Abstract
Background
Tuberculosis (TB) is considered one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent. Multidrug-resistant (MDR) TB can affect people of all age groups, including children (aged 0–15 years). However, very little is known about the extent of this problem in children. This systematic review aims to investigate the incidence of TB and drug-resistant (DR) TB among the pediatric population. It also reviews the therapeutic options available to treat the condition.
Methods
A comprehensive search for all relevant evidence was conducted. The following databases were searched: MEDLINE, CINAHL, and Web of Science. The searched time frame was limited from January 1990 to December 2020 with a focus on the incidence of TB and MDR-TB among pediatrics and the therapeutic options available.
Results
A total of 537 articles were obtained via the selected databases. After title and abstract screening, 418 articles were excluded leaving 119 articles. Full-text screening was conducted on 119 articles, excluding a further 110 articles. Thus, 9 articles were subject to quality assessment and included in this review. The 9 articles represented the age group of 0–15 years and included both males and females. All studies included were of retrospective study design.
Discussion
The included studies mentioned a moderate increase in TB cases among pediatrics exacerbated by malnutrition, lack of bacille Calmette-Guérin (BCG) vaccination, and human immunodeficiency virus (HIV) coinfection. MDR-TB prevalence was especially high in South Africa. Drug therapy for both TB and MDR-TB yielded favorable outcomes among pediatrics. However, one of the biggest challenges with drug therapy includes the dosage forms available.
Systematic review registration
Similar content being viewed by others
Background
Tuberculosis (TB) is an infectious disease [1]. It is considered one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent [1]. TB is caused by the bacillus Mycobacterium tuberculosis, which is spread when people who are sick with TB expel bacteria into the air; for example, by coughing [1]. The disease naturally affects the lungs (pulmonary TB) but can also affect others parts of the body (extra-pulmonary TB) [1]. Not every individual infected with TB bacteria becomes sick [2]. As a result, two TB-related conditions exist: latent TB infection and TB disease [2]. Latent TB infection occurs when a person has TB bacteria in his or her body, but does not have symptoms of the disease [3]. The infected person’s immune system fights off the TB organisms, and the TB remains inactive throughout life in most people who are infected [3]. This person would have a positive skin test, but a normal chest X-ray [3]. Whereas TB disease is characterized as a person who has signs and symptoms of an active infection [3]. The person would have a positive skin test and a positive chest X-ray [3].
Globally, an estimated 10.0 million people fell ill with TB in 2019 [1]. There were an estimated 1.2 million TB deaths among human immunodeficiency virus (HIV) negative people in 2019 and an additional 208,000 deaths among HIV-positive people [1]. Men (aged ≥ 15 years) reported 56% of the people who developed TB in 2019; women reported for 32% and children (aged < 15 years) for 12% [1].
Drug-resistant (DR) TB represents a major threat to the fight against TB globally [4, 5]. MDR-TB is the most common form of DR-TB defined as a disease caused by strains of Mycobacterium tuberculosis resistant to at least isoniazid and rifampicin, the two most powerful first-line anti-tuberculosis drugs [4, 5]. Globally, most cases of MDR remain undetected and untreated because of limited laboratory capacity to conduct tests for drug resistance and limited access to second-line treatment, which is long, toxic and expensive [4,5,6]. In some cases, even more severe drug-resistant TB may develop [7]. Extensively drug-resistant TB (XDR-TB) is a form of MDR-TB with additional resistance to more anti-TB drugs [7]. As of 2021, the World Health Organization (WHO) has updated its definition for extensively drug-resistant tuberculosis (XDR-TB) and has, for the first time, defined pre-XDR-TB [8]. Pre-XDR-TB is defined as disease caused by M. tuberculosis strains that are resistant to isoniazid, rifampicin, and any fluoroquinolone and XDR-TB is now defined as TB caused by Mycobacterium tuberculosis strains that are resistant to isoniazid, rifampicin, any fluoroquinolone, and either bedaquiline or linezolid (or both) [8]. Both of these conditions respond to even fewer available medicines [7].
As with other forms of tuberculosis, MDR-TB can affect people of all age groups, including children (aged 0–14 years). However, very little is known about the magnitude of this problem in children, although TB remains one of the top 10 causes of death among children worldwide [6, 9]. The diagnosis of MDR-TB is bacteriological by definition, based on the isolation of strains resistant to medicines [9, 10]. While isolating M. tuberculosis in adults with pulmonary tuberculosis is generally an easy procedure (the exception is patients who are immunocompromised), children mainly have paucibacillary disease, which means that specimens for culture and drug susceptibility testing are often difficult to obtain, particularly from the youngest who cannot expectorate sputum [10].
