Abstract
Background
Cutibacterium acnes is part of the anaerobic skin microbiome and resides in deeper skin layers. The organism is an agent of surgical site infections (SSI) in shoulder surgery. We hypothesized that prolonged skin preparation with an agent that penetrates deeply into the skin would be beneficial. Thus, we compared two classes of antiseptics, each combined with alcohol, each applied with two different contact times.
Methods
Using a cross-over arrangement, shoulders of 16 healthy volunteers were treated for 2.5 min (standard) or 30 min (prolonged) with alcohol-based chlorhexidine (CHG-ALC) or alcohol-based povidone-iodine (PVP-I-ALC). Skin sites were sampled before, immediately after, and 3 h after treatment, using a standardized cup-scrub technique.
Results
Aerobic skin flora was reduced more effectively by PVP-I-ALC than by CHG-ALC after 2.5 min application and immediate sampling (reduction factor [RF] 2.55 ± 0.75 vs. 1.94 ± 0.91, p = 0.04), but not after prolonged contact times and 3-h sampling. Coagulase-negative staphylococci were completely eliminated after PVP-I-ALC application, but still recovered from 4 of 32 samples after CHG-ALC application. Anaerobic flora was reduced more effectively by PVP-I-ALC than CHG-ALC after standard (RF 3.96 ± 1.46 vs. 1.74 ± 1.24, p < 0.01) and prolonged (RF 3.14 ± 1.20 vs. 1.38 ± 1.16, p < 0.01) contact times and immediate sampling, but not after 3-h sampling. No adverse events were reported.
Conclusions
PVP-I-ALC showed marginal benefits concerning the aerobic flora, but more substantial benefits over CHG-ALC concerning the anaerobic flora of the shoulder. Standard and prolonged contact times showed superiority for PVP-I-ALC for anaerobic flora at all immediate sampling points, but missed significance at 3-h sampling. The results underscore the need for protection against C. acnes and coagulase-negative staphylococci in orthopaedic surgery. The clinical relevance of these findings, however, should be studied with SSI as an endpoint.
Similar content being viewed by others
Introduction
The skin flora of patients is one of the most important factors in the pathogenesis of surgical site infections (SSI) [1,2,3,4]. Skin antisepsis constitutes an effective measure to reduce the numbers of microorganisms on skin. Therefore, it has been included as a key measure to prevent SSIs in recent international guidelines and recommendations [5,6,7].
The choice of the right preoperative skin antiseptic has been the topic of intense research, both microbiologically and in the form of clinical trials, and the subject of intense debate and controversies. The debate frequently focused on comparisons between “chlorhexidine and povidone-iodine” and which one of the two would be better; however, this did not take into account the important role of alcohols as potent ingredients in combination antiseptics [8]. In fact, many comparisons in the literature consisted of unequal two-against-one comparisons, for example, CHG-ALC combinations against aqueous PVP-I, or of comparisons of antiseptics with unknown or inadequate active ingredient content [9, 10]. Uncertainty surrounding these questions is also reflected by differences between recommendations in recent major guidelines; while the US Centers for Disease Control and Prevention guideline recommends an alcohol-based antiseptic with either CHG or PVP-I [6], the World Health Organization guideline recommends CHG-ALC over PVP-I-ALC [5].
The resident aerobic skin flora consists of organisms such as coagulase-negative staphylococci (CNS), Micrococcus luteus, Corynebacterium spp., Malassezia furfur and Acinetobacter spp. [11]. The anaerobic skin flora is located primarily in hair follicles and sebaceous glands. One of its main constituents is Cutibacterium acnes (formerly Propionibacterium acnes) [12, 13]. One study of lower limb surgery found common colonizing organism on skin and surgical wound edges to be CNS (80%), Corynebacterium spp. (25%) and Cutibacterium spp. (15%) [14]. Likely due to improvements in microbiological techniques, C. acnes is increasingly detected as a cause of SSIs, particularly in prosthetic joint infections [13].
In shoulder surgery, C. acnes predominate as the main anaerobic organism in SSI, particularly when prosthetic material is implanted [15,16,17]. The main reservoir for C. acnes is located deep in the skin within hair follicles and the pilo-sebaceous glands. In one study of superficial and deep intraoperative tissue samples collected during surgery, C. acnes was isolated in more than 36% of patients who received first-time shoulder surgery [15]. In another study, the chance of obtaining C. acnes-positive cultures was 2.5-fold greater in males and was smaller when patients reported to have hair loss [18]. In addition, C. acnes can be involved in infections after hip and knee joint replacements, after endo-prosthetic reconstructions of the femur [19], polyurethane-coated breast implants [20] and various other implants [21].
