Abstract
Background
Growth hormone (GH) deficiency is common in patients with Prader-Willi syndrome (PWS) and leads to short adult stature. The current study assessed clinical outcomes based on real-world observational data in pediatric patients with PWS who were treated with GH.
Methods
Data from patients previously naïve to treatment with GH who began therapy with somatropin were collected from 2006 to 2016 in the observational American Norditropin® Studies: Web-Enabled Research (ANSWER) Program® and NordiNet® International Outcome Study. Variables affecting change from baseline in height standard deviation scores (HSDS; n = 129) and body mass index standard deviation scores (BMI SDS; n = 98) were determined.
Results
Patients included in both HSDS and BMI SDS analyses were treated with a mean GH dose of 0.03 mg/kg/d (SD, 0.01 mg/kg/d). Results from the HSDS analysis revealed that baseline age and years on treatment had a significant impact on the change in HSDS. In the BMI SDS analysis, longer GH treatment time led to a greater change in BMI SDS from baseline, and patients with a higher BMI at the start of treatment had a greater decrease in BMI over time.
Conclusions
GH is effective in the management of children with PWS. Earlier treatment resulted in a greater gain in height, and a longer treatment period resulted in better outcomes for both height and BMI.
Trial registration
This study was registered with ClinicalTrials.gov (NCT01009905) on November 9, 2009.
Similar content being viewed by others
Background
Prader-Willi syndrome (PWS) is a rare multisystemic genetic disorder that arises from the lack of expression of paternally inherited genes known to be imprinted and located in the 15q11-q13 region. In approximately 70% of cases, PWS is caused by a non-inherited deletion in the paternally derived chromosome in that region, while maternal disomy 15 causes 25% of PWS cases, and genomic imprinting defects cause 3% of cases [1]. The remaining 2% of cases are caused by rare translocations. The overall clinical picture of PWS is characterized by hypotonia, poor feeding in infancy, hyperphagia with evolving obesity, hypogonadism, decreased adult height, and cognitive and behavioral disabilities [1, 2].
More than 50% of infants and children with PWS are, or will become, GH deficient as determined by standard testing protocols [2]. Children with PWS who have GH deficiency do not experience height gain acceleration typical of puberty, leading to short adult stature (mean final height of 155 cm and 148 cm in males and females, respectively). Diminished growth can be observed as early as the prenatal period and can be sustained through infancy. Severe hypotonia is associated with feeding difficulties; however, short stature in PWS due to GH deficiency is unrelated to nutritional deficiency or hypothyroidism [1, 3].
Data on the successful use of exogenous human GH to improve linear growth in children with PWS were first published in 1987 [4] and were supported by subsequent investigations that showed sustained treatment with GH could improve growth, body composition, physical strength and agility, bone mineral density, fat utilization, cognition, and adaptive functioning [5,6,7,8]. Around the same time, recombinant human GH (rhGH) became available [9]. Approved by the US Food and Drug Administration (FDA) for the treatment of PWS in 2000 [3], rhGH demonstrated efficacy in increasing short-term growth and adult height in patients with a range of growth disorders [10,11,12,13,14,15]. The most recent clinical guidelines for the use of rhGH for PWS recommend that infants and children begin with a GH dose of 0.5 mg/m2/d, incrementally titrating every 3 to 6 months based on clinical response toward a dose of 1.0 mg/m2 [2].
Somatropin (Norditropin®; Novo Nordisk A/S, Bagsværd, Denmark) is an rhGH indicated for multiple conditions associated with GH deficiency, with an approved dose of 0.034 mg/kg/d for pediatric patients with PWS in the United States and Switzerland [16]. The non-interventional American Norditropin® Studies: Web-Enabled Research (ANSWER) Program® and the NordiNet® International Outcome Study (IOS) were long-term observational studies designed to assess real-life clinical outcomes of pediatric and adult patients treated with somatropin as prescribed by physicians according to standard clinical practice [17]. Data collected through both initiatives provided the opportunity to detail effects of GH treatment in specific diagnostic and demographic populations in the United States (ANSWER Program®) and other countries (NordiNet® IOS) [14, 18, 19]. The current study used data from both initiatives to assess clinical outcomes of pediatric patients with PWS treated with GH.