Children with MDR-TB are treated in a parallel way to adults with MDR-TB [9]. One difference is that confirmation and drug susceptibility test (DST) may not possible so that empirical treatment is frequently required for children with suspected MDR-TB. Although outcome data in children are limited, the available evidence suggests that outcomes at least as good as those reported in adults can be achieved [11, 12].
Treating children with MDR tuberculosis is complex [13]. Few of the multiple drugs routinely used to treat MDR tuberculosis have been studied in children, and direction on drug regimens, dosages, appropriate monitoring, and duration of therapy is frequently established from adult data [13]. As young children metabolize drugs more rapidly than adults and generally have paucibacillary disease, this may not always be suitable [13].
Review question
What is the incidence of TB and MDR-TB in pediatrics and the therapeutic options available to treat the condition?
Objectives
-
To identify the incidence of TB in pediatrics.
-
To identify the incidence of MDR-TB in pediatrics.
-
To identify the therapeutic options available to treat TB and MDR in pediatrics.
-
To identify the therapeutic options available to treat MDR-TB in pediatrics.
-
To identify the dosage forms that are currently available for TB and MDR-TB in pediatric patients.
Methodological considerations
To attain our objectives, the following steps were followed:
-
1.
Development of a literature search strategy to identify the incidence of TB and MDR-TB, the treatment outcomes, and the dosage forms available among pediatrics.
-
2.
Screening of all the identified studies in objectives 1–5 for their relevance in addressing the research objectives.
-
3.
Critical appraisal of the evidence obtained from included studies.
-
4.
Extraction of relevant data from studies on the incidence of TB and MDR-TB and the treatment outcomes among pediatrics
Search strategy
A comprehensive search for all relevant evidence regardless of language or publication status was conducted. The following databases were searched: MEDLINE, CINAHL, and Web of Science. The search strategy can be found in Additional file 1. The search strategy used a combination of Medical Subject Heading terms and free-text words in titles, abstracts, and keywords. Terms related to TB or MDR-, incidence, drugs, and treatment outcomes were included. The searched time frame was limited from January 1990 to December 2020. SH was credited with the development of the search strategy. EW was credited with the review of the search strategy. Testing of the search strategy was conducted by SH and EW. Following the screening, studies that fulfilled the inclusion criteria and adequately address the research objectives shall be retained for full-text review. The reference lists of included studies and previous reviews will be explored to identify other eligible studies.
Selection criteria
Inclusion criteria
Population studied
The study was based on the evaluation of dosage forms available for the treatment of TB and MDR-TB, therefore, included the population from 0 to 15 years of age. Pediatric tuberculosis (TB) represents a major public health concern worldwide [14]. About 1 million children aged less than 15 years develop TB each year, contributing to 3–25% of the total TB caseload [14]. Both male and female pediatric patients will be considered for the review within the age limit as specified for the study. Participants of the study will not be limited to geographical locations or cultural backgrounds.
Investigated condition
The review will be assessing the incidence of TB and MDR-TB among the pediatric population.
Context of importance
The context includes all studies conducted in pediatric patients globally and is not limited to any cultural backgrounds.
Outcome
The outcome of the review is to assess the incidence of TB and MDR-TB among the pediatric population. It will also review the therapeutic options available to treat TB and MDR-TB in terms of the dosage forms available.
Types of studies
Studies published between the periods of January 1990 to December 2020 will be considered for the review. This review will only include studies that have been published in the English language. Studies that contain an abstract with a full text available will be considered.
Exclusion criteria
-
Studies that were published before January 1990 or after December 2020.
-
Studies that include children above the age of 15 years.
-
Studies in which the age of the children studied cannot be determined.
-
Studies that include both children and adults.
-
Studies that did not indicate the number/percentage of included children.
-
Studies that were not published in the English language—for this review, all the authors are only familiar with the English language.
-
Studies that do not include the outcome of the review—the outcome is the purpose of the review.
-
Studies published without abstracts - abstract assessments are the first step in quality assurance for the review.
-
Pilot studies—this type of study is a test study and not an actual study.