Thus, the anaerobic skin flora represents a major challenge for skin antisepsis. Lee et al. [22] reported that 7 out of 10 volunteers had C. acnes detectable in dermal punch biopsies after skin antisepsis with 2% chlorhexidine gluconate (CHG) with 70% isopropanol (IPA). Experiments with excised human skin in diffusion chambers showed that CHG penetrates relatively poorly into deep skin layers [23], while iodine released from povidone (PVP) molecules possesses substantially better penetration capabilities and penetrates through full-thickness skin in relevant concentrations in a time-dependent fashion [24]. Thus, PVP-I with alcohol (PVP-I-ALC) is hypothesised to have a greater antimicrobial effect against the deep resident skin flora when compared to CHG with alcohol (CHG-ALC). Although PVP-I in contrast to CHG has no appreciable residual antimicrobial effect [25], it exerts a long-lasting effectiveness on skin due to the delayed release of iodine from PVP-I by a second-order reaction.
The aim of this work was to conduct a study with healthy volunteers, following similar procedures as outlined by the US Food and Drug Administration (FDA) and the American Society for Testing and Materials (ASTM) [26,27,28,29], but modified to test the effects on both aerobic and anaerobic skin flora after standard and prolonged application times on the shoulder region. Competitor antiseptics were a commercially-available 2% w/v CHG with 55% w/v IPA preparation and a commercially-available antiseptic containing PVP-I and alcohol (3.24% w/v PVP-I, 38.9% w/v IPA, 37.3% w/v ethanol).
Methods
Study design
This study was conducted using a randomized cross-over design with participation of 16 healthy volunteers, 9 female and 7 male healthy individuals of Caucasian background and an average age of 31.3 years (range: 22–74 years). Two different skin antiseptics (CHG-ALC or PVP-I-ALC) and two different contact times for each antiseptic (2.5 min or 30 min) were applied on day 1 and 4, based on the assumption that a period of 3 days is sufficient for complete re-colonization of the skin. The application was carried out on both shoulders, using two separate shoulder areas for sampling (immediate and 3-h values) on the antero-lateral site of each shoulder on each day (Fig. 1). Participants were randomized by drawing opaque folded paper tickets from a container, such that two different antiseptic treatments per day were represented in a cross-over design, and each volunteer completed four different treatments. The Ethics Committee of the University of Greifswald approved the study (Reg. No. BB 109/10).
Volunteers
Inclusion criteria were age > 18 years, legal capacity, informed consent after the study procedure was explained, as well as willingness and ability to comply with the test protocol. Exclusion criteria were macroscopically visible lesions of the skin except acne juvenilis, infections of the shoulder areas, dermatosis except acne, participation in another study within the last 30 days, pregnancy, lactation, thyroid disorders, intolerance to CHG or iodine, age under 18, therapy with radioactive iodine, antiseptic procedures on the designated areas within in the last 7 days and systemic antibiotics within the last 7 days. Two female volunteers suffered from active acne juvenilis at the shoulder areas that was less pronounced in the face; this was confirmed dermatologically.
Tested compounds and application modes
Skin antisepsis was performed using the following commercially available products:
-
ChloraPrep® (CHG 2% w/v, IPA 55% w/v [70% v/v], coloured; CareFusion, Leawood, USA) with applicators: vigorous rubbing using the 26 ml applicator for 30 s, afterwards keeping the treated area moist for 2 min with the antiseptic.
-
Chlorhexidine 2% alcoholic (CHG 2% w/v, IPA 55% w/v [70% v/v]; B. Braun Medical, Sempach, CH): vigorous rubbing for 30 s using sterile forceps and gauze for 30 s, afterwards keeping the area moist with the antiseptic using a soaked sterile dressing (Zetuvit®, Hartmann, Heidenheim, Germany, 20 × 40 cm) for 29.5 min.
-
Betaseptic® (3.24% w/v PVP-I, 38.9% w/v IPA, 37.3% w/v ethanol; Mundipharma, Limburg, Germany): vigorous rubbing for 30 s by using sterile forceps and gauze for 30 s, afterward keeping the area moist with the antiseptic by using forceps and gauze for 2 min or a soaked sterile dressing (Zetuvit®, 20 × 40 cm) for 29.5 min.
While the two CHG-ALC preparations had identical composition, one came with applicators, the other without. The preparation without applicators was necessary for the 30-min application, in order to keep the skin areas moist with soaked dressings for 30 min. For the two CHG-ALC products, the IPA percentage was converted to 55% w/v from the manufacturers’ stated 70% v/v for the purpose of uniformity of measurements.