Methods
Data source
Data were extracted from the non-interventional ANSWER Program® and NordiNet® IOS, which aimed to evaluate long-term safety and effectiveness outcomes of pediatric and adult patients treated with somatropin in the United States and other countries, respectively. Details about both programs were described previously [17]. Briefly, the ANSWER Program® and NordiNet® IOS enrolled GH-naïve adults and children who were prescribed somatropin for treatment of growth disorders. Patient histories and physical examination data were entered by participating physician investigators using Web-based reporting forms. The doses of GH administered to participants were selected by treating physicians, and data on parameters such as baseline height standard deviation score (HSDS), weight, bone age, maximal stimulated serum GH concentration, serum insulin-like growth factor 1 (IGF-1) levels, and GH dose/frequency were collected [17]. Patient data from the ANSWER Program® were obtained over a 14-year period (June 24, 2002 to September 30, 2016) from 207 participating sites within the United States, while data from NordiNet® IOS were collected over 10 years (April 1, 2006 to December 31, 2016) from 469 clinics in 22 countries in Europe and the Middle East. The ANSWER Program® and NordiNet® IOS originally enrolled 20,204 and 17,995 pediatric patients, respectively [20].
Assessments
Change from baseline in HSDS and body mass index standard deviation scores (BMI SDS) were analyzed using available follow-up data. Model-based least squares (LS) mean estimates of the impact of baseline parameters on change in HSDS and BMI SDS from baseline were determined using a repeated measures model analysis that included baseline HSDS, BMI SDS, gender, age, target height, region (United States/Europe), and GH dose. Outputs comprised LS mean changes from baseline at years 1 to 4, but statistical comparisons were not made.
Results
Patient characteristics
An initial total of 145 patients with PWS from the ANSWER Program® (n = 78) and NordiNet® IOS (n = 67) were eligible for inclusion for effectiveness outcomes assessments. Based on availability of follow-up data to perform the current analyses, a total of 129 patients were included in the HSDS analysis and 98 in the BMI SDS analysis. The mean ± SD GH dose administered to patients in both registries was 0.03 ± 0.01 mg/kg/d.
HSDS analysis
Key demographic characteristics of the HSDS analysis are summarized in Table 1. Although proportions of males and females were balanced when combining both registries, taken individually, the ANSWER Program® and NordiNet® IOS provided higher proportions of females (59%) and males (60%), respectively. The overall mean (± SD) age at the start of GH treatment was 4.42 ± 4.51 years; however, patients were slightly younger in NordiNet® IOS (Table 1). The mean follow-up time was 2.48 ± 1.31 years and ranged up to 3.98 years.
At baseline, mean height of patients in the HSDS analysis was slightly greater in the ANSWER Program® (95.0 ± 33.47 cm) compared with NordiNet® IOS (88.8 ± 27.61 cm); with a similar trend observed in mean BA/CA ratios (1.04 ± 0.17 and 0.79 ± 0.31, respectively; Table 1). At the end of follow-up, the final recorded heights of patients from the ANSWER Program® and NordiNet® IOS were 124.2 ± 31.14 cm and 122.1 ± 27.65, respectively; while the final recorded BA/CA ratios were 1.04 ± 0.18 and 0.88 ± 0.17, respectively.
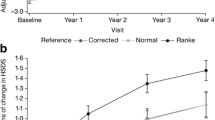
Mean baseline HSDS values were − 1.17 ± 1.40 and − 1.94 ± 1.52 from the ANSWER Program® and NordiNet® IOS, respectively. Results of the HSDS analysis revealed that baseline age (P = 0.014) and years on GH treatment (P < 0.0001) had significant impacts on changes in HSDS. Results from model-based mean estimates for change from baseline over time in HSDS are presented in Fig. 1. The findings demonstrated that patients in both the ANSWER Program® and NordiNet® IOS are expected to experience similar increases in HSDS over time.
