Abstract
Background
Frequency of extended-spectrum-β-lactamase-producing clinical isolates is increasing worldwide. This is a multi-center study which was aimed to check the frequency of third-generation cephalosporin resistance and distribution of the key genetic determinants of Extended-spectrum-β-lactamase-producing Clinical isolates in Pakistan.
Methods
A total of 2372 samples were processed in three tertiary care hospitals and one diagnostic research center of Lahore, Pakistan during Aug-2014 to Sep-2017. Analytical profile index (API 20-E) was used for biochemical characterization of isolates. Antibiotic susceptibility testing (AST) and third generation cephalosporin resistant (3GC-R) isolates were subjected to: double disc synergism test (DDST), combination disc test (CDST) and epsilometric test (E-test) for confirmation of ESBL-production. PCR amplification of isolates with plasmid and genomic DNA was performed. Amplicon sequences were checked for gene-variants and statistical analyses were performed to check the significance of data.
Results
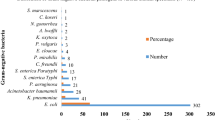
A total of 497/995 (50%) isolates including Escherichia coli 65% (n = 321), Klebsiella spp. 25% (n = 124) and Pseudomonas. 5% (n = 24), Enterobacter spp. 4% (n = 20) and Acinetobacter spp. 2% (n = 8) were screened as third generation cephalosporin resistant (3GC-R). Urine 56% (n = 278) followed by pus 20% (n = 99) and wound swab 6% (n = 29) were frequent sources. Incidence of ESBL-producers detected by combination disc test was 79% (n = 392). PCR revealed blaCTX − M (76%) gene followed by blaOXA (52%), blaTEM (28%) and blaSHV (21%) were most prevalent among ESBL-producers detected by CDST. blaCTX − M − 1(65%), blaOXA (78%) and blaTEM (57%) genes were carried on plasmids. Amplicon sequencing revealed blaCTX − M − 15 (75%), blaOXA − 1 (49%) and blaTEM − 1B (34%) and 21 (n = 28) isolates carried three genes in them.
Conclusion
Prevalence of ESBL-producing isolates has increased 1.13 folds during study years. Isolates had high prevalence of ESBL-encoding blaCTXM − 15 gene and narrow spectrum blaOXA − 1 and blaTEM − 1B were also prevalent.
Similar content being viewed by others
Background
Multidrug resistant clinical isolates have important clinical consequence in community and hospital settings [1]. They have evolved as a global concern, exacerbated by under reporting in some regions of the world [2]. The tendency of these isolates concurrently resistant to other groups of antibiotics significantly limits the selection of antibiotics for treatment of infections [3]. The development of resistance for third generation cephalosporin is attributed to production of β-lactamases including extended-spectrum-β- lactamases (ESBLs), AmpCs and carbapenemases [4]. The most significant β-lactamase genes are variants of CTX-M, SHV, TEM, VEB, GES, PER, TLA and OXA which have broadened the substrate specificity against ceftazidime, cefotaxime and ceftriaxone [4, 5]. These genes have broad host range but predominantly found in Escherichia coli and Klebsiella spp. [6]. While, OXA genes are found predominantly in Pseudomonas spp. and Acinetobacter spp. [7]. Moreover, many clinical pathogens harbor more than one β-lactam genes [8]. Plasmid association of these genes makes them easily spreadable. Due to the diversity of these enzymes, multiplex-PCR based detection methods have become a widely used tool for epidemiological surveys [8,9,10].
Asian countries are highly affected by extended spectrum-β-lactamase-producers inducing multidrug-resistant phenotype [11,12,13,14]. Several studies have reported the community-association of ESBL-producers [11, 14, 15]. In Pakistan, an increase in the number of ESBL-associated infections has been observed in last few decades [16,17,18,19,20,21]. Lack of regular surveillance programs at national or international levels, inadequate infection control agencies, lack of facilities and inappropriate diagnostic approaches contribute to the emergence of the antibiotic resistance in bacteria [2, 10, 22]. Moreover, dissemination of these isolates in the community demands the urgent call for surveillance of resistance and molecular characterization for extended-spectrum-β-lactamase-producers [23]. This study was designed to check molecular epidemiology of blaCTX−M, blaTEM, blaSHV and blaOXA genes among ESBL-producers in Pakistani population to have a generalized view about the situation in our region.