Sampling
Microbial skin counts were obtained before antiseptic application (pre-values), after application and air-drying of the sampling site (immediate post-values), and 3 h after treatment (3-h post-values). The cup-scrub technique according to ASTM E1874-14 [30] was used on 2.5 cm2 of skin, with 1 mL of sampling solution consisting of 0.9% sterile saline.
A first 10–1 dilution was prepared by adding 0.4 mL of sampling solution to 3.6 mL of neutralizer solution. For CHG-containing antiseptics, this was Lipofundin MCT 20% (B. Braun, Melsungen, Germany), for PVP-I-containing antiseptics, this was 3% Tween 80 (BioChemica, AppliChem, Darmstadt, Germany), 0.3% lecithin (AppliChem), 0.1% l-histidine (Roth, Nürnberg, Germany) and 0.5% sodium thiosulfate (Merck, Darmstadt, Germany).
Concurrently with the retrieval of the immediate post-values, another skin area of 4 × 4 cm was covered with a sterile dressing (Hydrofilm® transparent dressing 12 × 25 cm, Hartmann, Heidenheim, Germany) to protect a skin area where the 3-h samples were to be collected later.
Microbiological techniques
After 5 min neutralization in the first 10–1 dilution, further dilutions of 10–2 and 10–3 were prepared in the respective neutralization solution, and 0.1 mL of each dilution was plated onto Columbia agar with 5% sheep blood (Becton Dickinson, Heidelberg, Germany) for aerobic incubation (37 °C, 48 h) and onto Schaedler agar (BioMérieux, Nürtingen, Germany) for anaerobic incubation (37 °C, 7 days). The anaerobic atmosphere was generated in anaerobic jars using Anaerocult A sachets (Merck, Darmstadt, Germany). After aerobic incubation, the colony forming units (CFU) were counted and a representative sample of colonies was picked for identification, such that at least one colony of each morphologically different colony type was tested. Isolates were subjected to simple phenotypic identification, including Gram stain, catalase and coagulase tests, followed by VITEK® Cards (BioMérieux). The anaerobic CFUs were counted after 7 days incubation, and again representative colonies were analysed by Gram stain and VITEK® Cards.
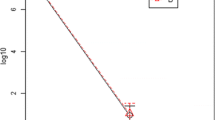
For uniformity of measurements, we converted the numbers of colonies counted to CFU per 5 cm2 of skin and expressed these as log10 values. Then, we calculated the reduction factors (RFs) as the differences between the log10 pre-values and the log10 post-values. To calculate the reduction factors and transform to log10, plates without growth were set to a value of 1.
Sampling and validation of neutralization
Skin bacteria from five volunteers were collected by the cup-scrub technique [30] and pooled. Using the methodology of ASTM E1054-08 [31], pooled bacteria were used to verify the effectiveness of Lipofundin to inactivate CHG, of sodium thiosulfate to diminish the oxidizing agent iodine, and to ensure that the inactivation solutions did not significantly influence the bacterial counts, quantitatively and qualitatively. Final concentrations of 0.4% CHG and 0.6% PVP-I in 1 mL 0.9% NaCl were tested for neutralizer effectiveness. The concentration of the active agent was calculated using the treated skin area of 300 cm2 (17.5 cm × 17.5 cm) with 17 mL of antiseptic solution from the applicator and 3 mL of additional antiseptic solution, which arises from the equilibrium of the soaked dressing with the antiseptic liquid film on the skin. Finally, an area of 2.5 cm2 served as the basis for the microbiological examinations after scrubbing.
Validation of neutralization was conducted according to the methodology of ASTM E1054-08 [31]. The suitable neutralizers, lecithin for inactivating biguanides and thiosulfate for quenching iodine, were derived from Table 1 in ASTM E1054 [31] and Annex B in EN 1040 [32] and EN 13727 [33]. Lipofundin containing 1.2% egg yolk lecithin inactivated 0.4% CHG without any inhibitory effect on growth of pooled skin bacteria after aerobic and anaerobic incubation, and sodium thiosulfate, the quenching agent for iodine in the neutralization mixture, was effective for 0.6% PVP-I without influencing bacterial counts. Similar results were obtained in tests of neutralizer effectiveness, neutralizer toxicity and organism viability under aerobic and anaerobic culture conditions, using test solutions containing the residual antimicrobial agent which were derived from volunteers after skin antisepsis. Therefore, it was ascertained that CHG or PVP-I were effectively inactivated by the respective neutralization solutions without influencing bacterial growth after aerobic and anaerobic incubation.