BMI SDS analysis
Key demographic characteristics of the BMI SDS analysis set are summarized in Table 2. At the start of GH treatment, mean patient age was 5.56 ± 4.79 years and mean duration of treatment was 2.45 ± 1.32 years. Baseline mean height of patients in the BMI SDS study was slightly greater from the ANSWER Program® (119.6 ± 25.24 cm) vs NordiNet® IOS (89.68 ± 28.29 cm), which was also reflected in mean baseline BA/CA ratios (1.06 ± 0.17 and 0.79 ± 0.31, respectively; Table 2). The last recorded heights of patients in the BMI SDS analysis from the ANSWER Program® and NordiNet® IOS were 140.3 ± 21.25 cm and 122.4 ± 27.55 cm, respectively; while the last recorded BA/CA ratios were 1.07 ± 0.17 and 0.88 ± 0.17, respectively.
For the BMI SDS analysis, mean baseline BMI was lower in NordiNet® IOS (18.04 ± 4.90 kg/m2) than in the ANSWER Program® (24.68 ± 7.75 kg/m2). The same was true for baseline BMI SDS values: 0.40 ± 1.54 in NordiNet® IOS and 1.80 ± 1.68 in the ANSWER Program®.
Longer GH treatment time led to a significantly greater change in BMI SDS from baseline (P < 0.0001). When considering the interaction between baseline BMI and time, patients with a higher BMI at the start of treatment had a greater decrease in BMI over time (P < 0.0001).
Results from model-based mean estimates for change from baseline over time in BMI SDS are presented in Fig. 2. These results were obtained based on a model using a GH dose of 1.04 mg/m2, baseline age of 5.91 years, and baseline BMI SDS of 1.26. Notably, the expected change from baseline declined over time in patients from the ANSWER Program® but increased over time in patients from NordiNet® IOS. Using this model, the average hypothetical patient in the ANSWER Program® had an increase of approximately 0.4 kg/m2 in BMI SDS at year 1, followed by a return to baseline by year 4. The average hypothetical patient in NordiNet® IOS experienced an initial decline in BMI SDS of − 0.16 kg/m2 at year 1 followed by a gradual increase to 0.38 kg/m2 over baseline by year 4.
Safety analysis
There were 8 non-serious adverse reactions and 0 serious adverse reactions reported in 6 patients with PWS in the ANSWER® registry. One death due to cardiopulmonary arrest was recorded; this occurred in a 2-year-old female patient and was considered unlikely to be related to treatment with somatropin. In the NordiNet® registry, 8 adverse reactions were reported in 6 patients with PWS, comprising 3 non-serious adverse reactions in 3 patients and 5 serious adverse reactions in 3 patients. Serious adverse reactions included type 1 and type 2 diabetes and scoliosis. In addition, 1 death of a 22-year old male was recorded; this sudden death was considered unlikely related to treatment with somatropin.
Discussion
This analysis of real-world data from patients with PWS who initiated treatment with GH revealed that the treatment was effective in improving growth and body composition.
Improvement in linear growth following GH therapy in young patients with PWS is well established based on a long history of randomized clinical trials [21,22,23,24,25,26,27,28,29,30,31,32] and registry/observational studies, resulting in GH becoming the standard of care for PWS [8, 33,34,35,36,37]. Initiation of treatment with GH is recommended as early as possible at the time of diagnosis [37], and evidence supports continued use in adults to maintain bone health and promote lean muscle mass [38]. The current study contributes to the field by demonstrating that, in real-world conditions, treatment with GH initiated at an earlier age and continuing for a longer duration is significantly associated with improvements in HSDS compared with later initiation and shorter duration. The continued improvements over 4 years are consistent with previous studies demonstrating ongoing improvements in growth for up to 15 years of GH therapy [34,35,36, 39, 40].
