Abstract
Background
Clinical practice of aerosol delivery in conjunction with respiratory support devices for critically ill adult patients remains a topic of controversy due to the complexity of the clinical scenarios and limited clinical evidence.
Objectives
To reach a consensus for guiding the clinical practice of aerosol delivery in patients receiving respiratory support (invasive and noninvasive) and identifying areas for future research.
Methods
A modified Delphi method was adopted to achieve a consensus on technical aspects of aerosol delivery for adult critically ill patients receiving various forms of respiratory support, including mechanical ventilation, noninvasive ventilation, and high-flow nasal cannula. A thorough search and review of the literature were conducted, and 17 international participants with considerable research involvement and publications on aerosol therapy, comprised a multi-professional panel that evaluated the evidence, reviewed, revised, and voted on recommendations to establish this consensus.
Results
We present a comprehensive document with 20 statements, reviewing the evidence, efficacy, and safety of delivering inhaled agents to adults needing respiratory support, and providing guidance for healthcare workers. Most recommendations were based on in-vitro or experimental studies (low-level evidence), emphasizing the need for randomized clinical trials. The panel reached a consensus after 3 rounds anonymous questionnaires and 2 online meetings.
Conclusions
We offer a multinational expert consensus that provides guidance on the optimal aerosol delivery techniques for patients receiving respiratory support in various real-world clinical scenarios.
Similar content being viewed by others
Introduction
Aerosol therapy has been broadly utilized in both inpatient and outpatient settings, due to its advantages of being non-invasive, easy-to-use, quick onset, lower dose, and with less systemic side effects than systemic administration [1]. Unlike most ambulatory patients, intensive care unit (ICU) patients often require respiratory support, including oxygen therapy (low and high flow) through a mask or nasal cannula, and ventilatory support, such as noninvasive ventilation (NIV) or invasive mechanical ventilation (MV), to help them breathe and maintain oxygenation. In most cases, to avoid the disruption of oxygen delivery and ventilation, medical aerosols need to be administered via respiratory support devices, such as high-flow nasal cannula (HFNC), NIV, and MV [2, 3]. Delivering medical aerosols inline with these devices can be challenging due to the interference of flows and positive pressure, while aerosol delivery for patients with low-flow oxygen therapy is similar to ambulatory patients. To date, no aerosol drug/device combination has been specifically approved by regulatory bodies for inline use with respiratory support devices, meaning drugs for inhalation during respiratory support are technically off-label and lacking manufacture guidance for administration. Therefore, this consensus document does not address the issue of delivering specific drugs.
Considerable research, from bench to bedside, has focused on evaluating the effectiveness of aerosol delivery via MV, NIV, and HFNC, and identifying factors that influence aerosol delivery in these settings [2,3,4,5,6]. Aerosol delivery effectiveness is primarily assessed by the responses in the target organ. Nebulization of bronchodilators targeted at the tracheobronchial tree can be assessed by its immediate response, such as the changes in airway resistance, intrinsic positive end-expiratory pressure, or lung compliance. However, other drugs with longer onset time, such as antibiotics and steroids, are challenging, as they require optimal techniques to reach desired levels of drug deposition in the lung parenchyma, and it may be difficult to assess the drug deposition and patient response [7, 8]. Factors that impact aerosol delivery include patient characteristics, breathing parameters, the severity of airway disease, the characteristics of aerosol devices, their integration into respiratory support devices and the interface of these devices to patients, ease of use, and patient comfort [1,2,3,4, 9, 10]. The present consensus document is focused on the technical conditions required to optimize aerosol delivery into the respiratory system.
Clinical practice of aerosol delivery in conjunction with respiratory support devices for adult ICU patients varies widely [11,12,13,14,15], with little consensus among clinicians and aerosol scientists. Thus, we performed a thorough search and literature review of aerosol delivery for adult ICU patients receiving various forms of respiratory support. We invited an international panel to review the evidence and make recommendations, with the aim to provide practical guidance on aerosol delivery for adult ICU patients and identify needs for future research.
Methods
This academic work was investigator-initiated and did not receive any funding from public or private entities. A modified Delphi method was adopted to achieve a consensus on aspects of aerosol delivery for adult critically ill patients receiving various forms of respiratory support, including MV, NIV, and HFNC.
Working group and panel
We set up a working group responsible for designing and implementing the study, including literature search, extracting and summarizing study findings, drafting and revising recommendations, communicating with panelists, summarizing the scores and comments for three rounds of review, and organizing the online meetings. Authors who had a minimum of three publications in aerosol research and H-index ≥ 10 were invited to participate in the panel, and they were tasked to evaluate the recommendation in light of available evidence, suggest missing literature, score and comment on the recommendations, and revise the manuscript. Details about participants in the panel can be found in Additional file 1: Appendix 1.
Literature search and preliminary recommendations generation
A literature search was conducted from the PubMed, Medline, and Scopus databases between January 1, 1990, and September 1, 2021. The key literature search strategy included (aerosol* OR nebuliz* OR inhal*) AND adult AND ((mechanical ventilation) OR (noninvasive ventilation) OR (high-flow nasal cannula)). Details of the research strategy are available in Additional file 1: Appendix 2. The working group screened the studies by titles and abstracts, and reviewed the relevant full manuscripts to select the studies included in the consensus. The study findings were extracted and summarized in tables for each question, with preliminary recommendations generated based on these findings. The preliminary recommendations, along with the summary tables and references, were provided to the panelists, who were invited to input and offer relevant references if any were missing.