Statistical analysis
The analysis of the raw data was performed using Graphpad Prism (GraphPad, La Jolla, CA, USA) and SPSS (IBM, Armonk, NY, USA) software. Mann–Whitney and Wilcoxon tests were calculated. A p-value < 0.05 was considered statistically significant. Presence of carry-over effects was tested using linear mixed models, including treatment, sequence, period and treatment × period interaction effects (MIXED procedure in SPSS). Sample size calculations for testing differences in RFs were based on two-sided Wilcoxon signed-rank tests for matched pairs at p = 0.05. Effect sizes (i.e. differences in RFs divided by the standard deviation of the differences) of 0.75 and 1.0 were assumed. Based on a required power of 0.80, results of sample size calculation indicated that a sample between n = 10 and n = 16 cases was required (G*Power 3.1).
Skin tolerability
All volunteers received a questionnaire for self-assessment of skin tolerability to evaluate the following parameters on an analogue scale from 1 to 10. Items “redness”, “burning”, “pruritus”, “scaliness”, and “pain” were assessed. In case of skin irritation, volunteers were asked to contact the investigators to have the nature of the irritation evaluated, and if necessary, to obtain treatment.
Results
Skin tolerability
The skin antiseptics were well tolerated after 2.5 and 30 min exposure without any irritations. None of the volunteers reported any of the five listed adverse events on the skin tolerability scale.
Pre-values
The validity of the cross-over design was confirmed by a comparison of the pre-values on day 1 and day 4. There was no significant difference (mean log value day 1 aerobically, 2.23, standard deviation [SD], 0.80, 95% confidence interval [CI], 1.80–2.66; mean log value day 4 aerobically, 2.17, SD, 0.88, 95% CI, 1.70–2.64; mean log value day 1 anaerobically, 3.68, SD, 1.47, 95% CI, 2.90–4.46; mean log value day 4 anaerobically, 3.80, SD 1.44, 95% CI, 3.03–4.57).
Aerobic skin flora
The aerobic flora consisted of more than 70% of CNS (mainly S. epidermidis, S. hominis, S. saprophyticus and S. lugdunensis). In addition, S. aureus (6% of aerobic flora) and M. luteus were found on the aerobic plates. PVP-I-ALC was significantly more effective than CHG-ALC when applied for 2.5 min, at the sampling time immediately after application (Tables 1 and 2), but this was not the case for the prolonged application time of 30 min and not for any 3-h values. There was no difference between the short and prolonged application times for each of the antiseptic agents, both immediately and 3 h after application (p = 0.09 and p = 0.16 for PVP-I-ALC; p = 0.37 and p = 0.51 for CHG-ALC). No statistically significant period or sequence effects or treatment × period interaction were found (p-values for immediate effect: 0.911 [period], 0.175 [sequence] and 0.987 [treatment × period]; p-values for 3 h effect: 0.197 [period], 0.213 [sequence] and 0.489 [treatment × period]).
While CNS were not found in any samples after PVP-I-ALC treatment under any of the application and sampling conditions, they were still recovered with counts of up to 5 CFU per sample after CHG-ALC treatment from 3 of 16 samples after 2.5-min application and 1 of 16 samples after 30 min application and immediate sampling, respectively. None of the samples collected after 3 h yielded CNS.
Anaerobic skin flora
The majority of the bacteria on anaerobic plates were C. acnes. Only a negligible number of other anaerobic species were recovered and a few anaerobic cultures yielded CNS. When comparing the RFs of the immediate post-values of PVP-I-ALC to the immediate post-values of CHG-ALC, the antisepsis with PVP-I-ALC was significantly more effective for both application times (Tables 1 and 2). Comparing the 3-h post-values, PVP-I-ALC applied for 30 min was significantly more effective than CHG-ALC applied for 2.5 min (p < 0.01), but not more effective than CHG-ALC applied for 30 min (p = 0.06; Table 2). When looking at the short (2.5 min) versus prolonged (30 min) application times, there was only one significant difference, in that PVP-I-ALC applied for 2.5 min appeared better than PVP-I-ALC applied for 30 min after immediate sampling, while all other 2.5–30 min comparisons showed no significant differences (p = 0.03 and p = 0.24 for PVP-I-ALC; p = 0.36 and p = 0.87 for CHG-ALC). However, we consider this single significant value a likely experimental outlier, because the log pre-values for the 2.5 min application of PVP-I were substantially higher (4.24 ± 1.27) than those for the 30 min application (3.50 ± 1.40), and in both instances, there was a majority of immediate post-values (12 and 13 of 16, respectively) with no CFU counts. Again, no statistically significant period or sequence effects or treatment × period interaction were found (p-values for immediate effect: 0.537 [period], 0.568 [sequence] and 0.584 [treatment × period]; p-values for 3 h effect: 0.392 [period], 0.230 [sequence] and 0.710 [treatment × period]).