Interestingly, in the BMI SDS analysis, results from our modeling studies showed opposite trends as BMI SDS increased and decreased over time for patients from NordiNet® IOS and the ANSWER Program®, respectively. This might be explained by the difference in the ages of patients in each registry, as the median age of enrollment (GH start) in the ANSWER Program® was 8 years (minimum 2 years) compared with 2 years (minimum 0.4 years) in NordiNet® IOS. Patients with PWS typically follow a series of age-dependent nutritional phases beginning with hypotonia and feeding difficulty up to 9 months of age, followed by normal feeding and growth until approximately 2 years of age when weight gain accelerates despite normal appetite. At approximately 4.5 years of age appetite increases, often leading to preoccupation with food and food-seeking behaviors that can result in obesity [41]. Due to this sequence of events, BMI SDS typically switches from below normal to above normal during the fourth year of life [42]; therefore, an increase or a decrease in BMI SDS could be considered an improvement, depending on the patient’s age and nutritional stage. As patients from the ANSWER Program® were, on average, older than those from NordiNet® IOS, it stands to reason that a larger proportion were in the insatiable, food-seeking phase compared with the younger patients in NordiNet® IOS. Also note that patients in the ANSWER Program® had higher mean baseline weight and BMI than patients in NordiNet® IOS, and similar age-dependent trends in BMI SDS in patients with PWS have also been observed in other studies [8, 33, 43,44,45,46,47].
Since the first demonstration over 30 years ago of the effectiveness of GH in improving growth in patients with PWS [4]. the use of GH has been widespread in these patients. The Global PWS Registry that includes approximately 2000 participants with PWS from 33 countries recently reported that 91% of patients are using (or previously used) GH, 71% of whom began treatment before 2 years of age. Of patients who used GH, 93% reported receiving benefits from the treatment [48, 49].
A notable strength of this study was the use of data from largest-to-date, real-world populations of patients treated with GH from NordiNet® IOS and the ANSWER Program®, totaling nearly 40,000 patients. However, by virtue of the size of these registries, data were collected at many sites by many different practitioners, presenting a likelihood of inconsistent data collection practices between sites, resulting in inconsistencies in patient numbers between parameters. Moreover, given that PWS is a rare disease, only a small overall number of patients was available for our analyses. Moreover, differences between NordiNet® IOS and the ANSWER Program®, such as the geographic/demographic backgrounds of patients, the aforementioned differences in patient ages, and the longer follow-up in NordiNet® IOS (median 3.1 years) vs the ANSWER Program® (median 2.25 years) may have produced volatility in the data and could underlie some of the differences in trends between registries, such as the different rates of change in height and BA/CA ratios. Finally, the study was limited more broadly by the general use of retrospective data.
Conclusions
In conclusion, GH was confirmed to be effective in the management of children with PWS, and earlier treatment resulted in a greater gain in height, and longer treatment period resulted in better outcomes for both height and BMI. This study supports early initiation and sustained use of GH therapy in patients with PWS, which aligns with the current practice of starting treatment with GH before age 2 in toddlers with PWS to gain positive effects on growth and BMI [37].
Availability of data and materials
The data that support the findings of this study are available from Novo Nordisk, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Novo Nordisk, Inc.
Abbreviations
- BMI:
-
Body mass index
- GH:
-
Growth hormone
- HSDS:
-
Height standard deviation score
- IOS:
-
International Outcome Study
- PWS:
-
Prader-Willi syndrome
- rhGH:
-
Recombinant human growth hormone
- SDS:
-
Standard deviation score
References
OMIM. Prader-Willi Syndrome; PWS. Available at: https://www.omim.org/entry/176270 Accessed 15 Oct 2019.
Deal CL, Tony M, Hoybye C, Allen DB, Tauber M, Christiansen JS. GrowthHormone research society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013;98(6):E1072–87.
Aycan Z, Bas VN. Prader-Willi syndrome and growth hormone deficiency. J Clin Res Pediatr Endocrinol. 2014;6(2):62–7.
Lee PD, Wilson DM, Rountree L, Hintz RL, Rosenfeld RG. Linear growth response to exogenous growth hormone in Prader-Willi syndrome. Am J Med Genet. 1987;28(4):865–71.
Festen DA, de Weerd AW, van den Bossche RA, Joosten K, Hoeve H, Hokken-Koelega AC. Sleep-related breathing disorders in prepubertal children with Prader-Willi syndrome and effects of growth hormone treatment. J Clin Endocrinol Metab. 2006;91(12):4911–5.