Rounds and rules for voting
A modified Delphi method (applying RAND rules) was used to collate the panelists’ views in 3 rounds of voting. Details about the rounds and rules can be found in Additional file 1: Appendix 3. During the review of the recommendations, panelists were requested to assign a Likert score of 1–9 (strongly disagree to strongly agree) to each recommendation and make comments based on their evaluation of the available evidence and their expertise. After each round of voting, the working group revised the recommendations based on panelists’ feedback. The revision and a report composed of the score distributions and a summary of anonymous comments were provided to the panelists in the next round of voting, and they were invited to vote again on both the revised and the recommendations that did not reach a consensus in the previous round. Finally, panelists discussed the final recommendations and next steps for the writing process via online meetings with attendance by ≥ 50% of the panelists. Detailed reports and results are available in Additional file 1: Appendix 4–11.
Trial registration: The study was registered on the Open Science Framework with registration digital object identifier https://osf.io/j8apu.
Level of consensus and recommendations
The perfect consensus was defined as all panelists scoring between 7 and 9 for agreement (or 1 and 3 for disagreement), while a very good consensus was defined as ≥ 80% of panelists scoring between 7–9 and 1–3 [16, 17]. Only those recommendations with perfect or very good consensus were included in the final recommendations. In contrast, recommendations that did not reach a consensus from the first three rounds and the final online meeting were withdrawn. The writing group consisted of the panel members and the working group who drafted the consensus, with circulation to the full panel for revision and approval of the final manuscript.
Results
The literature search and review were conducted between April 1, 2021, and September 10, 2021. 25 researchers met the inclusion criteria, and 21 accepted the invitation, of whom 18 panelists completed the scoring and comments in the first round of review, and the second round of review, while 17 panelists completed the third round of review. Two online meetings were held, with attendance by 10 and 13 panelists, respectively. Among the 17 panelists, 4 (22%) were female. The median H-index of the panel was 31 (21–60), representing pulmonologists, intensivists, anesthesiologists, physiotherapists, and respiratory care practitioners from North and South America, Europe, and Asia.
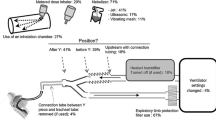
In the literature search, 3,342 articles were screened, and 102 full texts were reviewed. After the first round of review, 18 additional relevant articles were provided by the panelists (Fig. 1). In total, 120 studies were summarized in the tables of evidence for nebulization via various forms of respiratory support (the detailed list is available in Additional file 1: Appendix 2). In the first round of review, 53 recommendations were provided to the panel. Finally, panelists agreed to merge some recommendations, culminating in recommendations I–XX, all of which reached > 80% agreement. Detailed information on each round of deliberations is available in Fig. 2 and Table 1, and the recommendations are illustrated in Fig. 3. Recommendations include indications of the source of data, including in vitro (IV), clinical studies (CS), and animal studies (AS).
Aerosol delivery via invasive mechanical ventilation
Selection of aerosol device
Recommendation I During mechanical ventilation, vibrating mesh nebulizer or pressurized metered-dose inhaler with spacer are recommended for aerosol delivery, IV,CS with no preference between the devices. IV,CS The use of an inline continuous jet nebulizer results in changes in tidal volume, inspiratory flow patterns, and fraction of inspired oxygen, and aerosol delivery efficiency is low, thus continuous jet nebulizer is not preferred for aerosol delivery in this setting.
When comparing aerosol delivery via MV, in vitro [18,19,20,21,22,23,24,25,26] studies reported a higher aerosol delivery efficiency with vibrating mesh nebulizer (VMN) than continuous jet nebulizer (JN), regardless of the nebulizer placement and ventilator settings. A bioavailability study in mechanically ventilated patients also showed a higher percentage of urinary salbutamol levels with VMN than continuous JN [27]. Likewise, when pressurized metered-dose inhaler (pMDI) and spacer were placed in the inspiratory limb before the Y-piece, aerosol delivery efficiency with pMDI and spacer was higher than continuous JN in the in vitro studies [19, 28, 29]. However, three clinical studies reported no significant differences in reducing airway resistance for mechanically ventilated patients when inhaling albuterol via pMDI and spacer versus continuous JN [30,31,32]. Moreover, one randomized controlled trial did not find significant differences in the duration of mechanical ventilation among groups with VMN, JN, and pMDI with spacer for mechanically ventilated patients with asthma [33]. Notably, continuous JN is driven by an external compressed gas, which will affect the ventilation [34], including tidal volume, inspiratory flow patterns, trigger sensitivity, and the fraction of inspired oxygen (FIO2), in contrast to no influence when VMN, ultrasonic nebulizer (USN), and pMDI with spacer are utilized via MV. Breath-enhanced JNs designed for use with MV utilize less external gas flows and may reduce the impact on ventilation [35]. However, such nebulizers are not yet commercially available. Although ventilator-integrated JN does not affect ventilation, the aerosol delivery time is 2–3 times longer than continuous nebulizers [3], without consistent increases in delivery, limiting its use in clinical practice [11, 12]. Thus, VMN, USN, and pMDI with spacer are preferred over continuous JN. However, the heat generated during the use of USN has been associated with denaturing proteins, so its use with protein-containing drug should be avoided [36, 37]. When VMN and pMDI with spacer were placed at the inspiratory limb before the Y-piece, the inhaled dose of bronchodilator was similar between the two devices, and Dubosky et al. reported no differences in the VAP incidence with the use of VMN and pMDI with a spacer in their cohort study [38]. Thus, both VMN and pMDI with spacer are preferred for aerosol delivery during MV. Notably, VMNs are typically more expensive than JNs, thus it may be more cost-effective to reserve the use of VMN for patients who require frequent aerosol treatments or medications that are costly.