Discussion
The results of our study demonstrate that both antiseptic compounds, CHG-ALC and PVP-I-ALC effectively decreased the aerobic skin flora at shoulder sites. No growth at all was observed in 60 of 64 immediate post-values and in 49 of 64 3-h post-values. In aerobic cultures, PVP-I-ALC was better than CHG-ALC after 2.5 min contact time and immediate sampling (Table 2), but not in any other of the tested parameters, including prolonged application time and sampling after 3 h. The relative improvement of performance of CHG-ALC after prolonged application and late sampling appears consistent with the relatively slow skin penetration kinetics of CHG [23].
CHG-ALC did not completely eliminate CNS in some samples. This is consistent with findings from an earlier study in which CNS were frequently detected in the surgical field after 3 min of preoperative skin antisepsis with CHG-ALC (unpublished findings). This is also consistent with data from another study [4] that showed growth of residual bacteria directly after skin antisepsis with 70% v/v isopropanol in 35% of operations in orthopedic surgery. Among the isolates, 53% were identified as CNS [4]. These data underscore the need for protection against CNS in surgery by potent antisepsis.
More pronounced differences between the antiseptic compounds became apparent when tested against the anaerobic skin flora. PVP-I-ALC, when applied for 2.5 or 30 min, was better than CHG-ALC at all four immediate sampling points (Table 2), but not at the relevant 3-h sampling points when both received the equivalent applications times of 2.5 and 30 min, respectively. Overall, 6 of a total of 16 mean RFs obtained in this study were statistically significantly better for PVP-I-ALC than for CHG-ALC, and 15 out of 16 total measurement comparisons were in simple numerical terms better for PVP-I-ALC (Table 1). On the other hand, no relevant statistically significant differences were observed in any comparisons of the same agents between 2.5 min and 30 min application time.
Our results are in line with a clinical trial that compared the treatment of abdominal incision sites [34]. In this trial, 0.7% iodine povacrylex with 74% IPA was more effective in reducing SSI than 2% CHG with 70% IPA [34]. Another trial comparing 0.5% CHG with 70% alcohol and 1% PVP-I with 70% alcohol in hip and knee arthroplasty showed no difference in superficial wound complications [35]. However, on secondary endpoint analysis, skin antisepsis with CHG-ALC was associated with significantly higher odd ratios for overall SSI, including prosthetic joint infection [35]. In another trial, skin antisepsis with 7.5% PVP-I in aqueous solution did not show a significant difference in SSI rates to 2% CHG in 70% IPA after neurosurgical spine procedures in adults [36].
The shoulder region was chosen as test area because it is known to be colonized with C. acnes and has a high density of hair follicles and sebaceous glands [37]. C. acnes is a major pathogen of SSI in shoulder surgery [38, 39]. The standard method for preoperative skin antisepsis consists of treating the skin with either an applicator or with forceps and gauze for 30 s, followed by keeping the skin moist with the antiseptic for 2 min. The efficacy of both application methods is similar [40].
It is considered beneficial to use an extended contact time on skin areas that have a high density of sebaceous glands [41]. According to the manufacturer of the PVP-I-ALC solution that we used, the product’s recommended contact times are 1 min for skin with a low density and 10 min for skin with a high density of sebaceous glands. The contact time of 30 min in the present study was chosen because we reasoned that with an extended contact time, the deep skin flora would be targeted more effectively. If confirmed, this would have considerable implications for antiseptic preparation of the shoulder area. Indeed, after a contact time of 29.5 min under a soaked sterile dressing, the area still appeared to be moist.
It is thought that the physiological flora of the human skin is regenerated completely after 3 days, because the re-colonization starts about 60 min after alcohol-based skin antisepsis [42]. After 24 h, the skin flora is nearly completely regenerated [43]. Thus, the study was performed as a crossover study with an interval of 3 days between the tests. In line with our hypothesis, there was no significant difference between the pre-values on day 1 and day 4.
The validity and interpretation of our results depends heavily on the selection of effective neutralizing solutions. While this applies to all antiseptics, it is particularly relevant to CHG, because false-positive efficacy assessment in the absence of adequate neutralizers has been reported [44]. Soy bean or egg yolk lecithin is considered to be an effective neutralizer for CHG, and we used Lipofundin, which contains 1.2% egg yolk lecithin and has been previously shown to be suitable and effective [45].