Materials and Methods
Study design
This cross-sectional study was conducted at the Department of Microbiology and Molecular Genetics, University of the Punjab, Lahore in collaboration with the Department of Pathology, Allama Iqbal Medical College/ Jinnah Hospital, Lahore, Punjab Institute of Cardiology (PIC), Lahore, Doctors hospital, Lahore and Citilab and Research center, Lahore from August 2014 to September 2017. This study was approved by the ethical review board of the Citilab and Research Center, Lahore under reference: 28th-18 CLRC/ 28th.
Bacterial Isolates
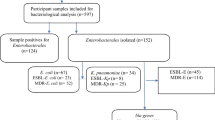
A total of 2,372 samples were processed during study period; 77 % (n=1835) cultures were positive and 54 % (n=995) gram negative non-duplicate clinical isolates from various sources were collected by standard culturing methods. Antibiotic susceptibility testing (AST) was performed according to the guidelines provided by clinical laboratory standards institute [24] by using standard antibiotic discs as mentioned in our previous study [16]. Multiple- antibiotics resistance (MAR) value was calculated as reported before [25]. E. coli ATCC 25922 was used as positive control and K. pneumoniae ATCC 700603 was used as negative control [26]. Analytical profile index (API 20-E) was used for biochemical characterization of isolates resistant to third generation cephalosporins.
Phenotypic confirmation of ESBL-producers
Third generation cephalosporin resistant (3GC-R) isolates as screened by Antibiotic susceptibility test (AST) were subjected to: double disc synergism test (DDST), combination disc test (CDST) and epsilometric test (E-test) for confirmation of ESBL-production [24]. In DDST, amoxicillin (AMC 20/10μg), cefuroxime (CRO 30μg), ceftazidime (CAZ 30μg) and cefotaxime (CTX 30μg) were applied [27]. In CDST, CAZ (30μg) and CTX (30μg) alone and in combination with clavulanic acid (CTC (40μg) and CZC (40μg) were used [16]. All discs used were from Oxoid, Inc (Canada). For E-test, CTX / CTX+ and CAZ/CAZ+ strips from AB BIODISK MICTM were used [24].
Molecular detection
The DNA used for multiplex-PCR was extracted by the heat lysis method [16]. In Multiplex-PCR, 2 μl whole cell lysate DNA for each isolate was used separately in 25 μl PCR-master mix and amplification primers as previously mentioned [16, 28, 29]. PCR amplification conditions were: Initial step of denaturation at 95°C for 5 min followed by 35 cycles of denaturation at 95°C for 1 min then annealing at 56°C for 1.5 min, extension at 95°C for 1 min and the final extension was done at 95°C for 10 min (Table 1).
Amplicon sequencing and in-silico analysis
PCR amplified products were sequenced by Advance Bioscience International, Pakistan in collaboration with 1st Base, Malaysia [30]. Nucleotide sequence similarity searches were performed using the services of National Centre for Biotechnology Information (NCBI) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). BLAST, CLUSTALX, and MEGA 7.0 software were used for sequence alignment of amplicon sequenced obtained with already submitted sequences of blaCTX−M, blaTEM, blaSHV and blaOXA in GenBank.
Statistical Analysis
All statistical analyses were performed using IBM-SPSS statistics 23. Bivariate analyses were performed using chi-square test for categorical variables. All p-values were two sided. The percentage values included in this article are the “valid percentages,” which exclude the missing data.
