Abstract
Background
The aim of our study was to determine through a systematic review and meta-analysis the incubation period of COVID-19. It was conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA). Criteria for eligibility were all published population-based primary literature in PubMed interface and the Science Direct, dealing with incubation period of COVID-19, written in English, since December 2019 to December 2020. We estimated the mean of the incubation period using meta-analysis, taking into account between-study heterogeneity, and the analysis with moderator variables.
Results
This review included 42 studies done predominantly in China. The mean and median incubation period were of maximum 8 days and 12 days respectively. In various parametric models, the 95th percentiles were in the range 10.3–16 days. The highest 99th percentile would be as long as 20.4 days. Out of the 10 included studies in the meta-analysis, 8 were conducted in China, 1 in Singapore, and 1 in Argentina. The pooled mean incubation period was 6.2 (95% CI 5.4, 7.0) days. The heterogeneity (I2 77.1%; p < 0.001) was decreased when we included the study quality and the method of calculation used as moderator variables (I2 0%). The mean incubation period ranged from 5.2 (95% CI 4.4 to 5.9) to 6.65 days (95% CI 6.0 to 7.2).
Conclusions
This work provides additional evidence of incubation period for COVID-19 and showed that it is prudent not to dismiss the possibility of incubation periods up to 14 days at this stage of the epidemic.
Similar content being viewed by others
Background
Since December 2019, the world is facing the pandemic of COVID-19. As of December 8, 2020, a total of cumulative confirmed cases were estimated at more than 68 million and 1.5 million cumulative deaths with a case fatality rate of 2.28% [1, 2].
While awaiting a vaccine, massive public health interventions such as social awareness, social distancing, isolation, quarantine, contact tracing, targeted screening, and border controls have been implemented nationally and globally to limit transmissibility and contain the epidemic since late January [3,4,5,6]. The incubation period, one of the key epidemiological parameters, is essential to epidemiological case definition, to determine the appropriate duration of quarantine and to estimate the size of the epidemics. Therefore, it was rapidly being studied from incoming case reports as the epidemic continues. Several studies have confirmed that cases are infectious during the asymptomatic period (latency period) prior to onset and that disease transmission may be carried out [7,8,9,10]. Up to this point, the quarantine and isolation duration of exposed or suspected cases is set at 14 days, which is the longest incubation time expected based on initial observations of SARS-CoV-2 and similarity to severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) [11].
The distribution of the incubation period in most of the literature is either described through a parametric model or its empirical distribution based on the observed incubation period from the contact-tracing data (specific data indicating the time of exposure). However, the contact-tracing data are challenging and expensive to obtain, and their accuracy can be highly influenced by recall bias. Therefore, previous population dynamics studies tend to make assumptions about the distribution of the incubation period without using the observed data called modeling studies, and we are talking here about an estimate of the incubation period.
Furthermore, estimating and standardizing the incubation period of COVID-19 may vary depending on climate [12], on age or the genetic of the individuals, their pathologies, or their treatment like the long-term use of glucocorticoids which might cause atypical infections and a long incubation period [13].
Thus, a specific maximum duration of the incubation period is needed to answer if the 14-day quarantine is sufficient to protect against the spread of the pandemic. In this systematic review and meta-analysis, we tried to identify studies that recruited symptomatic patients, regardless of sex or age diagnosed with COVID-19, and calculated or estimated the incubation period between December 2019 and December 2020.
Methods
Criteria for considering studies for this review
Types of studies
The protocol for this review was registered with PROSPERO (international prospective register of systematic reviews) under the number CRD42020196347 (https://www.crd.york.ac.uk/prospero/).
This systematic review was conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) to study the length of incubation period during the COVID-19 pandemic.
Criteria for eligibility were all published population-based primary literature dealing with incubation period of COVID-19, since December 2019. We included full-text publications and excluded all articles not accepted or peer reviewed, not written in English, editorials, perspective, letter to the editor, review, article info, and comments. We only took articles that used RT-PCR for the diagnosis of COVID-19. Since randomized controlled trials (RCTs) do not apply to this topic, only observational studies with no limit on the number of participants were included. There were no limitations given the types of outcome measures: we accepted all documents that presented results even without a statistical parameter of variability.