Our study has two important limitations. First, an ideal trial should compare CHG and PVP-I combined with the same alcohol species with identical concentrations, if conclusions concerning the activity of the CHG or PVP-I component are to be made. However, we had to use readily available commercial formulations, due to the fact that PVP-I formulations are too difficult to prepare in-house. The CHG comparator contained 55% w/v (70% v/v) straight IPA, and the PVP-I comparator contained 76% w/v of a mix between IPA and ethanol in nearly equal parts. Therefore, there is a possibility that this study’s main results may be due to the different alcohol compositions. Future studies may be able to address the microbicidal activity of CHG and PVP-I when combined with equal comparator alcohols. Second, a majority of our immediate and 3-h post-values had no detectable CFUs, and this was more frequently observed for PVI-ALC than for CHG-ALC (Table 1). This means that our study unintentionally captured measurement values that were commonly located at the bottom end of the measurable range of the experimental setup. This may be owed to the experimental conditions, including the size of the sampled skin sites and sample fractions plated on agar media, in combination with the relatively small number of 16 participants. As a result, it appears likely that any differences between the two antiseptics, contact times and immediate versus 3-h effects were underestimated. A scenario in which a larger number of the measured values would be located well within the measurable range would have a greater chance of showing statistically significant differences if they exist. This appears particularly likely for the comparison of PVP-I-ALC versus CHG-ALC after 30 min contact time and sampling after 3 h, where the P value was 0.06, but 10 samples showed no detectable growth for PVP-I-ALC versus 7 for CHG-ALC. Future studies may be able to address this with larger numbers of volunteers, larger sampled skin sites, larger sample volumes or lower starting dilutions (e.g. neat, 10–1, 10–2 instead of 10–1, 10–2, 10–3) plated on agar, or a combination of these variables.
Commonly recommended contact times for surgical skin preparation, including the 2.5 min chosen in this study, are neither experimentally nor clinically well founded. Starting from the hypothesis that a prolonged application time achieves better penetration and reaches deeper skin compartments and hair follicles, we decided to examine a contact time of 30 min in addition to 2.5 min. However, no relevant significant differences were observed between these contact times. In addition, the question arises whether a contact time of 30 min is practicable in a busy operating room setting. This means that contact times shorter than 30 min should be investigated in future studies. For example, some antiseptic preparations, when applied for 2.5 min on areas with high density of sebaceous glands, meet or even exceed the efficacy of the experimental reference antiseptic that is applied for 10 min [46]. The 3-h values in our study aimed at assessing the sustained activity of the antiseptic and the intraoperative skin re-colonization under surgical drapes, as would be expected during typical operations.
Common efficacy testing of skin antisepsis only assesses aerobic flora, and in Europe the samples are typically collected by swabbing the skin surface [47] and do not mobilise the deep resident skin flora to the same extent as the ASTM cup scrub method does [30]. One possibility to address this in future studies might be to take dermal punch biopsies after antisepsis, so that the effects of prolonged contact times can be measured in deeper skin layers.
Conclusions
While there was marginal superiority of PVP-I in combination with alcohol (3.24% w/v with ≥ 76% w/v alcohol) over 2% w/v CHG with 55% w/v IPA concerning the aerobic flora, there was more pronounced superiority concerning the anaerobic flora on the shoulder. PVP-I-ALC was clearly superior in its immediate efficacy in reducing the anaerobic skin flora. No significant difference was seen between standard and prolonged contact times of either agent. PVP-I-ALC seems to be a promising option for antisepsis on skin with a high density of sebaceous glands at a contact time of 2.5 min, especially in shoulder surgery. Future studies should expand upon these investigations with greater numbers of participants and contact times closer to 2.5 min, and ultimately should focus on clinical trials with SSIs as the endpoint.
Availability of data and materials
Original (de-identified) data are available from the corresponding author upon reasonable request.
Change history
26 April 2021
The original article has been corrected to include an OA funding note.
References
Altemeier WA, Culbertson WR, Hummel RP. Surgical considerations of endogenous infections—sources, types, and methods of control. Surg Clin N Am. 1968;48:227–40.
Bitkover CY, Marcusson E, Ransjo U. Spread of coagulase-negative staphylococci during cardiac operations in a modern operating room. Ann Thorac Surg. 2000;69:1110–5.
Kuhme T, Isaksson B, Dahlin LG. Wound contamination in cardiac surgery. A systematic quantitative and qualitative study of the bacterial growth in sternal wounds in cardiac surgery patients. Acta Pathol Microbiol Immunol Scand. 2007;115:1001–7.
Daeschlein G, Napp M, Layer F, et al. Antimicrobial efficacy of preoperative skin antisepsis and clonal relationship to postantiseptic skin-and-wound flora in patients undergoing clean orthopedic surgery. Eur J Clin Microbiol Infect Dis. 2015;34:2265–73.
World Health Organization. Global guidelines for the prevention of surgical site infection. Geneva: WHO; 2016.
Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784–91.
