E (72.3 mg AAE/g), and FD-L-50E (70.5 mg AAE/g). These results indicate that the antioxidant capacity of CA can be more potent when extracted with 50% or 70% EtOH than water alone. This is consistent with the result reported by Hamid et al. [22] who compared the antioxidant activity of CA in three different solvent extracts, such as water, ethanol, and light petroleum. Our results also demonstrated that freeze-dried CA extracts efficiently preserved its antioxidant capacity than those from heated-air-dried CA; this substantiates the rationale that the freeze-drying method is reportedly safer in preserving nutritional contents, including antioxidant components, than the heated-air drying method [23]. This is also consistent with the previous studies reported by Hsu et al. [13], Mao et al. [23], and Orak et al. [24], who compared the antioxidant activity of heated-air-dried and freeze-dried yam flours, daylily flowers, and strawberry trees, respectively. However, no apparent difference was observed in antioxidant activity between the leaf parts and whole plants of CA. A similar finding was reported by Zainol et al. [12], who investigated the antioxidant activity of different parts of CA and suggested that the antioxidant activity may be related to the following hypothesis: reduction of hydroperoxides, inactivation of free radicals, chelation of metal ions, or combinations thereof.
Total polyphenol and flavonoid contents
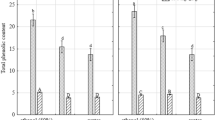
The TPC and TFC results are presented in Table 2. As expected, the water extract groups (CW and HW) showed relatively lower TPC and TFC contents than the ethanol extract groups (50E and 70E). The TPC was higher in FD-W-70E (140.2 mg GAE/g), FD-L-70E (135.7 mg GAE/g), HD-L-70E (132.6 mg GAE/g), and HD-W-70E (122.8 mg GAE/g) compared to the other extracts. The results indicated that TPC was generally higher in 70% EtOH extracts than in the other extracts, and freeze drying effectively enriched polyphenols in CA extract than heated-air drying. Furthermore, TFC was the highest in FD-L-70E (25.1 mg QE/g), followed by HD-L-50E (24.2 mg QE/g), FD-W-70E (22.4 mg QE/g), FD-W-50E (17.4 mg QE/g). These results also suggested that freeze-drying is better in enriching TFC than heated-air drying. Our result indicate that both TPC and TFC were higher in freeze-dried extracts than that of heated-air-dried extracts, which is similar to the previous study reported by Mao et al. [23] who investigated the effect of both drying methods on the phenolic compounds of daylily flower extracts. However, the present study is the first to establish the relationship between antioxidant activity and secondary metabolite contents and various extracts of CA depending on the drying methods, plant parts, and extraction conditions.
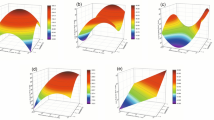
Meanwhile, scatter plot analysis was performed to compare the relationship between the antioxidant compounds (TPC and TFC) and antioxidant capacities (ABTS, DPPH, and FRAP) (Fig. 1). Linear regression on the scatterplot diagram indicated stronger positive correlations between TFC and antioxidant capacities (0.8547 < R2 < 0.9503), compared with the correlations between TPC and antioxidant capacities (0.7280 < R2 < 0.7734). Similar observation was reported by Singh et al. [21], who suggested that phenolic and flavonoid compounds are strongly correlated with the antioxidant capacity of CA.
Determination of various secondary metabolites by HPLC–DAD
Based on the above results, we next aimed to determine the known bioactive constituents in various CA extracts to investigate the correlation between the constituents and the antioxidant activity. As shown in Additional file 1: Fig. S1, we established the analytical conditions to quantify the 12 secondary metabolites, which were classified into three classes: triterpenes (madecassoside, asiaticoside, madecassic acid, and asiatic acid), polyphenolic acids (chlorogenic acid, caffeic acid, 3,4-dicaffeoylquinic acid, 1,5-dicaffeoylquinic acid, and ellagic acid), and flavonoids (cynaroside, scutellarin, and miquelianin). The chemical structures of the secondary metabolites were provided in Fig. 2. The contents of the individual secondary metabolites in various extracts are presented in Table 3. All the secondary metabolites analyzed were largely present in ethanolic extract groups (50E and 70E) than the water extract groups (CW and HW). In addition, as reported in many previous studies [25,26,27], our results showed that triterpene glycosides, mainly asiaticoside and madecassoside were the most abundant constituents in the CA extracts (Table 3). A similar study conducted by Alqahtani et al. [25] simultaneously analyzed a total of eight phytochemicals, including madecassic acid, madecassoside, asiatic acid, asiaticoside, in addition to three flavonoids (rutin, quercetin, and kaempferol) and one polyphenolic acid (chlorogenic acid) in CA extract.