Types of participants
We included individuals with a confirmed diagnosis of COVID-19 regardless of the severity of symptoms or associated comorbidities. There were no age, gender, or ethnicity restrictions. We excluded studies including populations with other coronavirus diseases (severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS)). We also excluded studies including populations with mixed viral diseases (e.g., COVID-19 plus influenza).
Types of outcome measures
The incubation period was defined as the amount of time between the exposure to SARS-CoV-2 and the onset of symptoms [14].
Estimated and calculated incubation period
-
1.
Incubation periods were calculated from observed data based on specific dates indicating the time of exposure. Measures of central tendency were ranges, mean, and median with appropriate dispersion parameters (interquartile range (IQR) and standard deviation (SD)).
-
2.
Incubation periods were estimated on incomplete data or imprecise exposure time using several models of distribution such as log-normal, Weibull, and Gamma distribution [15, 16]. They were presented by the mean and its 95% confidence interval (95% CI) with percentiles of the distribution in some studies.
Search methods for identification of studies
Electronic searches
The literature search was developed by WD and verified by a research librarian. It was carried out on Medline via its PubMed interface, through the following documentary query, as of 01/12/2020: (“Infectious Disease Incubation Period”[Mesh]) AND (“COVID-19”[All Fields] OR “COVID-2019”[All Fields] OR “severe acute respiratory syndrome coronavirus 2”[Supplementary Concept] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “2019-nCoV”[All Fields] OR “SARS-CoV-2”[All Fields] OR “2019nCoV”[All Fields] OR ((“Wuhan”[All Fields] AND (“coronavirus”[Mesh Terms] OR “coronavirus”[All Fields])) AND (2019/12[PDAT] OR 2020[PDAT]))). No filter was applied.
The research was also conducted on Science Direct through its advanced research (only the research article using COVID-19 and the incubation period in the title, abstract, or keywords specified by the author). The literature search was completed on 01/12/2020.
Data collection and analysis
Selection of studies and data extraction
The references were managed using the Zotero software. Firstly, and after exclusion of duplicates, all titles and abstracts of publications identified through the initial primary search were single reviewed for relevance.
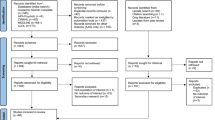
Secondly, the final selection of the articles was based on the full texts of papers by retrieving and checking for relevance by two authors (WD, AI) independently of each other, with referral to MJ in the case of discordant opinions. Studies were excluded if they were off topic or if they gave no number or statistics. One article was retracted from Medline during the process of final selection. We documented the study selection process in a flow chart and showed the total numbers of retrieved references and the numbers of included and excluded studies (Fig. 1).
One review author WD performed all data extractions. Two other review authors (AI and MJ) verified the accuracy and the plausibility of extractions.
All authors participated in quality assessment, level of evidence, and grades of recommendations. DW and AI independently reviewed all studies, with disagreements resolved by referral to MJ.
The following bibliometric, epidemiological data were extracted: authors, study design, country or geographical region, period of study, sample size, data and source collection, general characteristics of the studied population (age, sex ratio), exposure history, and duration of incubation period.
The meta-analysis was based on the mean of the distributions either in observed or estimated log-normal distribution data. The meta-analysis included all studies that reported the mean with its SD of the observed incubation period or the mean and corresponding CI of the normal log distribution. Excluded studies where those representing outcome reporting bias. The selection of studies to include in the meta-analysis was conducted by the primary author WD.