SH and EW ran a comprehensive search on the three selected databases. SH merged all the data from the databases to build a library on Endnote® X8.1 library. SH identified and removed any/all duplicates. SH and EW independently assessed the retrieved titles and abstracts of relevant studies for their eligibility to be included in the review. Relevant data will be extracted from each eligible article and retained for full-text review. SH and EW the performed independent screening of the full texts of retained articles with consensus in each stage. A third reviewer, KBM was consulted to resolve any or all disagreements. All studies that met the inclusion criteria were selected. If eligibility was unclear because of missing information, two attempts were made to contact the authors of the primary report. After two unsuccessful attempts, reports were excluded.
The inclusion and exclusion criteria for the study have been presented Table 1. These criteria were defined using the PICOS (Population, Intervention/Investigation, Comparator, Outcome, Study design) approach.
Methodological quality appraisal, data extraction, and cleaning
Crombie’s tool was used to assess the quality of included studies [15]. The methodological quality assessment tool was adopted and modified for this review due to the similarities this review shares with the study conducted by Tola et al. (2020) [16]. This tool contains six items for quality assessment of cross-sectional/prevalence studies. The quality of each article was given by the score ranged from 0 to 6. The quality assessment tool can be found in Additional file 2. Final inclusion of the study was decided through consensus of SH and EW and in the case of disagreement, KBM resolved.
SH and EW completed data extraction and compiled the findings together. KBM addressed any inconsistent data and checked for completeness.
Data synthesis and statistical analysis
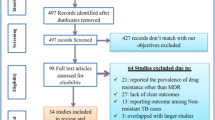
The review findings were analyzed and compiled in Fig. 1, Tables 2 and 3.
Results
The review yielded 533 results. Detailed citation screening resulted in 4 additional studies been added resulting in 537. There were no duplicates. The screening of the articles is depicted in Fig. 1. Title screening led to 418 articles being excluded resulting in 119 remaining for full-text screening. Full-text screening led to 9 articles being selected for the review.
Characteristics of the included studies
The characteristics of the 9 studies selected are detailed in Table 2. The study years ranged from 2006 to 2020. The studies represent the continents of Africa, Asia, and Europe. No studies were conducted in Australia, the North and South of America. All studies included both male and female participants. The general ages represented include 0 to 15 years. All studies were of the retrospective design. Four studies included participants with HIV co-infection. Of the 9 studies, the following variations of TB were mentioned, i.e.: TB, PTB, EPTB MDR-TB, TBM, and isoniazid-resistant tuberculosis.
Quality assessment of the included studies
According to the scoring tool, only 2 studies were of moderate quality and 7 studies were of high quality (Additional file 1). No studies were excluded based on the quality assessment tool. There was no disagreement between SH and EW.
Review findings
The summary of the findings of the studies included is detailed in Table 3.
Of the 9 studies, 5 studies focused on TB (EPTB, PTB, and TBM). Only Erkens et al. (2014) [20] study reported a significant reduction in the TB statistics whereas the other studies noted slight increases that occurred predominantly in the age group 5–14 years. The majority of studies noted an equal distribution of the condition among female and male participants.
Four articles mentioned MDR-TB. Three of these studies were conducted in South Africa. Although MDR-TB is growing in South Africa among the adult population, the statistics remain relatively low in the pediatric population but higher in South Africa in comparison to China [10, 18, 24, 25]. Zhou et al. (2020) mentioned that in Shandong, the prevalence of MDR-TB in childhood TB was low, at 5.6% [25].
Only three articles stated the therapeutic options available for the treatment of TB and DR-TB. The following drugs were mentioned: rifampicin (Rif), isoniazid (INH), pyrazinamide (PZA), ethambutol (Emb), ethionamide (Eth), ofloxacin, streptomycin, amikacin, terizadone, kanamycin, capreomycin, cycloserine, para-aminosalicylic acid, and linezolid. Padayatchi et al. reported only 1 favorable outcome and 7 deaths among the participants [13]. Fairlie et al. (2011) and Seddon et al. (2012) reported 76.9% and 82% favourable treatment outcomes [8, 17]. Therefore, children with MDR tuberculosis can be treated successfully.
In this data set, children infected with HIV were more likely to have confirmed MDR-TB. Three of the four studies were conducted in South Africa. South Africa has the biggest HIV epidemic in the world [25]. The HIV epidemic in southern Africa has exacerbated the spread and virulence of DR-TB [26]. On the other hand, malnutrition and TB are frequently associated conditions in children, and both represent a public health issue worldwide [26].