Results
Demographic data and distribution of clinical isolates
A total of 50 % (n=497/995) third generation cephalosporin resistant (3GC-R) clinical isolates were found among 995 gram-negative isolates. These include 65 % (n=321) Escherichia coli, 25 % (n=124) Klebsiella pneumoniae, 5 % (n=24) Pseudomonas aeruginosa and 4 % (n=20) were Enterobacter spp. and small number of Acinetobacter spp. 2 % (n=8) were found. These isolates were obtained from urine 59 % (n=278), pus 20 % (n=99), wound swab 6 % (n=29), Foley’s tip 3 % (n=17), sputum 3 % (n=16), tracheal secretion 3 % (n=14), body fluids 2 % (n=10), blood 8 % (n=40) and HVS 1 % (n=4) respectively with p-value <0.0001. Among study population, significantly (p-value <0.0001) higher number of strains were isolated from males 53 % (n=265) compared to females 47 % (n=232). Age group 41-60 years was prevalent 35 % (n=174) followed by 21-40 years 29 % (n=146) (p-value <0.0001) (Table 2).
Phenotypic screening and confirmation of ESBL-producers
Isolates had high resistance towards β-lactams including cefotaxime and cefaclor 100% (n = 497). While 98.6% (n = 490) and 96.4% (n = 479) isolates were resistant for cefuroxime and ceftazidime respectively. While, resistance for carbapenems was low 11% (n = 55). Moderate to high resistance towards aminoglycosides (67–89%) and quinolones (74–82%) was seen except amikacin 14% (n = 70). While isolates were quite susceptible to cefoparazone/sulbactam 6% (n = 30) and piperacillin/tazobactam 24% (n = 119). 60% (n = 303) of isolates had MAR-value in the range of 0.60 to 0.799, while 27% (n = 136) were having MAR-value of 0.8–1.0. Rest of the isolates 14% (n = 57) had MAR-value of 0.2–0.59. ESBL-positivity was as follows; double disc synergy test 55% (n = 273), combination disc test 79% (n = 392) and epsilometric-test showed 58% (n = 288). Year-wise data indicated frequency of ESBL-producers among 3GC-R has increased from 76% (n = 102) to 88% (n = 146) during study years (Table 2). E. coli 75% (n = 241), K. pneumoniae 80% (n = 99), Pseudomonas spp. 72% (n = 15) and Enterobacter spp. 75% (n = 15) had ceftazidime/ceftazidime+ MIC > 32/0.064 = 500 while 5.6% (n = 28) remained non-determined. Cefotaxime/cefotaxime+ > 16/0.016 = 1000 was most frequent MIC with E. coli 64% (n = 206), K. pneumoniae 69% (n = 85), Pseudomonas spp. 63% (n = 15), while 6% (n = 30) remained non-determined by cefotaxime/cefotaxime+ (Table 3).
Association analysis indicated among 82% (n = 262) ESBL-positive E. coli, females were more prone to such infection with 53% (n = 138). While, infectivity rate was high for males with ESBL-positive Klebsiella pneumoniae 54% (n = 47) and Enterobacter spp. 57% (n = 8) (Tables 4 and 5). High frequency of ESBL-producers 36% (n = 140) came from age group of 41–60 years. Age associated ESBL-infectivity rate was more confined to age group 41–60 years in E. coli 36% (n = 94), Klebsiella spp. 38% (n = 35) and Pseudomonas spp. 33% (n = 8) (Table 6). Urine samples were frequent source of ESBL-phenotype among E. coli 65% (n = 171), Klebsiella spp. 39% (n = 35) and Pseudomonas spp. 38% (n = 9) (Table 5).
Molecular detection
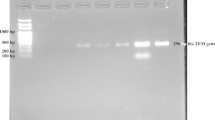
After screening, ESBL-producing isolates (n = 392) as detected by combination disc test were processed for the detection of blaCTX − M, blaSHV, blaTEM and blaOXA encoding genes by PCR. In Singleplex-PCR, blaCTX − M genes were predominant 76% (n = 303) followed by blaOXA 52% (n = 203), blaTEM 28% (n = 109) and blaSHV 21% (n = 82). Multiplex-PCR showed that blaCTX − M//SHV/TEM/OXA and blaOXA/TEM/SHV gene combination was present in 9% (n = 36) and 11% (n = 43) respectively. blaTEM/SHV and blaTEM/OXA combination was present in 13% (n = 51) and 27% (n = 105) respectively (Table 7, Fig. 1).