Pearson correlation analysis was performed to determine the relationship between each secondary metabolite and antioxidant capacity. As provided in Table 4, Pearson correlation coefficient (r) indicated that asiaticoside, scutellarin, madecassoside, chlorogenic acid, and ellagic acid were strongly positively correlated (0.70 < r < 0.89) with radical scavenging capacities (ABTS and DPPH) and reducing ability (FRAP). However, madecassic acid, asiatic acid, caffeic acid, and 3,4-dicaffeoylquinic acid did not show any significant correlation with the antioxidant activities.
Principal component analysis
To analyze the relationship between the contents of 12 secondary metabolites and their antioxidant activity, we performed multivariate statistical analysis using PCA. The aim of PCA is to reduce the complex dimensionality by transforming the correlated variability, which acts as a summary of features [28, 29]. James et al. [30] investigated the changes in major triterpene glycosides and their aglycones during cell culture of CA via metabolomic profiling using chromatographic techniques and a PCA model. Figure 3 shows the biplot for the PCA correlation matrix among the variables of 16 extracts, 12 secondary metabolites, and three antioxidant activities. The results showed that the first and second principal components (PC1 and PC2) accounted for 78.6% of the total variance (63.6% and 15.0%, respectively). The group of three closely horizontal and very tightly knit variable markers for antioxidant activities, including ABTS, DPPH, and FRAP, suggests a group of highly correlated markers, which is also strongly correlated with PC1 (represented by the horizontal axis). That is, the antioxidant variables were similarly aligned as PC1, indicating that they are highly correlated with each other and are the most important contributors to PC1. In addition, similar to the Pearson correlation results shown in Table 4, the antioxidant activity variables showed a strong correlation with chlorogenic acid, scutellarin, and cynaroside, compared to other secondary metabolites. Interestingly, based on the position of the extract variables on the biplot, four sampling sites were mostly distinguished without overlapping, which indicates that each site possesses its own characteristics correlated with its composition and antioxidant activity. First, an intergroup separation trend was observed between the EtOH and water extracts along PC1. The EtOH extracts prepared from freeze-dried CA (FD-W-50E, FD-W-70E, FD-L-50E, and FD-L-70E) were highly related to madecassoside, asiaticoside, miquelianin, and 1,5-dicaffeoylquinic acid, compared with the other extracts. Furthermore, EtOH extracts derived from heated-air-dried CA (HD-W-50E, HD-W-70E, HD-L-50E, and HD-L-70E) were closely associated with asiatic acid and madecassic acid than that of the other extracts. Only caffeic acid showed a strong correlation with the group of hot-water extracts, whereas the cold-water extracts (HD-W-CW, HD-L-CW, HD-W-CW, and HD-L-CW) did not show any correlation with any secondary metabolites. Given the antioxidant capacity of the extracts identified in Table 4, our PCA results indicate that madecassoside and asiaticoside play crucial roles in the antioxidant activity of CA, compared to other examined secondary metabolites. However, miquelianin and 1,5-dicaffeoylquinic acid are considered to have relatively minimal effect on the antioxidant activity because they are relatively less amounts compared to madecassoside and asiaticoside in CA. Consequently, the PCA results indicate that the triterpenoid glycosides (madecassoside and asiaticoside) and not their aglycone forms (madecassic acid and asiatic acid, respectively), are primarily responsible for the antioxidant activity of CA. Moreover, freeze-drying rather than heated-air drying the raw material can enrich these secondary metabolites in the final extract, when extracted with ethanolic solution, rather than only water. Interestingly, the enrichment of aglycone forms in the heated-air-dried CA extracts suggests that the induced breakdown of the triterpenoid glycoside forms into their corresponding aglycone forms due to heated-air drying method, results in a decrease in the antioxidant activity of the CA extracts. In addition, although no significant difference was observed between whole plant and leaf parts, the ethanolic solution (50% and 70%) was more effective than water in terms of increased antioxidant capacities of CA.