Nebulizer placement
Recommendation II When a vibrating mesh nebulizer or jet nebulizer is utilized during invasive ventilation with bias flow, it is recommended to place the nebulizer in the inspiratory limb, away from the Y-piece and toward the ventilator. IV,CS
With bias flow during MV, a higher inhaled dose is generally found with VMN placed close to the ventilator than when it is placed close to the patient [19, 26, 39,40,41]. However, in the absence of bias flow, the findings from two in vitro studies were contradictory [18, 41]. For continuous JN, a higher inhaled dose was found with placement close to the ventilator than close to the patient with no bias flow [18], whereas in the presence of bias flow similar inhaled dose was reported with both placements [19]. Furthermore, placing the JN close to the ventilator has the pragmatic advantage of less potential for contamination from the patient’s secretions.
The use of pMDI and spacer
Recommendation III When pressurized metered dose inhaler is utilized during invasive ventilation, it is recommended to be used with a spacer with a volume > 150 mL IV,CS and placed in the inspiratory limb before the Y-piece. IV,CS The pressurized metered dose inhaler is recommended to be actuated at the beginning of inspiratory flow from the ventilator. IV
When pMDI is utilized during MV, it needs to be used with an accessory device (adapter or spacer), which varies by design and size. The inhaled dose increased as the volume of the spacer/adapter increased, with a minimum volume requirement of 150 mL [24, 29, 42,43,44,45,46,47]. Among different placements, the inhaled dose was highest with the pMDI and spacer placed in the inspiratory limb 15 cm from the Y-piece [18, 48]. pMDI needs to be actuated with the onset of inspiratory flow from the ventilator, the inhaled dose was significantly reduced if the pMDI was actuated during exhalation [24, 49]. In addition, a minimum of 15 s intervals are required between actuations (puffs) [50].
Humidification
Recommendation IV For patients using an active heated humidifier, turning off the humidifier is not recommended for routine aerosol therapy; IV,CS for patients using a heat–moisture exchanger, removing or bypassing the heat moisture exchanger is recommended during aerosol delivery. IV
In vitro studies [18, 42, 49,50,51,52,53,54,55,56,57,58] identified a reduction of up to 50% in aerosol delivery efficiency during MV with heated humidification, compared to dry conditions, especially when JN or pMDI was utilized. In contrast, randomized trials reported no significant differences in urinary salbutamol concentrations [27], MV duration [33], and ICU length of stay [33] in groups of patients with or without humidification. Moreover, an in vitro study reported that aerosol delivery via pMDI and spacer immediately after turning off the humidifier was not improved, compared to aerosol delivery during heated humidification [57]. It might not be realistic for clinicians to wait for the circuit to dry or replace circuits after turning off the humidifier, and there is a risk for the clinician to forget to turn the humidifier on after nebulization is completed [57]. Therefore, considering the potential harms of administering dry gas to a patient airway, especially over a duration of several hours, and the time lapse required for a humidifier and circuits to cool down and dry, turning off the humidifier is not recommended for routine aerosol therapy.
Heat–moisture exchangers (HME) are generally considered a barrier to aerosol drug delivery in ventilated patients, with high-efficiency filter HMEs reported to reduce delivered doses to < 0.5% [20]. Thus, HMEs should be removed or bypassed from the pathway between the aerosol generator and the patient’s airway during aerosol delivery. However, removing HMEs from the ventilator circuit may cause lung de-recruitment [59] and increase the infection risk for both patients and care providers [60]. An alternative is to employ HMEs designed to allow aerosol delivery (HME-ADs) by bypassing the HME during aerosol administration. One in vitro study reported a similar inhaled dose with HME-AD compared to no HME [61].
Fill volume or diluent volume
Recommendation V When a nebulizer is utilized, changing the fill volume or diluent volume for the sole purpose of improving aerosol delivery efficiency is not recommended. IV,CS
When a VMN was utilized during MV, in vitro and in vivo studies reported a similar efficiency of aerosol delivery with dilution volumes of 6 versus 12 mL [62, 63]. In contrast, aerosol delivery efficiency with USN [64, 65] and inspiration-synchronized JN [64] was higher with the fill volume of ≥ 6 mL, compared to the fill volume of 3 mL. Of note, increasing the amount of drug placed in a JN induces additional manipulations and increases duration of treatment delivery, which need to be taken into account.