Assessment of risk of bias
Quality assessment was done according to recommendation of “Quality Assessment Tool for Quantitative Studies” developed in Canada by the Effective Public Health Practice Project (EPHPP) [17]. Once the assessment is fulfilled using a number pre-determined criteria, each examined practice receives a mark ranging between “strong,” “moderate,” and “weak” in three categories (study design, data collection practices, and selection bias). After discussing the ratings and resolving any discrepancy, the global rating for each paper was according to the sum of “weak” ratings given to the three categories (1: strong=0 “weak”; 2: moderate=1 “weak”; and 3: weak=≥2 “weak”) (Additional file 1).
Risk of bias was done according to Chapter 25 of Cochrane Handbook: assessing risk of bias in a non-randomized study [: /handbook/current/chapter-25]; assessing risk of bias was presented using Revman 5 tools (Review Manager (RevMan) [Computer program] version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.).
Level of evidence and grades of recommendations were assessed according to the Scottish Intercollegiate Guidelines Network (SIGN) [18].
Levels of evidence were graded into 8 levels from 1++ (high quality meta-analyses, systematic reviews of randomized controlled trials (RCTs), or RCTs with a very low risk of bias) to 4 (expert opinion).
There were 4 grades of recommendations (A, B, C, and D) based on the results of the level of evidence. D is given if the evidence level was 3 or 4 or extrapolated from studies rated as 2+ [18].
Data synthesis
A random effects meta-analysis was conducted in the Open Meta Analyst software [19], of the calculated and estimated mean of the log-normal distribution. Forest plots were produced using the same package. Heterogeneity between the studies was assessed using both the I2 statistic with a cutoff of 50% and the χ2 test with P-value <0.10 and investigated by conducting subgroup analyses of the data set following these moderator variables: population of studies (Chinese or not), severity (hospitalized or not), sex ratio (> or < 1), study quality, and method of calculation (estimated or calculated).
Results
Results of the search
We identified 117 records through Medline and Science Direct database searches. After removing 3 duplicates, we screened 114 records based on their titles and abstracts, leaving 48 full manuscripts to be assessed for eligibility (Fig. 1). As a result of this assessment, 42 studies met the inclusion criteria.
Study characteristics
Over the 42 observational studies, the quality assessment gave 9 strong, 19 moderate, and 14 weak studies (Additional file 1). Most of the studies had a retrospective data collection; 10 had a prospective one [7,8,9, 20,21,22,23,24,25,26]. The sampling methods and sample size recorded varied substantially across studies. In some cases, entire provinces or villages were selected [8, 9, 20, 24,25,26,27,28,29,30,31,32,33,34,35,36,37] whereas in others the focus was on serial cases or family clusters [7, 8, 24, 38,39,40,41,42], hospitals, and laboratory [43,44,45,46,47,48,49,50]. Almost studies were done in China (30 studies), including a study of around 8579 people in 30 provinces [23]. Four studies was conducted in Korea [9, 28, 34, 51], three in Singapore [21, 48, 52], one in France [41], one in Brunei [32], one in Argentina [33], one in Saudi Arabia [53], and two in Germany [37, 42]. The period of all studies was between January and May 2020 (Table 1).
Risk of bias within studies
All the 42 observational studies had the third level of evidence (non-analytic studies) with a grading D of recommendation.
Most of study had the risk of recall bias (Fig. 2).
Results of individual studies
Based on studies calculating incubation period for SARS-CoV-2
The median incubation period was calculated in 17 studies ranging from 2 to 12 days with an IQR lower bound of 2 days and higher bound of 14 days. In 9 studies, the mean was ranging from 3.9 to 8.98 days.
The total incubation period ranged in 9 studies from 0 to 26 days. One study was restricted to pediatric patients infected with SARS-CoV-2 from 7 months to 17 years old. The average incubation period was 8 days ranged from 1 day to 13 days [43] (Table 2).
Based on studies estimating incubation periods for SARS-CoV-2
The log-normal distribution was the best fitting to the data in 7 studies with an estimated mean ranging from 5.0 to 7.4 (95% CI, 2 to 20 days) (Table 3). The median was estimated in 9 studies and had a maximum value of 7.2 (95% CI, 6.4 to 7.9 days) [58]. The estimated 95th percentile of the distribution had a maximum value of 16.32 days. The maximum 97.5th and 99th percentile of the distribution was 11.5 days and 20.4 days respectively (Table 3).