Amplicon sequencing and subsequent analysis indicated blaCTX − M − 15 86% (n = 260) was prevalent among blaCTX − M − 1 group. blaOXA − 1 49% (n = 99) were found among blaOXA amplicons and blaTEM − 1B 63% (n = 69). 83% (n = 190) of E. coli had blaCTX − M − 15 followed by blaOXA − 1 55% (n = 69) and blaTEM − 1B 33% (n = 76). Klebsiella spp. contained blaCTX − M − 15 67% (n = 36) followed by blaSHV − 11 89% (n = 47) and blaTEM − 1B 34% (n = 19). While, Pseudomonas spp. and Acinetobacter baumannii had variants of OXA (blaOXA − 50, blaOXA − 144, blaOXA − 23, blaOXA − 371, blaOXA − 58, blaOXA − 68 and blaOXA − 94) (Table 8).
Discussion
Extensive use of antibiotics has resulted in resistance against variety of antibiotics including cephalosporins. They affect countries all over the world but control and prevention of ESBL-producers is severely compromised in underdeveloped countries [31,32,33].
Here, high prevalence of third generation cephalosporin resistant isolates (50%) was observed which has subsequently increased by 1.13-fold from 2014 to 2017. This high resistance also indicates high selection pressure for third generation cephalosporin resistant isolates [34]. This increase of resistance is worrisome as we are left with few treatment options including cephalosporins. Widespread usage of antibiotics might be the factor of such increase in resistance in our hospital settings [16, 35].
E. coli had high 3GC-R burden compared to Klebsiella spp. and Enterobacter spp. Bari et al., reported similar findings in a study conducted in 2013 in Lady Reading Hospital Peshawar [36]. These results are comparable to findings in Tanzania where 45% ESBL-producers have been reported [37]. Similar findings from different regions of the world were observed as previously studied [38, 39]. Nahid et al., reported very high prevalence of ESBL-producers (87.5%) but this is because she worked on Metallo-β-lactamase producers which are highly resistant organisms [40].
ESBL infectivity rate in males was moderately high as compared to females. This rate is quite similar to the rate reported by Afirdi et al. [41]. In our study ESBL infections were significantly higher in the mean age group of 41-60 years whereas, high infection rates have been reported in old age individuals who are immuno-compromised and hence, more prone to infections [42]. We have found isolates originating from females were more frequent ESBL-producers. According to many reports males have significantly higher rates of hospital-acquired infection and community-acquired infections are more prevalent in females [42,43,44,45,46]. These findings represent that males are more often exposed to the hospital settings compared to the females.
Studies indicated prevalence of ESBL-producers is variable in different regions of world as detected by phenotypic detection tests [47,48,49,50]. DDST determined only 54 % strains as ESBL-producers while CDST determined 79 % as ESBL-producers. Ejaz et al., reported similar detection efficiency of CDST as we reported here [17]. Prevalence of ESBL-producing isolates is quite higher than from other parts of the world including India (42.3 %), Bangladesh (37.8 %). Dalela et al., reported 90 % sensitivity of CDST for the detection of ESBL-producers [51]. E-test revealed that 61 % strains were ESBL-producers while 39 % remained non-determined by this technique. Mohanty et al. also reported 61 % positivity rate for ESBL-producers by E-test technique [52]. Such discrepancies between susceptibility data and phenotypic test results have increased the demand for more sensitive methods of ESBL-producer detection for implementation into routine susceptibility testing procedures.Despite of high resistance burden of ESBL-producers, the usage of molecular detection methods is not very common. A recent meta-analysis describes only 11% studies that reported PCR-based detection methods for screening of ESBL-producers in Pakistan [20]. Lack of knowledge and technical staff triggers the use of PCR-based methods as it is the rapid and reliable method of ESBL-producer detection [8]. It seems that blaCTX − M is predominant genotype in this region of the world. Another study from Pakistan indicated 72% of isolates had blaCTXM − 15 gene which was lower than prevalence of blaCTX-M gene found in this study [16]. Few studies from other parts of world have shown different prevalence of blaCTX-M gene among isolates including 84.7% (Chile), 98.8% (China) and 13.6% (Tanzania) [53,54,55]. We observed blaTEM and blaOXA genes were less common in our settings with 50% prevalence. Report from Hamad Medical Corporation, Qatar stated that CTX-M group has evolved through mutations in blaTEM and blaSHV genes and is recent endemic [56].