Artificial airways
Recommendation VI It is not recommended to change the endotracheal tube or tracheostomy tube to increase the internal diameter of the airway for the sole purpose of improving aerosol delivery efficiency. IV
When VMN and continuous JN were placed in line during MV [66, 67] or when a dry powder inhaler was utilized with a resuscitation bag [68], no significant differences in inhaled dose were found between the endotracheal tube and tracheostomy tube of the same size. Three in vitro studies reported no significant differences in aerosol delivery between size 7.0 and 9.0 mm airways [66, 68, 69]. Changing artificial airways imposes risks and adds to the costs of therapy for patients.
Heliox
Recommendation VII Adding heliox for the sole purpose of improving aerosol delivery efficiency is not recommended. IV,AS
While there may be some interest in using heliox to enhance aerosol delivery during MV, the use of this low-density gas mixture has fallen out of favor in clinical practice and a detailed discussion about the relative merits of using heliox for aerosol delivery is beyond the scope of this review. When heliox was used to drive the mechanical ventilator, one in vitro study [53] and one animal study [70] with noninfected piglets reported a higher inhaled dose than when the ventilator was driven by air or nitrogen–oxygen; however, no significant differences in inhaled dose were reported in infected piglets [70]. The cost of using heliox offsets the marginal benefits of increased aerosol delivery reported with heliox.
Filter on the expiratory limb
Recommendation VIII Placing a filter on the expiratory limb reduces fugitive aerosols and protects the ventilator expiratory sensors. Use of a expiratory filter with frequent changes (daily or more frequent based on aerosol administered and effect on filter resistance) is recommended. CS
During aerosol delivery via MV, most of the aerosols are emitted into the room air via the ventilator exhalation port. Those fugitive aerosols could pose a health hazard for bedside caregivers [63] and damage ventilator sensors at the exhalation port [71]. Thus, a filter should be placed at the exhalation port during aerosol delivery. High-efficiency particulate air filters are designed for this purpose and are therefore indicated, while heat and moisture exchanger filters should be avoided [72]. The resistance of the filter may increase as aerosols accumulate over time, and filters should be changed periodically.
Ventilator mode and parameter settings
Recommendations IX It is not recommended to change the ventilator mode and parameter settings for the sole purpose of improving aerosol delivery efficiency during routine nebulization in patients receiving invasive ventilation. IV,CS
Reported effects of ventilator mode on aerosol delivery vary. In vitro reports of no differences in the inhaled dose with pMDI and spacer between volume control (VC) and pressure support (PS) [52], which agrees with similar bronchodilation effects after inhaling salbutamol in VC and PS modes for 10 mechanically ventilated chronic obstructive pulmonary disease (COPD) patients [73]. In contrast, Dugernier et al. reported more radiolabeled aerosols delivered to the lung with VC than PS [74].
Reported effects of ventilator parameter settings on aerosol delivery are also contradictory. In vitro studies reported the inhaled dose increased as tidal volume increased over mechanical dead space but then was stable when pMDI with spacer [52] and USN [75] were used via MV. Similarly, Mouloudi et al. [76] did not find any significant differences in bronchodilation responses between tidal volumes of 8 mL/kg and 12 mL/kg in ventilated COPD patients. When VC mode was used, compared to constant flow, in vitro studies reported that decelerating flow decreased inhaled dose when VMN was used [40], but not for pMDI with spacer [77, 78] or inspiratory synchronized JN [78]. Six in vitro studies reported an increase in inhaled dose as the inspiratory time increased [50, 52, 64, 75, 78, 79], except for pMDI with spacer via MV. Two in vitro [75, 79] and one clinical [80] studies reported no significant differences in the inhaled dose or bronchodilation responses with versus without positive end-expiratory pressure. The use of an end-inspiratory pause of 5 s among 12 COPD mechanically ventilated patients did not improve bronchodilator effects [81].
Considering the contradictory reports and, more importantly, concerns that changing parameters may cause patient–ventilator asynchrony and harm, changing the ventilator mode or parameter settings for the sole purpose of improving aerosol delivery is not recommended.
Special considerations for antibiotics delivery via invasive mechanical ventilation
Delivering antibiotics to the infected lung parenchyma is challenging and discordant results in terms of patients’ outcomes were observed among clinical studies. High lung concentrations should theoretically be delivered to obtain a bactericidal effect in treating ventilator-associated pneumonia. Therefore, on a patient case-by-case basis, clinicians may consider changing ventilatory settings to improve drug delivery when deciding to implement such off-label therapy. No further consensus could be reached among panelists on this question which may deserve further investigations. The detailed discussions of the panel, pros and cons around several specific questions on this topic are provided in the supplementary Additional file 1: Appendix 11 (see pages 575 ~ 582).
Aerosol delivery via noninvasive ventilation
Aerosol delivery via noninvasive ventilation versus conventional aerosol therapy
Recommendation X Placing the nebulizer inline with noninvasive ventilation has similar or higher aerosol delivery efficiency than using the nebulizer with a mask or mouthpiece. Interrupting or discontinuing noninvasive ventilation to administer aerosol via a mask or mouthpiece is not recommended. IV,CS
When a JN is placed inline with NIV, two healthy volunteer studies[82, 83] and one in vitro study[84] reported a lower inhaled dose, while one healthy volunteer study reported a similar inhaled dose, compared to a JN via mask or mouthpiece. Likewise, in the study with stable asthma patients, the forced expiratory volume at the first second (FEV1) improvement was lower with JN via continuous positive airway pressure than with JN via mask or mouthpiece [85]. However, in three clinical studies among patients with asthma exacerbation, patient pulmonary function results were better with a JN via NIV with PS settings than JN via mask or mouthpiece [86,87,88].