Mean incubation period and meta-analysis
The estimated mean incubation period obtained from the included studies and the pooled mean are presented in Fig. 3. Out of the 10 included studies in the meta-analysis, 8 were conducted in China, 1 in Singapore, and 1 in Argentina. The pooled mean incubation period was 6.2 (95% CI 5.4, 7.0) days. Heterogeneity testing (I2 = 77.1%; p < 0.001) revealed notable differences among the included studies in the meta-analysis.
Moderator variables were analyzed to identify and eliminate the observed heterogeneity: population of studies, severity, sex-ratio, study quality, and method of calculation (Table 4).
The heterogeneity was decreased when we included the study quality and the method of calculation used as moderator variables (I2 0%). The mean incubation period ranged from 5.2 (95% CI 4.4 to 5.9) to 6.65 days (95% CI 6.0 to 7.2) (Fig. 3).
Discussion
This review includes 42 studies done predominantly in China showing a mean and median incubation period of maximum 8 days and 12 days respectively. The pooled mean incubation period for COVID-19 is 6.2 (95% CI 5.4, 7.0) and may vary depending on population of studies, severity, sex-ratio, study quality, and method of calculation. In various parametric models, the 95th percentiles were in the range 10.3–16 days, which was not consistent with the first WHO reports [61]. While it was difficult to estimate the right hand tail of the incubation period distribution based on small sample sizes, the highest 99th percentile would be as long as 20.4 days, and this indicates that long incubation periods are possible. Lauer et al. [56] estimated that 101 out of 10,000 cases (99th percentile=482) would develop symptoms after 14 days of active monitoring or quarantine. Wang et al. [57] reported that about 7.45% patients were overestimated with longer than 14 days of incubation periods. Although many studies did not match with the inclusion criteria in our review, they are worthy to be mentioned. In a research letter studying serial cases of 6 patients infected with SARS-CoV-2 in China, Bai et al. reported that the incubation period of patient 1 was 19 days [63]. Based on 175 case details reported by 64 web pages between January20, 2020, and February 12, 2020, Leung estimated a mean of 7.2 (95% CI 6.1 to 8.4) with a 95th percentile of the Weibull distribution of 14.6 days (95% CI, 12.1 to 17.1) [64]. On the other hand, in the beginning of the pandemic of COVID-19, some studies found that there is no observable difference between the incubation time for SARS-CoV-2, severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV) [11].
In our results, studies with contact tracing or exposure history of close contact showed a range of incubation period not exceeding 14 days [8, 38, 40, 41]. In fact, potential direct transmission could be related to a higher infecting dose and higher virulence of the strain that could lead to a shorter incubation period [65]. Indeed, Yu et al. showed that the incubation period was significantly shorter among patients who had multiple exposures to confirmed cases in the same province (Shanghai) (median 7.5 days; interquartile range (IQR) 5–7.9 days) compared with patients who had travel history in Wuhan (median 7.8days; (IQR) 5–8.2days) [30]. These results strengthen the hypothesis that a higher infecting dose could have been transmitted by the index case leading to a shorter incubation period compared with cases associated with “indirect” transmission.
In our study, there was considerable heterogeneity investigated with subgroup analysis. Several articles have shown that the incubation period differs between individuals according to their age or sex. Tan et al. showed that age-specific mean incubation periods were statistically significantly different across different age categories. The longest was observed among those aged 70+ (7.56 days, 95% CI 5.31–9.80) while the shortest was among those aged 60–69 years (4.69 days, 95% CI 3.86–5.52) and <30 years (4.95 days, 95% CI 4.31–5.58) [48]. However, Qin et al. concluded that there is no evidence that the incubation time depends on age [35].
Systematic reviews and meta-analyses conducted from 1 December 2019 to 11 March showed that the pooled incubation period mean was 5.68 (99% CI: 4.78, 6.59) days with heterogeneity testing (I2 = 98.4%) [66]. As in our findings, this heterogeneity test revealed notable differences among the included studies.