-Acinetobacter baumannii isolates had OXA variants (blaOXA − 23,58 and others) which are carbapenemase-encoding genes [57]. These variants have previously been isolated from France, Spain and Turkey which indicates the global spread [50]. blaOXA − 23 was amplified from pan-drug resistance A. baumannii only which is in accordance with our results [58]. But these Acinetobacter baumannii isolates did not carry any of the ESBL-encoding genes which terminate the co-existence of carbapenemase and ESBL-encoding genes. This is in accordance with already published article which states no significant relation between both groups [59]. Appearance of different variants might provide extra advantage for these isolates to spread them and complicate the therapeutics.
With the passage of time increase in co-resistance of different ESBL-producing genes is worrisome as co-existence of multiple genes hinders the detection of ESBL-producers and complicates the treatment strategy for clinicians. Moreover, high plasmid burden was found these plasmids are involved in gene-transfer and they also carry additional antibiotic resistance genes along with β-lactam antibiotics.
Conclusions and Recommendations
In conclusion, blaCTX−M-type ESBL-producing genes and blaOXA-type narrow spectrum-β-lactamases are prevalent among the isolates in our health care settings. Isolates had high resistance towards cephalosporins. Resistance towards cephalosporins and carbapenems has increased many folds during study period. Co-expression of multiple genes complicates the treatment strategy. blaCTXM−15, a pandemic genotype is quite prevalent and their plasmid association is a big thread for the community. There is a dire need for efficient molecular diagnostic tools for the detection of bla genes at laboratory level.
Abbreviations
- CDST:
-
Combination disc test
- DDST:
-
Double disc synergy test
- ESBL:
-
Extended-spectrum β-lactamases
- ESBLs:
-
Extended-spectrum-β-lactamase-producing strains
- E-Test:
-
Epsilometric test
- MAR:
-
Multiple- antibiotics resistance
- MIC:
-
Minimum inhibitory concentration
References
O'Connell N, Keating D, Kavanagh J, Schaffer K. Detection and characterization of extended-spectrum beta-lactamase-producing Enterobacteriaceae in high-risk patients in an Irish tertiary care hospital. J Hosp Infect. 2015;90(2):102–7.
Molton JS, Tambyah PA, Ang BS, Ling ML, Fisher DA. The global spread of healthcare-associated multidrug-resistant bacteria: a perspective from Asia. Clin Infect Dis. 2013;56(9):1310–8.
Rudresh S, Nagarathnamma T. Extended spectrum β-lactamase producing Enterobacteriaceae & antibiotic co-resistance. Indian J Med Res. 2011;133(1):116.
Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol. 2010;300(6):371–9.
Oteo J, Navarro C, Cercenado E, Delgado-Iribarren A, Wilhelmi I, Orden B, et al. Spread of Escherichia coli strains with high-level cefotaxime and ceftazidime resistance between the community, long-term care facilities, and hospital institutions. J Clin Microbiol. 2006;44(7):2359–66.
Bajpai T, Pandey M, Varma M, Bhatambare GS. Prevalence of TEM, SHV, and CTX-M Beta-Lactamase genes in the urinary isolates of a tertiary care hospital. Avicenna J Med. 2017;7(1):12.
Page M. Extended-spectrum β-lactamases: structure and kinetic mechanism. Clin Microbiol Infect. 2008;14(s1):63–74.
Sharma J, Sharma M, Ray P. Detection of TEM & SHV genes in Escherichia coli & Klebsiella pneumoniae isolates in a tertiary care hospital from India. Indian J Med Res. 2010;132:332–6.
Sharma M, Pathak S, SrivaStava P. Prevalence and antibiogram of Extended Spectrum β-Lactamase (ESBL) producing Gram negative bacilli and further molecular characterization of ESBL producing Escherichia coli and Klebsiella spp. J Clin Diagn Res. 2013;7(10):2173.
Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–5.