Siemensma EPC. Tummers-de Lind van Wijngaarden RFA, Festen DAM, et al. beneficial effects of growth hormone treatment on cognition in children with Prader-Willi syndrome: a randomized controlled trial and longitudinal study. J Clin Endocrinol Metab. 2012;97(7):2307–14.
Lo ST, Festen DAM. Tummers-de Lind van Wijngaarden RFA, Collin PJL, Hokken-Koelega ACS. Beneficial effects of long-term growth hormone treatment on adaptive functioning in infants with Prader-Willi syndrome. Am J Intellect Dev Disabil. 2015;120(4):315–27.
Bakker NE, Lindberg A, Heissler J, Wollmann HA, Camacho-Hubner C, Hokken-Koelega AC. Growth hormone treatment in children with Prader-Willi syndrome: three years of longitudinal data in prepubertal children and adult height data from the KIGS database. J Clin Endocrinol Metab. 2017;102(5):1702–11.
Lindholm J. Growth hormone: historical notes. Pituitary. 2006;9(1):5–10.
Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2007;3:CD004440.
Crabbe R, von Holtey M, Engrand P, Chatelain P. Recombinant human growth hormone for children born small for gestational age: meta-analysis confirms the consistent dose-effect relationship on catch-up growth. J Endocrinol Investig. 2008;31(4):346–51.
Lee PA, Savendahl L, Oliver I, et al. Comparison of response to 2-years’ growth hormone treatment in children with isolated growth hormone deficiency, born small for gestational age, idiopathic short stature, or multiple pituitary hormone deficiency: combined results from two large observational studies. Int J Pediatr Endocrinol. 2012;2012(1):22.
Maiorana A, Cianfarani S. Impact of growth hormone therapy on adult height of children born small for gestational age. Pediatrics. 2009;124(3):e519–31.
Ross JL, Lee PA, Gut R, Germak J. Attaining genetic height potential: analysis of height outcomes from the ANSWER Program® in children treated with growth hormone over 5 years. Growth Hormon IGF Res. 2015;25(6):286–93.
Ross JL, Lee PA, Gut R, Germak J. Increased height standard deviation scores in response to growth hormone therapy to near-adult height in older children with delayed skeletal maturation: results from the ANSWER Program®. Int J Pediatr Endocrinol. 2015;2015(1):1.
Norditropin (somatropin) injection [package insert]. Plainsboro: Novo Nordisk Inc.; 2018.
Höybye C, Sävendahl L, Christesen HT, et al. The NordiNet® International Outcome Study and NovoNet® ANSWER Program®: rationale, design, and methodology of two international pharmacoepidemiological registry-based studies monitoring long-term clinical and safety outcomes of growth hormone therapy (Norditropin®). Clin Epidemiol. 2013;5:119–27.
Lee PA, Germak J, Gut R, Khutoryansky N, Ross J. Identification of factors associated with good response to growth hormone therapy in children with short stature: results from the ANSWER Program®. Int J Pediatr Endocrinol. 2011;2011:6.
Ross J, Lee PA, Gut R, Germak J. Factors influencing the one- and two-year growth response in children treated with growth hormone: analysis from an observational study. Int J Pediatr Endocrinol. 2010;2010:494656.
Sävendahl L, Polak M, Backeljauw P, et al. Treatment of children with GH in the United States and Europe: long-term follow-up from NordiNet® IOS and ANSWER Program®. J Clin Endocrinol Metab. 2019;104(10):4730–42.
Carrel AL, Moerchen V, Myers SE, Bekx MT, Whitman BY, Allen DB. Growth hormone improves mobility and body composition in infants and toddlers with Prader-Willi syndrome. J Pediatr. 2004;145(6):744–9.
Carrel AL, Myers SE, Whitman BY, Allen DB. Growth hormone improves body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome: a controlled study. J Pediatr. 1999;134(2):215–21.
Carrel AL, Myers SE, Whitman BY, Allen DB. Sustained benefits of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome are dose-dependent. J Pediatr Endocrinol Metab. 2001;14(8):1097–105.