On the other hand, and based on the log-normal distribution, McAloon et al. [67] found in a meta-analysis conducted from December 1, 2019, to April 8, 2020, a mean of 5.8 days (95% CI 5.0–6.7) for the corresponding incubation period. However, our results with an estimated incubation mean of 5.2 (95% CI, 4.4–5.9) were more reliable since the heterogeneity test was zero.
Our study has some notable limitations. First, in most studies, the data were collected retrospectively, resulting in a recall bias (uncertain exact dates of exposure) and some missing data that would inevitably influence our assessment. Second, due to urgent timeline for data extraction and analysis, many studies have estimated the incubation period in a limited case number in a short period of time, which necessitates the cautious interpretation of the generalizability of our findings. The numbers were too small to detect systematic differences in incubation time with age or sex. Third, not all studies (except Tian et al. [44]) paid attention to asymptomatic patients, so our review may represent an erroneous incubation period. Although there is interest on asymptomatic transmission, we were unable to address this point in our review, and further studies should be done to better understand disease transmissibility of asymptomatic cases.
Conclusions
This work provides additional evidence of incubation period for COVID-19 and showed that it is prudent not to dismiss the possibility of incubation periods up to 14 days at this stage of the epidemic. As the epidemic continues, it remains important to collect more information on incubation periods through longitudinal studies with more patients so that we can conduct subgroup analysis and better understand the transmissibility of.
Availability of data and materials
Not applicable
Abbreviations
- EPHPP:
-
Effective Public Health Practice Project
- MERS-CoV:
-
Middle East respiratory syndrome coronavirus
- IQR:
-
Interquartile range
- RCTs:
-
Randomized controlled trials
- SARS-CoV:
-
Severe acute respiratory syndrome coronavirus
- SD:
-
Standard deviation
- SIGN:
-
Scottish Intercollegiate Guidelines Network
- 95% CI:
-
95% confidence interval
References
Coronavirus COVID-19 (2019-nCoV) [Internet]. [cited 2020 Dec 8]. Available from: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.
Weekly operational update on COVID-19 - 7 December 2020 [Internet]. [cited 2020 Dec 8]. Available from: https://www.who.int/publications/m/item/weeklyoperational-update-on-covid-19%2D%2D-7-december-2020.
Quilty BJ, Clifford S, Flasche S, Eggo RM, CMMID nCoV working group. Effectiveness of airport screening at detecting travellers infected with novel coronavirus (2019-nCoV). Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(5).
Gostic K, Gomez AC, Mummah RO, Kucharski AJ, Lloyd-Smith JO. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. eLife. 9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7060038/. [cited 2020 Dec 8].
Tang B, Wang X, Li Q, Bragazzi NL, Tang S, Xiao Y, et al. Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions. J Clin Med. 2020;9(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074281/. [cited 2020 Dec 8].
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400.
Gao Y, Shi C, Chen Y, Shi P, Liu J, Xiao Y, et al. A cluster of the corona virus disease 2019 caused by incubation period transmission in Wuxi, China. J Infect. 2020;80(6):666–70. https://doi.org/10.1016/j.jinf.2020.03.042.
Huang L, Zhang X, Zhang X, Wei Z, Zhang L, Xu J, et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect. 2020;80(6):e1–13. https://doi.org/10.1016/j.jinf.2020.03.006.
Chun JY, Baek G, Kim Y. Transmission onset distribution of COVID-19. Int J Infect Dis. 2020;99:403–7. https://doi.org/10.1016/j.ijid.2020.07.075.
Jing Q-L, Liu M-J, Zhang Z-B, Fang L-Q, Yuan J, Zhang A-R, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(10):1141–50. https://doi.org/10.1016/S1473-3099(20)30471-0.