Song J-H, Jung S-I, Ko KS, Kim NY, Son JS, Chang H-H, et al. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antmicrob Agents Chemother. 2004;48(6):2101–7.
Hsueh PR, Hoban DJ, Carmeli Y, Chen SY, Desikan S, Alejandria M, et al. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J Inf Secur. 2011;63(2):114–23.
Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791.
Jean S-S, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37(4):291–5.
Kucheria R, Dasgupta P, Sacks S, Khan M, NJPmj S. Urinary tract infections: new insights into a common problem. Postgrad Med J. 2005;81(952):83–6.
Abrar S, Vajeeha A, Ul-Ain N, Riaz S. Distribution of CTX-M group I and group III β-lactamases produced by Escherichia coli and Klebsiella pneumoniae in Lahore, Pakistan. Microb Pathog. 2017;103:8–12.
Ejaz H. Detection of extended-spectrum β-lactamases in Klebsiella pneumoniae: Comparison of phenotypic characterization methods. Pak J Med Sci. 2013;29(3):768.
Jabeen K, Zafar A, RJJoPMA H. Frequency and sensitivity pattern of extended spectrum beta lactamase producing isolates in a tertiary care hospital laboratory of Pakistan. J Pak Med Assoc. 2005;55(10):436.
Khan E, Ejaz M, Zafar A, Jabeen K, Shakoor S, Inayat R, et al. Increased isolation of ESBL producing Klebsiella pneumoniae with emergence of carbapenem resistant isolates in Pakistan: report from a tertiary care hospital. J Pak Med Assoc. 2010;60(3):186.
Abrar S, Hussain S, Khan RA, Ain NU, Haider H, Riaz S. Prevalence of extended-spectrum-β-lactamase-producing Enterobacteriaceae: first systematic meta-analysis report from Pakistan. Antimicrob Resist Infect Control. 2018;7(1):26.
Mumtaz S, Ahmed J, Ali L, Hussain H. Prevalence of extended spectrum beta lactamases (ESBL) in clinical isolates from a teaching hospital in Peshawar, Pakistan. Afr J Microbiol Res. 2011;5(19):2880–4.
Vernet G, Mary C, Altmann DM, Doumbo O, Morpeth S, Bhutta ZA, et al. Surveillance for antimicrobial drug resistance in under-resourced countries. Emerg Infect Dis. 2014;20(3):434.
Bhardwaj AK, Vinothkumar K. Evolution of MDRs. Quorum Sensing vs Quorum Quenching: A Battle with No End in Sight. India: Springer; 2015. p. 9–22.
LS CaI. Performance standards for antimicrobial disk susceptibility testing: approved standard. Natl Comm Clin Lab Stand. 2014;6:285–9.
Christopher AF, Hora S, Ali Z. Investigation of plasmid profile, antibiotic susceptibility pattern multiple antibiotic resistance index calculation of Escherichia coli isolates obtained from different human clinical specimens at tertiary care hospital in Bareilly-India. Ann Trop Med Public Health. 2013;6(3):285.
Alfaresi M, Elkoush A. Real-time polymerase chain reaction for rapid detection of genes encoding SHV extended-spectrum β-lactamases. Indian J Med Microbiol. 2010;28(4):332.
Drieux L, Brossier F, Sougakoff W, Jarlier V. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: review and bench guide. Clin Microbiol Infect. 2008;14(s1):90–103.
Pitout JD, Hossain A, Hanson ND. Phenotypic and Molecular Detection of CTX-M-β-Lactamases Produced by Escherichia coli and Klebsiella spp. J Clin Microbiol. 2004;42(12):5715–21.
Ain NU, Iftikhar A, Bukhari SS, Abrar S, Hussain S, Haider MH, et al. High frequency and molecular epidemiology of metallo-β-lactamase-producing gram-negative bacilli in a tertiary care hospital in Lahore, Pakistan. Antimicrob Resist Infect Control. 2018;7:128.
Kumar V, Sun P, Vamathevan J, Li Y, Ingraham K, Palmer L, et al. Comparative genomics of Klebsiella pneumoniae strains with different antibiotic resistance profiles. Antimicrob Agents Chemother. 2011;55(9):4267–76.
Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6(1):47.
Reardon S. Antibiotic resistance sweeping developing world: bacteria are increasingly dodging extermination as drug availability outpaces regulation. Nature. 2014;509(7499):141–3.
Carlet J, Jarlier V, Harbarth S, Voss A, Goossens H, Pittet D. Ready for a world without antibiotics? The pensières antibiotic resistance call to action: Springer; 2012.
Vounba P, Arsenault J, Bada-Alambedji R, Fairbrother JM. Prevalence of antimicrobial resistance and potential pathogenicity, and possible spread of third generation cephalosporin resistance, in Escherichia coli isolated from healthy chicken farms in the region of Dakar, Senegal. PLoS One. 2019;14(3):e0214304.
Peymani A, Naserpour-Farivar T, Zare E, Azarhoosh K. Distribution of blaTEM, blaSHV, and bla CTX-M genes among ESBL-producing P. aeruginosa isolated from Qazvin and Tehran hospitals, Iran. J Prev Med Hyg. 2017;58(2):E155.
Bari F, Shah H, Wazir R. Frequency and Detection of Extended Spectrum Betalactamase in Escherichia coli and Klebsiella pneumoniae: A Study At Lady Reading Hospital Peshawar. J Postgrad Med Inst. 2016;29(4).
Moyo SJ, Aboud S, Kasubi M, Lyamuya EF, Maselle SY. Antimicrobial resistance among producers and non-producers of extended spectrum beta-lactamases in urinary isolates at a tertiary Hospital in Tanzania. BMC Res Notes. 2010;3(1):348–52.
Bindayna K, Khanfar HS, Senok AC, Botta GA. Predominance of CTX-M genotype among extended spectrum beta lactamase isolates in a tertiary hospital in Saudi Arabia. Saudi Med J. 2010;31(8):859–63.
Maina D, Revathi G, Kariuki S, Ozwara H. Genotypes and cephalosporin susceptibility in extended-spectrum beta-lactamase producing enterobacteriaceae in the community. J Infect Dev Ctries. 2011;6(06):470–7.
Nahid F, Khan AA, Rehman S. Zahra RJJoi, health p. Prevalence of metallo-β-lactamase NDM-1-producing multi-drug resistant bacteria at two Pakistani hospitals and implications for public health. J Infect Public Health. 2013;6(6):487–93.
Afridi FI, Farooqi BJ, Hussain A. Frequency of extended spectrum beta-lactamase producing Enterobacteriaceae among urinary pathogen isolates. J Coll Physicians Surg Pak. 2011;21(12):741–4.
Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES, et al. A multinational survey of risk factors for infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49(5):682–90.
Ahmad M, Hassan M, Khalid A, Tariq I, Asad MH, Samad A, et al. Prevalence of Extended Spectrum beta-Lactamase and Antimicrobial Susceptibility Pattern of Clinical Isolates of Pseudomonas from Patients of Khyber Pakhtunkhwa, Pakistan. Biomed Res Int. 2016;2016:6068429.
Casella T, Rodríguez MM, Takahashi JT, Ghiglione B, Dropa M, Assunção E, et al. Detection of bla CTX-M-type genes in complex class 1 integrons carried by Enterobacteriaceae isolated from retail chicken meat in Brazil. Int J Food Microbiol. 2015;197:88–91.
Anwar M, Chaudhry I, Ahmad I, Bhatti K, Jaffery G, Tayyab M, et al. Frequency of extended spectrum β-lactamase producing Klebsiella pnumoniae and Escherichia coli isolates. Biomedica. 2007;23:34–8.
Bourjilat F, Bouchrif B, Dersi N, Claude JDPG, Amarouch H, Timinouni M. Emergence of extended-spectrum beta-lactamases-producing Escherichia coli in community-acquired urinary infections in Casablanca, Morocco. J Infect Dev Countr. 2011;5(12):850–5.
Bonnet R. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48(1):1–14.
Falagas M, Karageorgopoulos DE. Extended-spectrum β-lactamase-producing organisms. J Hosp Infect. 2009;73(4):345–54.