Hansis M, Kramer A, Mittelmeier W, et al. Prevention of surgical site infections—recommendations of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert-Koch-Institute. Bundesgesundheitsblatt. 2018;61:448–73.
Maiwald M, Chan ES. The forgotten role of alcohol: a systematic review and meta-analysis of the clinical efficacy and perceived role of chlorhexidine in skin antisepsis. PLoS ONE. 2012;7:e44277.
Maiwald M, Petney TN, Assam PN, et al. Use of statistics as another factor leading to an overestimation of chlorhexidine’s role in skin antisepsis. Infect Control Hosp Epidemiol. 2013;34:872–3.
Maiwald M, Widmer AF. WHO’s recommendation for surgical skin antisepsis is premature. Lancet Infect Dis. 2017;17:1023–4.
Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82.
Funke G, Graevenitz A, Clarridge JE, et al. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997;10:125–59.
Boisrenoult P. Cutibacterium acnes prosthetic joint infection: Diagnosis and treatment. Orth Traumatol Surg Res. 2018;104:S19–24.
Turtiainen J, Hakala T, Hakkarainen T, et al. The impact of surgical wound bacterial colonization on the incidence of surgical site infection after lower limb vascular surgery: a prospective observational study. Eur J Vasc Endovasc Surg. 2014;47:411–7.
Hudek R, Sommer F, Kerwat M, et al. Propionibacterium acnes in shoulder surgery: true infection, contamination, or commensal of the deep tissue? J Shoulder Elbow Surg. 2014;23:1763–71.
Kadler BK, Mehta SS, Funk L. Propionibacterium acnes infection after shoulder surgery. Int J Shoulder Surg. 2015;9:139–44.
Brolin TJ, Hackett DJ, Abboud JA, et al. Routine cultures for seemingly aseptic revision shoulder arthroplasty: are they necessary? J Shoulder Elbow Surg. 2017;26:2060–6.
Hudek R, Sommer F, Abdelkawi AF, et al. Propionibacterium acnes in shoulder surgery: is loss of hair protective for infection? J Shoulder Elbow Surg. 2016;25:973–80.
Dramis A, Aldlyami E, Grimer RJ, et al. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33:829–33.
Rieger UM, Djedovic G, Pattiss A, et al. Presence of biofilms on polyurethane-coated breast implants: preliminary results. J Long Term Eff Med Implants. 2016;26:237–43.
Portillo ME, Corvec S, Borens O, et al. Propionibacterium acnes: An underestimated pathogen in implant-associated infections. Biomed Res Int. 2013;2013:804391.
Lee MJ, Pottinger PS, Butler-Wu S, et al. Propionibacterium persists in the skin despite standard surgical preparation. J Bone Joint Surg Am. 2014;96:1447–50.
Karpanen TJ, Worthington T, Conway BR, et al. Penetration of chlorhexidine into human skin. Antimicrob Agents Chemother. 2008;52:3633–6.
Nesvadbova M, Crosera M, Maina G, et al. Povidone iodine skin absorption: an ex-vivo study. Toxicol Lett. 2015;235:155–60.
Müller G, Langer J, Siebert J, et al. Residual antimicrobial effect of chlorhexidine digluconate and octenidine dihydrochloride on reconstructed human epidermis. Skin Pharmacol Physiol. 2014;27:1–8.
Food and Drug Administration (FDA). Tentative final monograph for health-care antiseptic drug products; proposed rule. 21 CFR parts 333 and 369. Fed Regist. 1994;59(116):31402–52.
American Society for Testing and Materials. ASTM E1173-15. Standard test method for evaluation of preoperative, precatheterization, or preinjection skin preparations. West Conshohocken: ASTM International; 2015.
Art G. Comparison of the safety and efficacy of two topical antiseptic products: chlorhexidine gluconate + isopropyl alcohol and povidone-iodine + isopropyl alcohol. JAVA. 2007;12(3):156–63.
Bashir MH, Olson LKM, Walters SA. Suppression of regrowth of normal skin flora under chlorhexidine gluconate dressings applied to chlorhexidine gluconate-prepped skin. Am J Infect Control. 2012;40:344–8.
American Society for Testing and Materials. ASTM E1874-14. Standard test method for recovery of microorganisms from skin using the cup scrub technique. West Conshohocken: ASTM International; 2014.
American Society for Testing and Materials. ASTM E1054-08. Standard test methods for evaluation of inactivators of antimicrobial agents. West Conshohocken: ASTM International; 2013.
European Committee for Standardization. EN 1040:2005. Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of basic bactericidal activity of chemical disinfectants and antiseptics. Test method and requirements (phase 1). Brussels: CEN; 2006.