Jiang X, Rayner S, Luo M-H. Does SARS-CoV-2 has a longer incubation period than SARS and MERS? J Med Virol. 2020;92(5):476–8. https://doi.org/10.1002/jmv.25708.
Şahin M. Impact of weather on COVID-19 pandemic in Turkey. Sci Total Environ. 2020;728:138810. https://doi.org/10.1016/j.scitotenv.2020.138810.
Han Y, Jiang M, Xia D, He L, Lv X, Liao X, et al. COVID-19 in a patient with long-term use of glucocorticoids: a study of a familial cluster. Clin Immunol Orlando Fla. 2020;214:108413. https://doi.org/10.1016/j.clim.2020.108413.
Armenian HK, Lilienfeld AM. Incubation period of disease. Epidemiol Rev. 1983;5(1):1–15. https://doi.org/10.1093/oxfordjournals.epirev.a036254.
Virlogeux V, Li M, Tsang TK, Feng L, Fang VJ, Jiang H, et al. Estimating the distribution of the incubation periods of human avian influenza A(H7N9) virus infections. Am J Epidemiol. 2015 Oct 15;182(8):723–9. https://doi.org/10.1093/aje/kwv115.
Nishiura H. Early efforts in modeling the incubation period of infectious diseases with an acute course of illness. Emerg Themes Epidemiol. 2007 May 11;4(1):2. https://doi.org/10.1186/1742-7622-4-2.
Quality assessment tool for quantitative studies [Internet]. Effective Public Healthcare Panacea Project. [cited 2020 Dec 8]. Available from: https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/
Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323(7308):334–6. https://doi.org/10.1136/bmj.323.7308.334.
OpenMetaAnalyst: Wallace, Byron C, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, et al. Closing the gap between methodologists and end-users: R as a computational back-End. J Statistical Software. 49(2012):5.
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–207.
Pung R, Chiew CJ, Young BE, Chin S, Chen MI-C, Clapham HE, et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. The Lancet. 2020 Mar 28;395(10229):1039–46. https://doi.org/10.1016/S0140-6736(20)30528-6.
Song Y-S, Hao Y-B, Liu W-W, Zhang S-S, Wang P, Fan T-L. Clinical features of 17 patients with 2019-nCoV. Eur Rev Med Pharmacol Sci. 2020;24(20):10896–901. https://doi.org/10.26355/eurrev_202010_23454.
Zhang J, Litvinova M, Wang W, Wang Y, Deng X, Chen X, et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis. 2020 Jul 1;20(7):793–802. https://doi.org/10.1016/S1473-3099(20)30230-9.
Bi Q, Wu Y, Mei S, Ye C, Zou X, Zhang Z, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–9. https://doi.org/10.1016/S1473-3099(20)30287-5.
Wang K, Gao J, Wang H, Wu X, Yuan Q, Guo F, et al. Epidemiology of 2019 novel coronavirus in Jiangsu Province, China after wartime control measures: a population-level retrospective study. Travel Med Infect Dis. 2020;35:101654.
Yang L, Dai J, Zhao J, Wang Y, Deng P, Wang J. Estimation of incubation period and serial interval of COVID-19: analysis of 178 cases and 131 transmission chains in Hubei province, China. Epidemiol Infect. 2020;148:e117.
Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Ki M. Task Force for 2019-nCoV. Epidemiologic characteristics of early cases with 2019 novel coronavirus (2019-nCoV) disease in Korea. Epidemiol. Health. 2020;42:e2020007.
Linton NM, Kobayashi T, Yang Y, Hayashi K, Akhmetzhanov AR, Jung S, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9(2) Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074197/. [cited 2020 Dec 9].
Yu X, Sun X, Cui P, Pan H, Lin S, Han R, et al. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai. China. Transbound Emerg Dis. 2020;67(4):1697–707. https://doi.org/10.1111/tbed.13604.
Xiao Z, Xie X, Guo W, Luo Z, Liao J, Wen F, et al. Examining the incubation period distributions of COVID-19 on Chinese patients with different travel histories. J Infect Dev Ctries. 2020;14(4):323–7.