The use of pMDI with spacer
Recommendation XI During noninvasive ventilation, placing a pressurized metered-dose inhaler with a spacer between exhalation valve and mask, with actuation at the beginning of inspiration is recommended. IV,CS
In a randomized crossover study with 18 stable COPD patients, Nava et al. reported that compared to the same dose of albuterol delivery via pMDI and a spacer during spontaneous breathing, the pMDI and spacer placed in line with NIV generated similar improvement of FEV1 and greater improvement in forced volume capacity [89]. Notably, Branconnier et al. found a lower inhaled dose with pMDI actuated during exhalation than inhalation when pMDI was used in line with NIV [90].
Nebulizer placement
Recommendation XII During noninvasive ventilation using a single-limb circuit, the continuous nebulizer is recommended to be placed between the exhalation valve and the mask. When available, vibrating mesh nebulizer is preferred over jet nebulizer. IV,CS
During NIV using a single-limb circuit with a non-vented mask, the inhaled dose with continuous nebulizers (JN and VMN) placed at the ventilator outlet was lower compared to placing the nebulizers between the exhalation valve and the mask [91,92,93]. During NIV using a dual-limb circuit, little evidence about comparative nebulizer placement is available, nebulizer may be placed in the inspiratory limb the same way as in a dual-limb invasive ventilation circuit [94]. When placing the continuous nebulizer inline with NIV, both in vitro [91,92,93, 95,96,97,98,99] and in vivo studies [100, 101] reported higher inhaled doses with VMN than JN, regardless of the nebulizer placement and ventilator settings. In addition, JN is driven by an external compressed gas, which may affect the tidal volume and FIO2 delivered by the ventilator, whereas these parameters are unlikely to be affected when VMN is utilized. Thus, when available, VMN should be preferred over JN for aerosol delivery in this setting.
Vented mask versus non-vented mask
Recommendation XIII When a continuous nebulizer is placed inline with noninvasive ventilation, aerosol administration with a non-vented mask is preferred over a vented mask. IV When a non-vented mask is used, there is no recommendation for the use of single versus dual limb circuits for aerosol delivery. IV
When a continuous nebulizer is placed inline with NIV, the aerosol delivery efficiency is higher with a non-vented mask than with a vented mask, regardless of the ventilator settings and nebulizer types [90]. One in vitro study [94] reported no significant differences in inhaled dose when the VMN was placed at the optimal placements in a single-limb noninvasive ventilator or a dual-limb critical care ventilator.
Humidification
Recommendation XIV During aerosol delivery via noninvasive ventilation, turning off the humidifier is not recommended. IV,CS
Unlike the impact of humidification on aerosol delivery via MV, both in vivo and in vitro studies reported no significant effect of humidification on aerosol delivery via NIV, regardless of nebulizer types [97, 98, 101]. This difference may be explained by the lower temperatures and humidification of the inspired gas used during NIV than MV, as it traverses the nose. Thus, there is no supporting information to turn off the humidifier during aerosol delivery via NIV. Off note, if an HME is used during NIV (pros and cons of this practice is beyond the scope of this work), it should be removed during aerosol delivery similar to recommendations during dual-limb invasive mechanical ventilation.
Fill volume
Recommendation XV When a continuous nebulizer is utilized during noninvasive ventilation, increasing the fill volume for the sole purpose of improving aerosol delivery efficiency is not recommended. IV,CS
When a JN was utilized during NIV, higher aerosol delivery was reported when the fill volume was increased from 1 to 2 mL [98, 102]. However, when the fill volume was increased from 2 to 4 mL, two in vitro studies reported a small increment of the inhaled dose but a significant extension of nebulization time [97, 102]. When VMN was utilized during NIV, no significant differences were reported with different fill volumes [97, 98, 102]. Considering that the standard fill volume for most nebulization treatments is 2 mL or higher, increasing the fill volume for improving aerosol delivery is not recommended.
Ventilation mode and parameter settings
Recommendation XVI During aerosol delivery via noninvasive ventilation, changing the mode or parameters for the sole purpose to improve aerosol delivery efficiency is not recommended. IV,CS
Four in vitro studies of JN during NIV reported that inhaled doses increased as pressure support settings increased [103,104,105,106]. However, in an randomized controlled trial with 36 severe asthma patients, a greater improvement in patients’ pulmonary function was found with JN via NIV with inspiratory/expiratory pressure settings of 15/10 and 15/5 cmH2O than JN via a mask, particularly with setting of 15/10 cmH2O [87]. The discrepancies might be explained by the tidal volume changes during NIV. In the in vitro settings, tidal volume increased as pressure support increased, resulting in a higher inhaled dose. When continuous positive airway pressure was used, both in vitro and in vivo studies reported no significant differences between settings. Clinically, ventilator settings need to be adjusted based on the patient’s needs and it is not recommended to change the ventilator settings for the sole purpose of improving aerosol delivery efficiency.