Limitations
Limitations of the review
-
The restriction of the literature search timeline to the range of January 1990 to December 2020.Inclusion of studies published only in the English language.
-
Limiting the literature search to three databases.
-
All included studies were of retrospective design; hence, there could be missing or incomplete information.
-
The review did not have a representative of studies conducted in North America, South America and Australia.
The above limitations can contribute to selection bias and have an impact on the results of this review.
Discussion
Childhood TB adds nearly 15–20% of all TB cases worldwide [25,26,27]. TB can imitate signs and symptoms of many common childhood diseases, including pneumonia, malnutrition, and HIV infection which pose diagnostic difficulties. Childhood TB has increasingly been recognized as contributing a substantial proportion of the global TB caseload; however, pioneering work has been done spanning diagnosis, prevention, management, and the impact of HIV [28]. This is evidenced by the moderate increases in the condition over the years.
One of the contributing factors to an increase in pediatric TB is the subject of household contact. According to the published literature, a single pulmonary TB (PTB) patient can infect 10 to 15 persons on average [29], having close contact, within a community whereas 90% of the TB transmission in the community is due to sputum smear-positive [30, 31]. Household contacts are highly susceptible to acquire TB infection from the index cases because of their proximity. The goal of contact tracing and their screening for TB could lead to the detection of additional cases of TB, maximizing the impact of case detection and effective treatment [32, 33]. The prevalence of TB disease is particularly soaring among children who are close contacts of a TB patient. Hence, screening children as contacts are generally recommended, though practiced in rarity [34].
Malnutrition is highly prevalent in children living in tuberculosis endemic countries and contributes to 2.2 million deaths in children under 5 years of age globally [35]. Poverty, overcrowding, food insecurity, and human immunodeficiency virus (HIV) further set the stage for both malnutrition and poor infection control [36].
Several studies conducted in Europe noted that an increase in immigration of people from high TB incidence areas has recently contributed to the resurgence of this disease in these countries [24, 37]. Many countries have developed immigration TB screening programs to suit the needs of adults, but have not focused much attention on migrant children [38].
Around 3% of children with TB have MDR-TB, amounting to between 25,000 and 32,000 children developing MDR-TB disease each year [39]. Only 3–4% of them are diagnosed and treated and, as a result, around 21% of children with MDR-TB likely die [39]. Increased implementation of household contact investigations could help to close the treatment gap [39]. According to the included studies in this review, South Africa represented the highest incidence of MDR-TB among the pediatric population.
Diagnosing MDR-TB is especially problematic in children, from whom it is difficult to isolate a bacteriologic specimen that can be used to directly detect drug resistance [40].
Seddon et al. (2014) conducted a cohort study in children in South Africa [41]. In this cohort of children with either bacteriologically confirmed and/or clinically diagnosed disease, treatment was well tolerated overall with few significant adverse events. Treatment outcomes were excellent, with over 90% of children cured or probably cured. Many children were identified and started on treatment early, following the diagnosis of an MDR-TB source case, illustrating the importance of contact tracing. The three children who died either presented late with severe TB disease and concomitant HIV infection or had the extensive disease and had defaulted care [41]. This is supportive of other literature and the studies included in this review. One of the major challenges with drug therapy is very few drugs are produced in pediatric formulations, and the pharmacokinetics are incompletely studied in children [42]. This means that optimal dosing of second-line drugs is unknown and that tablets must be broken or cut, potentially leading to inaccurate dosages and blood concentrations that are sub-therapeutic or toxic [42]. The taste of medications is often unpalatable and a number of the drugs can cause vomiting and diarrhoea. This may affect the amount of the drug absorbed [42].