European Committee for Standardization. EN 13727:2012 + A2:2015. Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of bactericidal activity in the medical area. Test method and requirements (phase 2, step 1). Brussels: CEN; 2015.
Swenson BR, Hedrick TL, Metzger R, et al. Effects of preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol. 2009;30:964–71.
Peel TN, Dowsey MM, Buising KL, et al. Chlorhexidine-alcohol versus iodine-alcohol for surgical site skin preparation in an elective arthroplasty (ACAISA) study: a cluster randomized controlled trial. Clin Microbiol Infect. 2019;25:1239–45.
Ghobrial GM, Wang MY, Green BA, et al. Preoperative skin antisepsis with chlorhexidine gluconate versus povidone-iodine: a prospective analysis of 6959 consecutive spinal surgery patients. J Neurosurg Spine. 2018;28:209–14.
Patel A, Calfee RP, Plante M, et al. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg. 2009;18:897–902.
Athwal GS, Sperling JW, Rispoli DM, et al. Deep infection after rotator cuff repair. J Shoulder Elbow Surg. 2007;16:306–11.
Singh JA, Sperling JW, Schleck C, et al. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21:1534–41.
Ulmer M, Lademann J, Patzelt A, et al. New strategies for preoperative skin antisepsis. Skin Pharmacol Physiol. 2014;27:283–92.
Hübner NO, Kellner NB, Partecke LI, et al. Determination of antiseptic efficacy of rubs on the forearm and consequences for surgical hand disinfection. J Hosp Infect. 2011;78:11–5.
Falk-Brynhildsen K, Söderquist B, Friberg O, et al. Bacterial recolonization of the skin and wound contamination during cardiac surgery: a randomized controlled trial of the use of plastic adhesive drape compared with bare skin. J Hosp Infect. 2013;84:151–8.
Dettenkofer M, Jonas D, Wiechmann C, et al. Effect of skin disinfection with octenidine dihydrochloride on insertion site colonization of intravascular catheters. Infection. 2002;30:282–5.
Kampf G, Reichel M, Hollingsworth A, Bashir M. Efficacy of surgical hand scrub products based on chlorhexidine is largely overestimated without neutralizing agents in the sampling fluid. Am J Infect Control. 2013;41(1):e1–5.
Welk A, Zahedani M, Beyer C, et al. Antibacterial and antiplaque efficacy of a commercially available octenidine-containing mouth rinse. Clin Oral Invest. 2016;20:1469–76.
Kampf G, Pitten FA, Heeg P, et al. Efficacy of two ethanol-based skin antiseptics on the forehead at shorter application times. BMC Microbiol. 2007;7:85.
Reichel M, Heisig P, Kohlmann T, et al. Alcohols for skin antisepsis at clinically relevant skin sites. Antimicrob Agents Chemother. 2009;53:4778–82.
Acknowledgements
We thank Dr. John C. Allen, Duke-National University of Singapore Graduate Medical School, Singapore, for statistical advice.
Funding
Open Access funding enabled and organized by Projekt DEAL. Mundipharma GmbH provided funding for this work. All three antiseptic companies (Mundipharma GmbH, B. Braun Medical AG, CareFusion Inc.) provided antiseptics free of charge, and Hartmann AG provided sterile dressings free of charge.
Author information
Authors and Affiliations
Contributions
D.D., A.K., and G.D. had the idea for the study and planned and supervised the experiments, analysed and interpreted the data, and drafted the first version of the manuscript. G.M. and R.H. participated in the technical design of the study, performed laboratory measurements and analysed and interpreted the data. G.K., M.M., and O.A. participated in the study’s design and coordination, analysed and interpreted the data, and revised the manuscript. T.K. analysed and interpreted the data, and conducted the statistical analyses. All authors participated in drafting the manuscript and revising it critically for important intellectual content, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the University of Greifswald (Reg. No. BB 109/10).
Consent for publication
Not applicable.
Competing interests
Mundipharma GmbH, the sponsor of the study, had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors declare no conflict of interest in relation to this work. O. Assadian and A. Kramer report institutional and consultant advisory grants from B. Braun Medical AG, Becton Dickinson, CareFusion Inc., J&J/Ethicon, Mundipharma GmbH, Schülke & Mayr GmbH, Smith & Nephew Ltd., and Uluru Inc. outside the submitted work in the past.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dörfel, D., Maiwald, M., Daeschlein, G. et al. Comparison of the antimicrobial efficacy of povidone-iodine-alcohol versus chlorhexidine-alcohol for surgical skin preparation on the aerobic and anaerobic skin flora of the shoulder region. Antimicrob Resist Infect Control 10, 17 (2021). https://doi.org/10.1186/s13756-020-00874-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-020-00874-8