Aerosol delivery via high-flow nasal cannula
The effectiveness of aerosol delivery via HFNC versus conventional aerosol therapy
Recommendation XVII The aerosol delivery efficiency with a nebulizer via high-flow nasal cannula is similar to that with a nebulizer and a mask or mouthpiece. Discontinuing high-flow nasal cannula treatment to administer a nebulizer with a mask or mouthpiece is not recommended. IV,CS Placing a nebulizer with a mask or mouthpiece with concurrent high-flow nasal cannula treatment should be avoided. IV
Compared to HFNC alone, albuterol delivery via HFNC significantly improved FEV1 and peak expiratory flow during COPD exacerbation [107] and in stable patients with reversible airflow obstruction [108]. Compared to conventional aerosol delivery via JN with a mask or mouthpiece, placing a VMN or JN inline with HFNC generated a comparable improvement of FEV1 for stable COPD patients [108, 109]. For patients who require HFNC therapy, discontinuing HFNC to use a conventional nebulizer adds the risk of interrupting oxygen and positive pressure. Moreover, placing a nebulizer with a mask or mouthpiece while the patient is concurrently receiving HFNC oxygen therapy significantly reduces the inhaled dose of the aerosolized drug to a negligible level, and this practice is not recommended.
Selection of nebulizer: VMN versus JN
Recommendation XVIII During aerosol delivery via high-flow nasal cannula, a vibrating mesh nebulizer is preferred over a jet nebulizer. IV,CS The nebulizer is recommended to be placed at the inlet of the humidifier. IV
During aerosol delivery via HFNC, both in vitro [110] and in vivo studies [111, 112] reported a higher efficiency of aerosol delivery with VMN than JN. Moreover, JN is driven by compressed oxygen or air, the introduction of the additional gas flow would affect flows or FIO2 delivery during HFNC treatment, whereas VMN is unlikely to influence flows or FIO2. Thus, VMN is preferred over JN. When HFNC gas flow was ≥ 10 L/min, the inhaled dose was higher with a nebulizer placed at the inlet of the humidifier compared to the nebulizer placed close to the nasal cannula [110, 113].
The use of pMDI and spacer
Recommendation XIX When pressurized metered dose inhaler is placed inline with high-flow nasal cannula, it is recommended to be used with a spacer and placed close to the nasal cannula with the aerosol plume directed toward the patient. IV
When pMDI was placed inline with HFNC, the use of a spacer increased the inhaled dose by 2–5 times in comparison with no spacer, regardless of pMDI placement and HFNC flow settings [114]. The inhaled dose was higher with the spacer placed close to the nasal cannula than close to the humidifier. When the spacer was placed with the gas flow, i.e., the aerosol plume was directed toward the patient, the inhaled dose was higher than when the pMDI was actuated into the spacer with the plume directed against the direction of gas flow.
Humidification
Recommendation XX During aerosol delivery via high-flow nasal cannula, turning off the humidifier is not recommended. CS
Aerosol deposition in the lung was higher with aerosol delivery via HFNC using dry gas than heated humidified gas [115]. However, this improvement in aerosol delivery only existed with gas flow ≥ 30 L/min, which might not be tolerated by patients and might cause potential harm, such as nose bleeding.
Additional information and results from the consensus can be found in Additional file 1: Appendix 11.
Discussion
Unlike aerosol therapy for ambulatory patients, aerosol delivery for critically ill patients, especially inline placement with various respiratory support equipment, is affected by several factors [116]. However, evidence to support the optimal aerosol delivery via respiratory support for patients is limited. In this consensus, most of the evidence is from in vitro studies, in vivo evidence especially clinical evidence on patient outcomes remains largely unknown and, in many cases, impractical. As a result, the panelist group carefully reviewed the currently available evidence and profoundly discussed the clinical benefits versus harms of applying those findings. Finally, this consensus was made with caution. Even after extensive discussion, consensus could not be reached on some topics among the panelists, such as ventilator settings and humidification for aerosolized antibiotics during MV, we provided the pros and cons of our debates for readers to review in the Additional file 1: Appendix. Clearly, more research is needed to provide firm guidelines for aerosol delivery in a variety of clinical settings encountered among critically ill patients receiving respiratory support.
Similar to other translational research, many of the in vitro findings could not be translated directly into clinical effectiveness, due in large part to the complicated mechanisms at play in the human body and the difficulty of quantifying the actual inhaled dose and the relevant clinical response. Critically ill patients, often receive multiple treatments simultaneously, making it challenging to evaluate the effects of aerosol treatments unless the aerosolized medication has a short onset and a measurable result. As such, albuterol is the most frequently used medication in clinical studies, using the rapid onset of bronchodilation effects to indirectly assess the aerosol deposition in the lung. However, due to the steep dose–response curve, a relatively small inhaled dose can cause patients to reach a plateau response, resulting in insignificant differences in clinical response between various administration settings. A more sensitive clinical measure is needed in future clinical studies. For aerosolized medications that do not have quick onset but are expensive, such as inhaled antibiotics, surfactants, gene therapy, and others, individualized dosing to reach the effective target concentration might play a key role in ensuring treatment success.