Conclusion
A large percentage of pediatric TB and/DR-TB cases remained undetected, or not reported; however, in recent years, a growing number of children are contracting DR-TB, mainly through household transmission. It is critical when adults test positive for DR-TB, that the entire household is screened. Diagnosis of DR-TB is more difficult in children as the disease in children is generally pauci-bacillary. The sputum is rarely produced resulting in the specimens being hard to obtain. The diagnosis in most of cases is clinical, and only rarely bacteriologically confirmed. The treatment of DR-TB in children is guided by the same principles and uses the same second-line drugs as the treatment in adults, although optimal durations of regimens are not known. Children face challenges to appropriate treatment. Not only because of the difficulty in swelling pills, but also because of the challenges to achieve the right dosage and blood concentration, to obtain efficacy of the therapy and to avoid toxicity. With increased MDR-TB rates evident especially in high HIV prevalence areas such as South Africa in this study, infection control needs to be addressed urgently. More data on morbidity and long-term mortality in HIV-infected and HIV-uninfected children, are needed. Knowing the TB burden in the pediatric population, globally and at country level, is fundamental and developing more child-friendly formulations to treat DR-TB would be ideal in combating DR-TB.
Recommendations
The therapeutic management of children with MDR- andXDR-TB is complicated by a lack of knowledge and the fact that many potentially suitable drugs are not registered for pediatric use and there are no formulations suitable for children. This review highlights the need for more research to be done to assess the incidence and prevalence of DR-TB in pediatrics and the possible demand for the availability of suitable dosage forms to treat the condition.
Availability of data and materials
Data and materials are available from the corresponding author.
Change history
22 October 2022
A Correction to this paper has been published: https://doi.org/10.1186/s13643-022-02101-4
Abbreviations
- BCG:
-
Bacille Calmette-Guérin
- DR:
-
Drug-resistant
- DST:
-
Drug susceptibility test
- Emb:
-
Ethambutol
- EPTB:
-
Extrapulmonary tuberculosis
- Eth:
-
Ethionamide
- HIV:
-
Human immunodeficiency virus
- INH:
-
Isoniazid
- MDR-TB:
-
Multi-drug-resistant tuberculosis
- MTB:
-
Mycobacterium tuberculosis
- PTB:
-
Pulmonary tuberculosis
- PZA:
-
Pyrazinamide
- Rif:
-
Rifampicin
- TB:
-
Tuberculosis
- TBM:
-
Tuberculosis meningitis
- XDR-TB:
-
Extensively drug-resistant tuberculosis
References
Tuberculosis South Africa Project. WHO Global Tuberculosis Report 2020: USAID, Publication; 2020. Available at: https://tbsouthafrica.org.za/resources/who-global-tuberculosis-report-2020#background. Accessed 22 May 2021, 11:00
Latent TB infection and TB disease. Division of Tuberculosis Elimination, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Page last reviewed December 11th 2020. Available at: https://www.cdc.gov/tb/topic/basics/tbinfectiondisease.htm. Accessed 11 June 2022, 11:27.
Tuberculosis. John Hopkins Medicine. Available at: https://www.hopkinsmedicine.org/health/conditions-and-diseases/tuberculosis-tb. Accessed 11 June 2022, 11:40.
World Health Organization. Global Tuberculosis Report 2012. Geneva: WHO Press; 2012.
Falzon D, Jaramillo E, Schunemann HJ, Arentz M, Bauer M, Bayona T, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011. Eur Respir J. 2011;38:516–28. https://doi.org/10.1183/09031936.00073611 Falzon D., Gandhi N., Migliori G.B., Sotgiu G., Cox H.S., Holtz H.T., Hollm-Delgado M., Keshavjee S., DeRiemer K., Centis R., D’Ambrosio L., Lange C.G., Bauer M., Menzies D. on behalf of the Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Published in the European Respiratory Journal 2013 42: 156-168; DOI: 10.1183/09031936.00134712.
Tuberculosis: Multidrug-resistant tuberculosis (MDR-TB). WHO. Available at: https://www.who.int/news-room/questions-and-answers/item/tuberculosis-multidrug-resistant-tuberculosis-(mdr-tb)#:~:text=Multidrug%2Dresistant%20TB%20(MDR%2D,person%2Dto%2Dperson%20transmission. Accessed 12 June 2022, 18:59.
WHO announces updated definitions of extensively drug-resistant tuberculosis. WHO. Updated 27 January 2021. Available at: https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis. Accessed 12 June 2022, 19:05.
Maais BJ, Schaaf HS. Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin N Am. 2010;24:727–49.
Seddon JA, Hesseling CA, Willemse M, Donald PR, Schaaf SH. Culture-confirmed multidrug-resistant tuberculosis in children: clinical features, treatment, and outcome. Clin Infect Dis J. 2012;54(2):157–66.