Donze SH, Kuppens RJ, Bakker NE, van Alfen-van der Velden J, Hokken-Koelega ACS. Bone mineral density in young adults with Prader-Willi syndrome: a randomized, placebo-controlled, crossover GH trial. Clin Endocrinol. 2018;88(6):806–12.
Hauffa BP. One-year results of growth hormone treatment of short stature in Prader-Willi syndrome. Acta Paediatr Suppl. 1997;423:63–5.
Kuppens RJ, Bakker NE, Siemensma EP, Donze SH, Stijnen T, Hokken-Koelega AC. Metabolic health profile in young adults with Prader-Willi syndrome: results of a 2-year randomized, placebo-controlled, crossover GH trial. Clin Endocrinol. 2017;86(2):297–304.
Kuppens RJ, Bakker NE, Siemensma EPC, et al. Beneficial effects of GH in young adults with Prader-Willi syndrome: a 2-year crossover trial. J Clin Endocrinol Metab. 2016;101(11):4110–6.
Lindgren AC, Hagenas L, Muller J, et al. Effects of growth hormone treatment on growth and body composition in Prader-Willi syndrome: a preliminary report. The Swedish National Growth Hormone Advisory Group. Acta Paediatr Suppl. 1997;423:60–2.
Lindgren AC, Hagenas L, Ritzen EM. Growth hormone treatment of children with Prader-Willi syndrome: effects on glucose and insulin homeostasis. Swedish National Growth Hormone Advisory Group. Horm Res. 1999;51(4):157–61.
Myers SE, Carrel AL, Whitman BY, Allen DB. Physical effects of growth hormone treatment in children with Prader-Willi syndrome. Acta Paediatr Suppl. 1999;88(433):112–4.
Myers SE, Carrel AL, Whitman BY, Allen DB. Sustained benefit after 2 years of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome. J Pediatr. 2000;137(1):42–9.
Myers SE, Whitman BY, Carrel AL, Moerchen V, Bekx MT, Allen DB. Two years of growth hormone therapy in young children with Prader-Willi syndrome: physical and neurodevelopmental benefits. Am J Med Genet A. 2007;143a(5):443–8.
Lin HY, Lin SP, Tsai LP, et al. Effects of growth hormone treatment on height, weight, and obesity in Taiwanese patients with Prader-Willi syndrome. J Chin Med Assoc. 2008;71(6):305–9.
Meinhardt U, Christiansen JS, Farholt S, et al. The efficacy and safety of long-term Norditropin® treatment in children with Prader-Willi syndrome. Horm Metab Res. 2013;45(7):532–6.
Obata K, Sakazume S, Yoshino A, Murakami N, Sakuta R. Effects of 5 years growth hormone treatment in patients with Prader-Willi syndrome. J Pediatr Endocrinol Metab. 2003;16(2):155–62.
Iughetti L, Tornese G, Street ME, et al. Long-term safety and efficacy of Omnitrope®, a somatropin biosimilar, in children requiring growth hormone treatment: Italian interim analysis of the PATRO children study. Ital J Pediatr. 2016;42(1):93.
Duis J, van Wattum PJ, Scheimann A, et al. A multidisciplinary approach to the clinical management of Prader-Willi syndrome. Mol Genet Genomic Med. 2019;7(3):e514.
Mogul HR, Lee PD, Whitman BY, et al. Growth hormone treatment of adults with Prader-Willi syndrome and growth hormone deficiency improves lean body mass, fractional body fat, and serum triiodothyronine without glucose impairment: results from the United States multicenter trial. J Clin Endocrinol Metab. 2008;93(4):1238–45.
Hoybye C. Growth hormone treatment of Prader-Willi syndrome has long-term, positive effects on body composition. Acta Paediatr. 2015;104(4):422–7.
Angulo MA, Castro-Magana M, Lamerson M, Arguello R, Accacha S, Khan A. Final adult height in children with Prader-Willi syndrome with and without human growth hormone treatment. Am J Med Genet A. 2007;143a(13):1456–1461.
Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155a(5):1040–9.
Butler JV, Whittington JE, Holland AJ, McAllister CJ, Goldstone AP. The transition between the phenotypes of Prader-Willi syndrome during infancy and early childhood. Dev Med Child Neurol. 2010;52(6):e88–93.
Lindgren AC, Ritzen EM. Five years of growth hormone treatment in children with Prader-Willi syndrome. Swedish National Growth Hormone Advisory Group. Acta Paediatr Suppl. 1999;88(433):109–11.
Crino A, Di Giorgio G, Manco M, Grugni G, Maggioni A. Effects of growth hormone therapy on glucose metabolism and insulin sensitivity indices in prepubertal children with Prader-Willi syndrome. Horm Res. 2007;68(2):83–90.
Reinehr T, Lindberg A, Koltowska-Haggstrom M, Ranke M. Is growth hormone treatment in children associated with weight gain?--longitudinal analysis of KIGS data. Clin Endocrinol. 2014;81(5):721–6.
Stipancic G, Pozgaj Sepec M, La Grasta Sabolic L. Effect of growth hormone therapy in children with Prader-Willi syndrome - our first experiences. Acta Clin Croat. 2018;57(4):744–55.
Corripio R, Tubau C, Calvo L, et al. Safety and effectiveness of growth hormone therapy in infants with Prader-Willi syndrome younger than 2 years: a prospective study. J Pediatr Endocrinol Metab. 2019;32(8):879–84.
PWS Registry Data. Available at: https://www.fpwr.org/blog/pws-registry-data-91-percent-have-used-growth-hormone-for-pws-infographic Accessed 17 Oct 2019.
Bohonowych J, Miller J, McCandless SE, Strong TV. The Global Prader-Willi Syndrome Registry: development, launch, and early demographics. Genes (Basel). 2019;10(9):713.
Acknowledgments
This study was sponsored by Novo Nordisk Inc., Plainsboro, NJ, and is registered with ClinicalTrials.gov (NCT01009905). The authors acknowledge the medical writing assistance of PRECISIONscientia, Yardley, PA, which was supported financially by Novo Nordisk. These data were presented as a poster at ENDO 2019; March 23-26, 2019; New Orleans, LA, USA. Select data was presented as a poster titled “Outcomes in children treated with growth hormone for Prader-Willi Syndrome: Data from the ANSWER Program and NordiNet International Outcome Study (IOS)” at the ENDO 2019 annual meeting.
Funding
This study was funded by Novo Nordisk.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization and design of the study; MJA was a study investigator; VO supervised the study, obtained funding, and participated in the data analysis; VO and NK provided study resources; and all authors participated in reviewing and editing the manuscript, gave their final approval of the version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval was obtained from relevant ethics committees, and written consent was obtained from all participants or guardians/legal representatives. ANSWER was conducted in accordance with the Declaration of Helsinki, Guideline for Good Pharmacoepidemiology Practices, and regulatory requirements.
Consent for publication
Not applicable.
Competing interests
MA has nothing to disclose. MJA has received research support from AstraZeneca. She also serves as a research investigator for Novo Nordisk, Ascendis, Soleno, Levo, Millendo, and Rhythm; as a speaker for Novo Nordisk and Rhythm; and as an advisory board member for Ascendis and Rhythm. AP, VO, and NK are employees of Novo Nordisk. MT is an advisory board member for Novo Nordisk, Pfizer, Ipsen, and Merck Serono, a consultant for Alizé Pharma, a recipient of grants from Novo Nordisk, Pfizer, Ipsen, and Sandoz, a research investigator for Novo Nordisk and Alizé Pharma, and a speaker for Novo Nordisk, Pfizer, Ipsen, and Merck Serono.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Angulo, M., Abuzzahab, M.J., Pietropoli, A. et al. Outcomes in children treated with growth hormone for Prader-Willi syndrome: data from the ANSWER Program® and NordiNet® International Outcome Study. Int J Pediatr Endocrinol 2020, 20 (2020). https://doi.org/10.1186/s13633-020-00090-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13633-020-00090-6