Principal component analysis (PCA) biplot of C. asiatica extracts depending on postharvest drying methods, extraction conditions, secondary metabolites, and antioxidant activities. The ellipses mean clusters of correlated extracts according to first and second principal components (PC1 and PC2). The PC1 (63.6% explained variance) and PC2 (15.0% explained variance) accounted for 78.6% of the total variance. The group of three closely horizontal and very tightly knit variable markers for antioxidant activities, including ABTS, DPPH, and FRAP, indicates a group of highly correlated markers, whch is strongly correlated with PC1 (represented by the horizontal axis). 1,5-DCQA, 1,5-dicaffeoylquinic acid; 3,4-DCQA, 3,4-dicaffeoylquinic acid, AA, asiatic acid; AS, asiaticoside; CaA, caffeic acid; ChA, chlorogenic acid; Cy, cynaroside (Luteolin 7-O-β-glycoside); EA, ellagic acid; MA, madecassic acid; MS, madecassoside; Mi, miquelianin (quercetin 3-O-β-glucuronide); Sc, scutellarin (scutellarein 7-O-β-glucuronide)
The interesting pharmacological effects of madecassoside and asiaticoside have been investigated for the treatment of several diseases [31]. Numerous recent studies reported that triterpenoid glycosides, mainly madecassoside and asiaticoside, can attribute to various biological activities, such as neuro-protective and neuro-regenerative properties [32], wound healing and other dermatological properties [33], hepato-protective [34], pancreas-protective [35], anti-cancer [36], anti-inflammatory [37, 38], anti-obesity [39] properties. A previous study showed that cultivation with low water and low temperature affected the increase of madecassoside and asiaticoside contents in CA [40], but another study reported that silver nitrate treatment could enhance those contents during the cultivation period [40]. However, to our knowledge, the present study is the first to demonstrate the crucial role of triterpene glycosides in enhancing the expression of antioxidant activity in CA extract.
In the present study, various conditions including postharvest drying methods, plant parts, and extraction conditions were compared considering the secondary metabolite content and antioxidant capacity to optimize the extraction methods for CA. The application of statistical method, such as correlation analysis and PCA, is a useful tool in estimating activity-compound correlations. Our results indicate that the antioxidant activity of CA was primarily affected by triterpene glycosides, mainly madecassoside and asiaticoside, which can be enriched by EtOH extraction from freeze-dried CA. However, there was no difference in antioxidant capacities between the whole plant and leaf parts. Our results provide a valuable groundwork for the development of optimal extraction processes from medicinal herbs, including CA. However, further studies should assess the relationship between secondary metabolites and other biological activities.
Availability of data and materials
Not included, available upon request.
References
Gohil KJ, Patel JA, Gajjar AK (2010) Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci. https://doi.org/10.4103/0250-474X.78519
Brinkhaus B, Lindner M, Schuppan D, Hahn EG (2000) Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine. https://doi.org/10.1016/s0944-7113(00)80065-3
Pittella F, Dutra RC, Junior DD, Lopes MT, Barbosa NR (2009) Antioxidant and cytotoxic activities of Centella asiatica (L) Urb. Int J Mol Sci. https://doi.org/10.3390/ijms10093713
Nasution MY, Restuati M, Pulungan ASS, Pratiwi N, Diningrat DS (2018) Antimicrobial activities of Centella asiatica leaf and root extracts on selected pathogenic micro-organisms. J Med Sci. https://doi.org/10.3923/jms.2018.198.204
Masola B, Oguntibeju OO, Oyenihi AB (2018) Centella asiatica ameliorates diabetes-induced stress in rat tissues via influences on antioxidants and inflammatory cytokines. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2018.02.115
Hashim P (2011) Centella asiatica in food and beverage applications and its potential antioxidant and neuroprotective effect. Int Food Res J 18:1215–1222
Rao SB, Chetana M, Devi PU (2005) Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol Behav. https://doi.org/10.1016/j.physbeh.2005.07.019
Setorki M (2020) Medicinal herbs with anti-depressant effects. J Herbmed Pharmacol. https://doi.org/10.34172/jhp.2020.39