Guidelines for the programmatic management of drug resistant tuberculosis - 2011 update. Geneva: World Health Organization; 2011 (WHO/HTM/TB/2011.6).
Ettehad D, Schaff HS, Seddon JA, Cooke GS, Ford N. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(6):449–56. PMID: 22373593. https://doi.org/10.1016/S1473-3099(12)70033-6.
Berti E, Galli L, Venturini E, de Martini M, Chiappini E. Tuberculosis in childhood: a systematic review of national and international guidelines. BMC Infect Dis. 2014;14(1):1–0 Available at: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-14-S1-S3. Accessed 25 May 2021, 10:28.
Gledovic Z, Grgurevic A, Pekinezovic T. Childhood tuberculosis in Serbia. Pediatr Infect Dis J. 2006;25(3):269–70 Available at: https://journals.lww.com/pidj/Fulltext/2006/03000/Childhood_Tuberculosis_in_Serbia.24.aspx. Accessed 13 June 2021.
Zeng X, Zhang Y, Kwong JSW, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10.
Tola HH, Khadoura KJ, Jimma W, Nedjat S, Majdzadeh R. Multidrug resistant tuberculosis treatment outcome in children in developing and developed countries: a systematic review and meta-analysis. Int J Infect Dis. 2020;96:12–8.
Loebstein R, Koren G. Clinical pharmacology and therapeutic drug monitoring in neonates and children. Pediatr Rev. 1998;19:423–8.
Padayatchi N, Bamber S, Dawood HF, Bobat R. Multidrug-resistant tuberculous meningitis in children in Durban, South Africa. Pediatr Infect Dis J. 2006;25(2):147–50.
Fairlie L, Beylis NC, Reubenson G, Moore DP, Madhi S. High prevalence of childhood multi-drug resistant tuberculosis in Johannesburg, South Africa: a cross sectional study. BMC Infect Dis J. 2011;11:1–9 Available at: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-11-28. Accessed 12 June 2021.
Erkens CGM, de Vries G, Keizer ST, Slump E, van den Hof S. The epidemiology of childhood tuberculosis in the Netherlands: still room for prevention. BMC Infect Dis. 2014;14 Available at: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-14-295. Accessed 15 June 2021.
Nabukeera-Barungi N, Wilmshurst J, Rudzani M, Nuttall J. Presentation and outcome of tuberculous meningitis among children: experiences from a tertiary children's hospital. Afr Health Sci J. 2014;14(1):143–9 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4449047/. Accessed 16 June 2021.
Ben A, Gargouri H, Koubaa L, Rekik M, Hammemi K, Ben Jemaa F, et al. The growing burden of childhood tuberculosis in Southern Tunisia: temporal trends across two decades: 1995-2016. Electron J Gen Med. 2019;16(4):em145. https://doi.org/10.29333/ejgm/109660.
Wang DM, Li QF, Zhu M, Wu GH, Li X, Xu YH, et al. Epidemiological, clinical characteristics and drug resistance situation of culture-confirmed children TBM in southwest of China: a 6-year retrospective study. BMC Infect Dis. 2020;20(1) Available at: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-020-05041-3. Accessed 21 June 2021.
Zhou XL, Chen QP, Wang MS. Prevalence of multidrug-resistant tuberculosis in suspected childhood tuberculosis in Shandong, China: a laboratory-based study. J Int Med Res. 2020;48(1) Available at: https://journals.sagepub.com/doi/10.1177/0300060519869715. Accessed 18 June 2021.
Wu XR, Yin QQ, Jiao AX, Xu BP, Sun L, Jiao WW, et al. Pediatric tuberculosis at Beijing Children’s Hospital: 2002-2010. Pediatrics. 2012;130(6):E1433–40.
World Health Organization. Tuberculosis In Women. Available at: www.who.int/tb. Accessed 18 July 2021.
Getahun H, Sculier D, Sismanidis C, Grzemska M, Raviglione M. Prevention, diagnosis, and treatment of tuberculosis in children and mothers: evidence for action for maternal, neonatal, and child health services. J Infect Dis. 2012;205:12.
Elettra B, Venturini LG, de Martini E, Chiappini M, E. Tuberculosis in Childhood: a Systematic Review of National and International Guidelines. BMC Infect Dis. 2014;14(53):10.
Zar HJ, Eley B, Nicol MP, Figaji A, Hawkridge A. Advances in childhood tuberculosis. S Afr Med J. 2012; Available at: http://www.samj.org.za/index.php/samj/article/view/5552/4240. Accessed: 18 July 2021.