Currently, there is significant variation in the clinical practice of aerosol delivery for patients receiving respiratory support [12, 13], one size does not fit all, but the aim of this consensus statement is to clarify the numerous technical factors influencing aerosol delivery in this setting. Clinicians could use it as a reference to guide their practice based on their resources and conditions, such as the available aerosol and respiratory support devices, as well as human resources. More importantly, via this consensus statement and debates among clinical aerosol panelists (Additional file 1: Appendix 12), future directions in clinical aerosol research are suggested in Table 2.
The authors of this document recognize that there are several limitations to this approach. First, although we performed a thorough search of panelists in clinical aerosol research, we might still have missed some, especially those who published aerosol research in non-English journals. Second, due to various reasons, some panelists could not participate in this consensus. Third, the invited panelists are from a limited number of countries. Although all of them have clinical backgrounds and most of them are working with medical aerosols on a daily basis, they represent a very small proportion of clinicians worldwide. Fourth, due to the lack of robust clinical evidence, we could not use more explicit assessments such as GRADE to make the recommendations, thus the level of most recommendations is low and clinicians are advised to take this into account. Finally, this consensus only evaluates evidence from the adult population, and the recommendations in this document may not apply to aerosol delivery in infants and children receiving various forms of respiratory support.
Availability of data and materials
Not applicable.
Abbreviations
- ICU:
-
Intensive care unit
- NIV:
-
Noninvasive ventilation
- MV:
-
Mechanical ventilation
- HFNC:
-
High-flow nasal cannula
- IV:
-
In vitro study
- CS:
-
Clinical study
- AS:
-
Animal study
- VMN:
-
Vibrating mesh nebulizer
- pMDI:
-
Pressurized metered-dose inhaler
- JN:
-
Jet nebulizer
- FIO2 :
-
Fraction of inspired oxygen
- USN:
-
Ultrasonic nebulizer
- HME:
-
Heat–moisture exchanger
- VC:
-
Volume control
- PS:
-
Pressure support
- COPD:
-
Chronic obstructive pulmonary disease
- FEV1 :
-
Forced expiratory volume in the first second
References
Dhanani J, Fraser JF, Chan HK, Rello J, Cohen J, Roberts JA. Fundamentals of aerosol therapy in critical care. Crit Care. 2016;20(1):269.
Dugernier J, Ehrmann S, Sottiaux T, Roeseler J, Wittebole X, Dugernier T, et al. Aerosol delivery during invasive mechanical ventilation: a systematic review. Crit Care. 2017;21(1):264.
Lin HL, Fink JB, Ge H. Aerosol delivery via invasive ventilation: a narrative review. Ann Transl Med. 2021;9(7):588.
Dhand R. Aerosol therapy in patients receiving noninvasive positive pressure ventilation. J Aerosol Med Pulm Drug Deliv. 2012;25(2):63–78.
Wong FJ, Dudney T, Dhand R. Aerosolized antibiotics for treatment of pneumonia in mechanically ventilated subjects. Respir Care. 2019;64(8):962–79.
Li J, Fink JB, MacLoughlin R, Dhand R. A narrative review on trans-nasal pulmonary aerosol delivery. Crit Care. 2020;24(1):506.
Rello J, Bougle A, Rouby JJ. Aerosolised antibiotics in critical care. Intensive Care Med. 2023. https://doi.org/10.1007/s00134-023-07036-5.
Motos A, Yang H, Li Bassi G, Yang M, Meli A, Battaglini D, et al. Inhaled amikacin for pneumonia treatment and dissemination prevention: an experimental model of severe monolateral pseudomonas aeruginosa pneumonia. Crit Care. 2023;27(1):60.
Rouby JJ, Bouhemad B, Monsel A, Brisson H, Arbelot C, Lu Q, et al. Aerosolized antibiotics for ventilator-associated pneumonia: lessons from experimental studies. Anesthesiology. 2012;117(6):1364–80.
Rello J, Rouby JJ, Sole-Lleonart C, Chastre J, Blot S, Luyt CE, et al. Key considerations on nebulization of antimicrobial agents to mechanically ventilated patients. Clin Microbiol Infect. 2017;23(9):640–6.
Ehrmann S, Roche-Campo F, Bodet-Contentin L, Razazi K, Dugernier J, Trenado-Alvarez J, et al. Aerosol therapy in intensive and intermediate care units: prospective observation of 2808 critically ill patients. Intensive Care Med. 2016;42(2):192–201.
Lyu S, Li J, Yang L, Du X, Liu X, Chuan L, et al. The utilization of aerosol therapy in mechanical ventilation patients: a prospective multicenter observational cohort study and a review of the current evidence. Ann Transl Med. 2020;8(17):1071.
Li J, Tu M, Yang L, Jing G, Fink JB, Burtin C, et al. Worldwide clinical practice of high-flow nasal cannula and concomitant aerosol therapy in the adult ICU setting. Respir Care. 2021;66(9):1416–24.
Sole-Lleonart C, Roberts JA, Chastre J, Poulakou G, Palmer LB, Blot S, et al. Global survey on nebulization of antimicrobial agents in mechanically ventilated patients: a call for international guidelines. Clin Microbiol Infect. 2016;22(4):359–64.
Sole-Lleonart C, Rouby JJ, Chastre J, Poulakou G, Palmer LB, Blot S, et al. Intratracheal administration of antimicrobial agents in mechanically ventilated adults: an international survey on delivery practices and safety. Respir Care. 2016;61(8):1008–14.