Bylka W, Znajdek-Awiżeń P, Studzińska-Sroka E, Brzezińska M (2013) Centella asiatica in cosmetology. Postepy Dermatol Alergol. https://doi.org/10.5114/pdia.2013.33378
Sangwan RS, Tripathi S, Singh J, Narnoliya LK, Sangwan NS (2013) De novo sequencing and assembly of Centella asiatica leaf transcriptome for mapping of structural, functional and regulatory genes with special reference to secondary metabolism. Gene. https://doi.org/10.1016/j.gene.2013.04.057
Suntornsuk L, Anurukvorakun O (2005) Precision improvement for the analysis of flavonoids in selected Thai plants by capillary zone electrophoresis. Electrophoresis. https://doi.org/10.1002/elps.200410203
Zainol MK, Abd-Hamid A, Yusof S, Muse R (2003) Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chem. https://doi.org/10.1016/S0308-8146(02)00498-3
Hsu C-L, Chen W, Weng Y-M, Tseng C-Y (2003) Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chem. https://doi.org/10.1016/S0308-8146(03)00053-0
Niamnuy C, Charoenchaitrakool M, Mayachiew P, Devahastin S (2013) Bioactive compounds and bioactivities of Centella asiatica (L.) Urban prepared by different drying methods and conditions. Dry Technol. https://doi.org/10.1080/07373937.2013.839563
Que F, Mao L, Fang X, Wu T (2008) Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int J Food Sci Technol. https://doi.org/10.1111/j.1365-2621.2007.01590.x
Kim TH, Kim WJ, Park SY, Kim H, Chung DK (2021) In vitro anti-wrinkle and skin-moisturizing effects of evening primrose (Oenothera biennis) sprout and identification of its active components. Processes. https://doi.org/10.3390/pr9010145
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. https://doi.org/10.1038/nprot.2007.102
Marinova D, Ribarova F, Atanassova M (2005) Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metallurgy 3:255–260
Reimann C, Filzmoser P, Garrett R, Dutter R (2011) Statistical data analysis explained: applied environmental statistics with R. In: Reimann C, Filzmoser P, Garrett R, Dutter R (eds) Principal component analysis (PCA) and factor analysis (FA). Wiley, Hoboken, pp 211–232
Papoutsis K, Pristijono P, Golding JB, Stathopoulos CE, Bowyer MC, Scarlett CJ et al (2017) Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int J Food Sci Technol. https://doi.org/10.1111/ijfs.13351
Singh S, Singh DR, Banu VS, A. N, (2014) Functional constituents (micronutrients and phytochemicals) and antioxidant activity of Centella asiatica (L.) Urban leaves. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2014.06.045
Hamid AA, Shah ZM, Muse R, Mohamed S (2002) Characterisation of antioxidative activities of various extracts of Centella asiatica (L) Urban. Food Chem. https://doi.org/10.1016/S0308-8146(01)00384-3
Mao L-C, Pan X, Que F, Fang X-H (2006) Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur Food Res Technol. https://doi.org/10.1007/s00217-005-0007-0
Orak HH, Aktas T, Yagar H, İsbilir SS, Ekinci N, Sahin FH (2012) Effects of hot air and freeze drying methods on antioxidant activity, colour and some nutritional characteristics of strawberry tree (Arbutus unedo L) fruit. Food Sci Technol Int. https://doi.org/10.1177/1082013211428213
Alqahtani A, Tongkao-on W, Li KM, Razmovski-Naumovski V, Chan K, Li GQ (2015) Seasonal variation of triterpenes and phenolic compounds in australian Centella asiatica (L.) Urb. Phytochem Anal. https://doi.org/10.1002/pca.2578
Hashim P, Sidek H, Helan MHM, Sabery A, Palanisamy UD, Ilham M (2011) Triterpene composition and bioactivities of Centella asiatica. Molecules. https://doi.org/10.3390/molecules16021310
Randriamampionona D, Diallo B, Rakotoniriana F, Rabemanantsoa C, Cheuk K, Corbisier A-M et al (2007) Comparative analysis of active constituents in Centella asiatica samples from Madagascar: application for ex situ conservation and clonal propagation. Fitoterapia. https://doi.org/10.1016/j.fitote.2007.03.016
Shin HY, Kim H, Kim JE, Lee KH, Bae YJ, Yu KW (2020) Bioactive compounds, anti-oxidant activities and anti-inflammatory activities of solvent extracts from Centella asiatica cultured in Chungju. Korean J Food Nutr. https://doi.org/10.9799/ksfan.2020.33.6.692
Lever J, Krzywinski M, Altman N (2017) Principal component analysis. Nat Methods. https://doi.org/10.1038/nmeth.4346