Reinert RR, Low DE, Rossi F, Zhang X, Wattal C, Dowzicky MJ. Antimicrobial susceptibility among organisms from the Asia/Pacific Rim, Europe and Latin and North America collected as part of TEST and the in vitro activity of tigecycline. J Antimicrob Chemother. 2007;60(5):1018–29.
Chander A, Shrestha CD. Prevalence of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae urinary isolates in a tertiary care hospital in Kathmandu, Nepal. BMC Res Notes. 2013;6(1):487.
Dalela G. Prevalence of extended spectrum beta-lactamase (ESBL) producers among gram-negative bacilli from various clinical isolates in a tertiary care hospital at Jhalawar, Rajasthan, India. J Clin Diagn Res. 2012;6(2):182–7.
Mohanty S, Gaind R, Ranjan R, Deb M. Use of the cefepime-clavulanate ESBL Etest for detection of extended-spectrum beta-lactamases in AmpC co-producing bacteria. J Infect Dev Countr. 2009;4(01):024–9.
Sonda T, Kumburu H, van Zwetselaar M, Alifrangis M, Mmbaga BT, Lund O, et al. Prevalence and risk factors for CTX-M gram-negative bacteria in hospitalized patients at a tertiary care hospital in Kilimanjaro, Tanzania. Eur J Clin Microbiol Infect Dis. 2018;37:1–10.
Zhao D, Quan J, Liu L, Du X, Chen Y, Jiang Y, et al. High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J Antimicrob Chemother. 2016;72(1):273–80.
Pavez M, Troncoso C, Osses I, Salazar R, Illesca V, Reydet P, et al. High prevalence of CTX-M-1 group in ESBL-producing enterobacteriaceae infection in intensive care units in southern Chile. Braz J Infect Dis. 2019.
Sid Ahmed MA, Bansal D, Acharya A, Elmi AA, Hamid JM, Sid Ahmed AM, et al. Antimicrobial susceptibility and molecular epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae from intensive care units at Hamad Medical Corporation, Qatar. Antimicrob Resist Infect Control. 2016;5(1):4–9.
Evans BA, Amyes SGB. OXA β-Lactamases. Clinical microbiology reviews. 2014;27(2):241–63.
Braun SD, Jamil B, Syed MA, Abbasi SA, Weiss D, Slickers P, et al. Prevalence of carbapenemase-producing organisms at the Kidney Center of Rawalpindi (Pakistan) and evaluation of an advanced molecular microarray-based carbapenemase assay. Future Microbiol. 2018;13:1225–46.
Safari M, Nejad ASM, Bahador A, Jafari R, Alikhani MY. Prevalence of ESBL and MBL encoding genes in Acinetobacter baumannii strains isolated from patients of intensive care units (ICU). Saudi J Biol Sci. 2015;22(4):424–9.
Acknowledgements
We would like to pay our gratitude towards Microbiology section of Allama Iqbal Medical College/Jinnah Hospital, Lahore, Punjab Institute of cardiology (PIC), Doctors hospital, Lahore and Cililab and Research Center, Lahore to provide assistance in collection of bacterial isolates.
Funding
No funding was provided for this study
Availability of data and materials
All the data files generated during this study are with authors of this and can be provided on demand.
Author information
Authors and Affiliations
Contributions
Study concept and design of the study: SR; data collection: (SA and HL); FR (helps in managing data and strains from Allama Iqbal Medical College); reviewing the manuscript and editing (SH, NuA and SR); Major experiment work (SA, HL and SH). All authors approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by local ethics committee (CitiLab and Research Centre Ref # 28th -18 CLRC/ 28th).
Consent for publication
Not applicable
Competing interests
This study is part of PhD thesis of Ms. Samyyia Abrar. All other authors declare that the work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Abrar, S., Ain, N.U., Liaqat, H. et al. Distribution of blaCTX − M, blaTEM, blaSHV and blaOXA genes in Extended-spectrum-β-lactamase-producing Clinical isolates: A three-year multi-center study from Lahore, Pakistan. Antimicrob Resist Infect Control 8, 80 (2019). https://doi.org/10.1186/s13756-019-0536-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-019-0536-0