World Health Organization. Global tuberculosis control: epidemiology, strategy, financing: WHO report 2009. World Health Organization; 2009.
Gebregergs G, Alemu W. Household contact screening adherence among tuberculosis patients in northern Ethiopia. PLoS One. 2015;10(5):e0125767.
Sekandi J, Neuhauser D, Smyth K, Whalen C. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int J Tuberc Lung Dis. 2009;13(4):508–13.
Gupta M, Saibannavar A, Kumar V. Household symptomatic contact screening of newly diagnosed sputum smears positive tuberculosis patients - an effective case detection tool. Lung India. 2016;33(2):159–62.
Begun M, Newall A, Marks G, Wood J. Contact tracing of tuberculosis: a systematic review of transmission modelling studies. PLoS One. 2013;8(9):e72470.
World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. Geneva: World Health Organization; 2013.
Black RE, Allen LH, Bhutta AZ, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60.
Jaganath D, Mupere E. Childhood Tuberculosis and Malnutrition. J Infect Dis. 2012; Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3502375/. Accessed: 18 Jul 2021.
Chiappini E, Bonsignori F, Orlandini E, Sollai S, Venturini E, Galli L, et al. Increasing incidence of tuberculosis in Tuscan Youth, 1997 to 2011. Pediatr Infect Dis J. 2013;32(11):1289–91 Available at: https://journals.lww.com/pidj/Fulltext/2013/11000/Increasing_Incidence_of_Tuberculosis_in_Tuscan.30.aspx. Accessed 15 June 2021.
Alvarez GG, Clark M, Altpeter E, Douglas P, Jones J, Paty MC, et al. Pediatric tuberculosis immigration screening in high-immigration, low-incidence countries. Int J Tuberc Lung Dis. 2010;14(12):1530–7.
Jenkins HE, Yuen MC. The burden of multidrug-resistant tuberculosis in children. Int J Tuberc Lung Dis. 2018;22(5):3–6 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5975247/. Accessed 18 Jul 2021.
Abubakar I, Zignol M, Falzon D, Raviglione M, Ditiu L, Masham S, et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013;13:529–39.
Seddon JA, Hesseling AC, Godfrey-Faussett P, Schaaf HS. High treatment success in children treated for multidrug-resistant tuberculosis: an observational cohort study. Thorax. 2014; Available at: https://thorax.bmj.com/content/69/5/458. Accessed 18 Jul 2021.
Management of drug-resistant TB in children. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children. 2nd ed. Geneva: World Health Organization; 2014. Available at: https://www.ncbi.nlm.nih.gov/books/NBK214453/. Accessed 18 Jul 2021
Funding
No funding was required for this review study.
Author information
Authors and Affiliations
Contributions
SH is credited with the conception of the review, the coordination of the systematic review, the development of the search strategy, the search and selection of studies to be included in the review, the extraction and management of quantitative and qualitative data, the assessment of methodological quality, the filtering of all reference materials, the integration and interpretation of the data, the drafting of the manuscript and is the principal reviewer. EW is credited with the review of the search strategy, the search and selection of studies to be included in the review, the extraction and management of quantitative and qualitative data, the assessment of methodological quality, the integration and interpretation of the data, and the review of the manuscript. KBM is credited with the review of the search strategy, the assessment of the studies before data extraction, the review of the manuscript, and the co-supervisor of the review. VB is credited with the review of the manuscript, the coordination of the systematic review, and the co-supervisor of the review. FO is credited with the conception of the review, the review of the manuscript, and the overall supervision of the review. All authors have reviewed and accepted the final manuscript of the review for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was not required as this review is based on previously published articles. The review does not include the enrolment of animal or human subjects.
Consent for publication
None.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: There is an error on the publication with respect to the affiliation of authors with Kofi Boamah Mensah & Frasia Oosthuizen. Affiliation 3 should be affiliated to Kofi Boamah Mensah.
Supplementary Information
Additional file 1.
Search strategy.
Additional file 2.
Quality assessment tool.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Harichander, S., Wiafe, E., Mensah, K.B. et al. The incidence of TB and MDR-TB in pediatrics and therapeutic options: a systematic review. Syst Rev 11, 157 (2022). https://doi.org/10.1186/s13643-022-02023-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-022-02023-1