James JT, Tugizimana F, Steenkamp PA, Dubery IA (2013) Metabolomic analysis of methyl jasmonate-induced triterpenoid production in the medicinal herb Centella asiatica (L.) urban. Molecules. https://doi.org/10.3390/molecules18044267
Winitthana T, Niwattisaiwong N, Patarapanich C, Tantisira MH, Lawanprasert S (2011) In vitro inhibitory effects of asiaticoside and madecassoside on human cytochrome P450. Toxicol In Vitro. https://doi.org/10.1016/j.tiv.2011.02.010
Matthews DG, Caruso M, Murchison CF, Zhu JY, Wright KM, Harris CJ et al (2019) Centella asiatica improves memory and promotes antioxidative signaling in 5XFAD mice. Antioxidants. https://doi.org/10.3390/antiox8120630
Hou Q, Li M, Lu YH, Liu DH, Li CC (2016) Burn wound healing properties of asiaticoside and madecassoside. Exp Ther Med. https://doi.org/10.3892/etm.2016.3459
Zhang L, Li H-Z, Gong X, Luo F-L, Wang B, Hu N et al (2010) Protective effects of asiaticoside on acute liver injury induced by lipopolysaccharide/D-galactosamine in mice. Phytomedicine. https://doi.org/10.1016/j.phymed.2010.01.008
Wu K, Yao G, Shi X, Zhang H, Zhu Q, Liu X et al (2021) Asiaticoside ameliorates acinar cell necrosis in acute pancreatitis via toll-like receptor 4 pathway. Mol Immunol. https://doi.org/10.1016/j.molimm.2020.11.018
Zhou X, Lv Y, Lin T, Ke C, Dong F, Ren C et al (2020) Asiaticoside suppresses cell proliferation by inhibiting the NF-κB signaling pathway in colorectal cancer. Int J Mol Med. https://doi.org/10.3892/ijmm.2020.4688
Li H, Gong X, Zhang L, Zhang Z, Luo F, Zhou Q et al (2009) Madecassoside attenuates inflammatory response on collagen-induced arthritis in DBA/1 mice. Phytomedicine. https://doi.org/10.1016/j.phymed.2008.11.002
Sasmita AO, Ling APK, Voon KGL, Koh RY, Wong YP (2018) Madecassoside activates anti-neuroinflammatory mechanisms by inhibiting lipopolysaccharide-induced microglial inflammation. Int J Mol Med. https://doi.org/10.3892/ijmm.2018.3479
Sun B, Hayashi M, Kudo M, Wu L, Qin L, Gao M et al (2021) Madecassoside inhibits body weight gain via modulating SIRT1-AMPK signaling pathway and activating genes related to thermogenesis. Front Endocrinol. https://doi.org/10.3389/fendo.2021.627950
Ranjith GP, Jisha S, Hemanthakumar AS, Saji CV, Shenoi RA, Sabu KK (2021) Impact of potential stimulants on asiaticoside and madecassoside levels and expression of triterpenoid-related genes in axenic shoot cultures of Centella asiatica (L.) Urb. Phytochemistry. https://doi.org/10.1016/j.phytochem.2021.112735
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (PJ015285042021), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
HYS: Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft. HK: Investigation, Supervision, Writing—original draft, Writing—review and editing. SJ: Data curation, Methodology, Software, Validation. K-HL: Investigation, Project administration. Y-JB: Methodology, Validation, Visualization. HJS: Resources, Methodology, Validation. K-WY: Funding acquisition, Project administration, Supervision, Writing—original draft, Writing—review and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Extraction yield of various C. asiatica extracts. Figure S1. HPLC chromatogram of standard references and secondary metabolites of C. asiatica extracts. Standard materials and extracts were run in the HPLC system coupled with a PDA detector at 205 nm (A and C) and 325 nm (B and D). (a) Chromatogram of standard references (250 μg/mL, each), including madecassoside (MS), asiaticoside (AS), madecassic acid (MA), and Asiatic acid (AA); (b) Chromatogram of standard references (250 μg/mL, each), including chlorogenic acid (ChA), caffeic acid (CaA), cymaroside (CY), 3,4-dicaffeoylquinic acid (3,4-DCQA), 1,5-dicaffeoylquinic acid (1,5-DCQA), scutellarin (SC), miquelianin (MI), and ellagic acid (EA); (c) Representative chromatogram of extract (HD-L-50E; 5 mg/mL) to determine terpenoids. (d) Representative chromatogram of the extract (HD-L-50E; 5 mg/mL) to determine polyphenolic acids and flavonoids.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, H.Y., Kim, H., Jung, S. et al. Interrelationship between secondary metabolites and antioxidant capacities of Centella asiatica using bivariate and multivariate correlation analyses. Appl Biol Chem 64, 82 (2021). https://doi.org/10.1186/s13765-021-00656-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-021-00656-9