Wong J, Chaw L, Koh WC, Alikhan MF, Jamaludin SA, Poh WWP, et al. Epidemiological investigation of the first 135 COVID-19 cases in Brunei: implications for surveillance, control, and travel restrictions. Am J Trop Med Hyg. 2020;103(4):1608–13. https://doi.org/10.4269/ajtmh.20-0771.
Viego V, Geri M, Castiglia J, Jouglard E. Incubation period and serial interval of Covid-19 in a chain of infections in Bahia Blanca (Argentina). Cienc Saude Coletiva. 2020;25(9):3503–10. https://doi.org/10.1590/1413-81232020259.20852020.
Ryu S, Ali ST, Jang C, Kim B, Cowling BJ. Effect of nonpharmaceutical interventions on transmission of severe acute respiratory syndrome coronavirus 2, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2406–10. https://doi.org/10.3201/eid2610.201886.
Qin J, You C, Lin Q, Hu T, Yu S, Zhou X-H. Estimation of incubation period distribution of COVID-19 using disease onset forward time: a novel cross-sectional and forward follow-up study. Sci Adv. 2020;6(33):eabc1202.
You C, Deng Y, Hu W, Sun J, Lin Q, Zhou F, et al. Estimation of the time-varying reproduction number of COVID-19 outbreak in China. Int J Hyg Environ Health. 2020;228:113555. https://doi.org/10.1016/j.ijheh.2020.113555.
Wieland T. A phenomenological approach to assessing the effectiveness of COVID-19 related nonpharmaceutical interventions in Germany. Saf Sci. 2020;131:104924. https://doi.org/10.1016/j.ssci.2020.104924.
Pung R, Chiew CJ, Young BE, Chin S, Chen MI-C, Clapham HE, et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet Lond Engl. 2020;395(10229):1039–46.
Song R, Han B, Song M, Wang L, Conlon CP, Dong T, et al. Clinical and epidemiological features of COVID-19 family clusters in Beijing. China. J Infect. 2020;81(2):e26–30. https://doi.org/10.1016/j.jinf.2020.04.018.
Xia X-Y, Wu J, Liu H-L, Xia H, Jia B, Huang W-X. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID-19. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2020;127:104360. https://doi.org/10.1016/j.jcv.2020.104360.
Bernard Stoecklin S, Rolland P, Silue Y, Mailles A, Campese C, Simondon A, et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Eurosurveillance. 2020;25(6) Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7029452/. [cited 2020 Dec 9].
Böhmer MM, Buchholz U, Corman VM, Hoch M, Katz K, Marosevic DV, et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20(8):920–8. https://doi.org/10.1016/S1473-3099(20)30314-5.
Chen J, Zhang Z-Z, Chen Y-K, Long Q-X, Tian W-G, Deng H-J, et al. The clinical and immunological features of pediatric COVID-19 patients in China. Genes Dis. 2020;7(4):535–41. https://doi.org/10.1016/j.gendis.2020.03.008.
Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, et al. Characteristics of COVID-19 infection in Beijing. J Infect. 2020;80(4):401–6. https://doi.org/10.1016/j.jinf.2020.02.018.
Zheng Y, Xiong C, Liu Y, Qian X, Tang Y, Liu L, et al. Epidemiological and clinical characteristics analysis of COVID-19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol Res. 2020;157:104821. https://doi.org/10.1016/j.phrs.2020.104821.
Zhao C, Xu Y, Zhang X, Zhong Y, Long L, Zhan W, et al. Public health initiatives from hospitalized patients with COVID-19. China. J Infect Public Health. 2020;13(9):1229–36. https://doi.org/10.1016/j.jiph.2020.06.013.
Wang X, Zhou Q, He Y, Liu L, Ma X, Wei X, et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55(6).
Tan WYT, Wong LY, Leo YS, Toh MPHS. Does incubation period of COVID-19 vary with age? A study of epidemiologically linked cases in Singapore. Epidemiol Infect. 2020;148:e197.
Li J, Ding J, Chen L, Hong L, Yu X, Ye E, et al. Epidemiological and clinical characteristics of three family clusters of COVID-19 transmitted by latent patients in China. Epidemiol Infect. 2020;148:e137.
Lou B, Li T-D, Zheng S-F, Su Y-Y, Li Z-Y, Liu W, et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur Respir J. 2020;56(2).
Lee H, Kim K, Choi K, Hong S, Son H, Ryu S. Incubation period of the coronavirus disease 2019 (COVID-19) in Busan, South Korea. J Infect Chemother Off J Jpn Soc Chemother. 2020;26(9):1011–3. https://doi.org/10.1016/j.jiac.2020.06.018.
Tindale LC, Stockdale JE, Coombe M, Garlock ES, Lau WYV, Saraswat M, et al. Evidence for transmission of COVID-19 prior to symptom onset. eLife. 2020;22:9.
Alsofayan YM, Althunayyan SM, Khan AA, Hakawi AM, Assiri AM. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Public Health. 2020;13(7):920–5. https://doi.org/10.1016/j.jiph.2020.05.026.
Xu T, Chen C, Zhu Z, Cui M, Chen C, Dai H, et al. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2020;94:68–71.
Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25(5).
Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;10 [cited 2020 Dec 9]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7081172/.
Wang P, Lu J, Jin Y, Zhu M, Wang L, Chen S. Statistical and network analysis of 1212 COVID-19 patients in Henan, China. Int J Infect Dis. 2020;95:391–8. https://doi.org/10.1016/j.ijid.2020.04.051.
Yu P, Zhu J, Zhang Z, Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757–61.
Zhang H, Shang W, Liu Q, Zhang X, Zheng M, Yue M. Clinical characteristics of 194 cases of COVID-19 in Huanggang and Taian. China. Infection. 2020;48(5):687–94. https://doi.org/10.1007/s15010-020-01440-5.
Nie X, Fan L, Mu G, Tan Q, Wang M, Xie Y, et al. Epidemiological characteristics and incubation period of 7015 confirmed cases with coronavirus disease 2019 outside Hubei Province in China. J Infect Dis. 2020;222(1):26–33.
Coronavirus disease (COVID-19) situation reports [Internet]. [cited 2020 Dec 9]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
Liu J, Liao X, Qian S, Yuan J, Wang F, Liu Y, et al. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis. 2020;26(6):1320–3. https://doi.org/10.3201/eid2606.200239.
Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Apr 14;323(14):1406–7. https://doi.org/10.1001/jama.2020.2565.
Leung C. The difference in the incubation period of 2019 novel coronavirus (SARS-CoV-2) infection between travelers to Hubei and nontravelers: the need for a longer quarantine period. Infect Control Hosp Epidemiol. 2020;41(5):594–6. https://doi.org/10.1017/ice.2020.81.
Virlogeux V, Fang VJ, Wu JT, Ho L-M, Malik Peiris JS, Leung GM, et al. Incubation period duration and severity of clinical disease following severe acute respiratory syndrome coronavirus infection. Epidemiol Camb Mass. 2015;26(5):666–9. https://doi.org/10.1097/EDE.0000000000000339.
Khalili M, Karamouzian M, Nasiri N, Javadi S, Mirzazadeh A, Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e130.
McAloon C, Collins Á, Hunt K, Barber A, Byrne AW, Butler F, et al. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10(8):e039652.
Acknowledgements
Not applicable.
Funding
No funding
Author information
Authors and Affiliations
Contributions
WD: conceptualization, research literature, and writing—original draft.
IA and JM: research literature and quality assessment. NZ, RG, SBF: contributor in writing the manuscript. HG: revision of all manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Quality assessment of the included studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dhouib, W., Maatoug, J., Ayouni, I. et al. The incubation period during the pandemic of COVID-19: a systematic review and meta-analysis. Syst Rev 10, 101 (2021). https://doi.org/10.1186/s13643-021-01648-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-021-01648-y