Hussain A, Via G, Melniker L, Goffi A, Tavazzi G, Neri L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702.
Wong A, Galarza L, Forni L, De Backer D, Slama M, Cholley B, et al. Recommendations for core critical care ultrasound competencies as a part of specialist training in multidisciplinary intensive care: a framework proposed by the european society of intensive care medicine (ESICM). Crit Care. 2020;24(1):393.
Ari A, Areabi H, Fink JB. Evaluation of aerosol generator devices at 3 locations in humidified and non-humidified circuits during adult mechanical ventilation. Respir Care. 2010;55(7):837–44.
Ari A, Atalay OT, Harwood R, Sheard MM, Aljamhan EA, Fink JB. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010;55(7):845–51.
Ari A, Dang T, Al Enazi FH, Alqahtani MM, Alkhathami A, Qoutah R, et al. Effect of heat moisture exchanger on aerosol drug delivery and airway resistance in simulated ventilator-dependent adults using jet and mesh nebulizers. J Aerosol Med Pulm Drug Deliv. 2018;31(1):42–8.
ElHansy MHE, Boules ME, El Essawy AFM, Al-Kholy MB, Abdelrahman MM, Said ASA, et al. Inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during invasive mechanical ventilation. Pulm Pharmacol Ther. 2017;45:159–63.
ElHansy MHE, Boules ME, Farid H, Chrystyn H, El-Maraghi SK, Al-Kholy MB, et al. In vitro aerodynamic characteristics of aerosol delivered from different inhalation methods in mechanical ventilation. Pharm Dev Technol. 2017;22(6):844–9.
Liu CY, Ko HK, Fink JB, Wan GH, Huang CC, Chen YC, et al. Size distribution of colistin delivery by different type nebulizers and concentrations during mechanical ventilation. Pharmaceutics. 2019;11(9):459.
Naughton PJ, Joyce M, Mac Giolla Eain M, O’Sullivan A, MacLoughlin R. Evaluation of aerosol drug delivery options during adult mechanical ventilation in the COVID-19 era. Pharmaceutics. 2021;13(10):1574.
Hou H, Xu D, Dai B, Zhao H, Wang W, Kang J, et al. Position of different nebulizer types for aerosol delivery in an adult model of mechanical ventilation. Front Med (Lausanne). 2022;9:950569.
Rajendran RR, Kumaran S, Banerjee A, Berlinski A. A hybrid in vitro in silico framework for albuterol delivery through an adult ventilator circuit to a patient-specific lung airway model. J Aerosol Sci. 2021;158:105844.
Moustafa IOF, Ali MRA, Al Hallag M, Rabea H, Fink JB, Dailey P, et al. Lung deposition and systemic bioavailability of different aerosol devices with and without humidification in mechanically ventilated patients. Heart Lung. 2017;46(6):464–7.
Fuller HD, Dolovich MB, Posmituck G, Pack WW, Newhouse MT. Pressurized aerosol versus jet aerosol delivery to mechanically ventilated patients. Comparison of dose to the lungs. Am Rev Respir Dis. 1990;141(2):440–4.
Marik P, Hogan J, Krikorian J. A comparison of bronchodilator therapy delivered by nebulization and metered-dose inhaler in mechanically ventilated patients. Chest. 1999;115(6):1653–7.
Manthous CA, Hall JB, Schmidt GA, Wood LD. Metered-dose inhaler versus nebulized albuterol in mechanically ventilated patients. Am Rev Respir Dis. 1993;148(6 Pt 1):1567–70.
Guerin C, Chevre A, Dessirier P, Poncet T, Becquemin MH, Dequin PF, et al. Inhaled fenoterol-ipratropium bromide in mechanically ventilated patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1036–42.
Duarte AG, Momii K, Bidani A. Bronchodilator therapy with metered-dose inhaler and spacer versus nebulizer in mechanically ventilated patients: comparison of magnitude and duration of response. Respir Care. 2000;45(7):817–23.
Moustafa IOF, ElHansy MHE, Al Hallag M, Fink JB, Dailey P, Rabea H, et al. Clinical outcome associated with the use of different inhalation method with and without humidification in asthmatic mechanically ventilated patients. Pulm Pharmacol Ther. 2017;45:40–6.
Ehrmann S, Lyazidi A, Louis B, Isabey D, Le Pennec D, Brochard L, et al. Ventilator-integrated jet nebulization systems: tidal volume control and efficiency of synchronization. Respir Care. 2014;59(10):1508–16.
Cuccia AD, Ashraf S, McPeck M, Samuel J, Smaldone GC. Wet-side breath-enhanced jet nebulization: controlling drug delivery during mechanical ventilation. Respir Care. 2020;65(8):1077–89.
Niven RW, Ip AY, Mittelman S, Prestrelski SJ, Arakawa T. Some factors associated with the ultrasonic nebulization of proteins. Pharm Res. 1995;12(1):53–9.
O’Riordan TG. Formulations and nebulizer performance. Respir Care. 2002;47(11):1305–12.
Dubosky MN, Chen YF, Henriksen ME, Vines DL. Vibrating mesh nebulizer compared with metered-dose inhaler in mechanically ventilated subjects. Respir Care. 2017;62(4):391–5.
Anderson AC, Dubosky MN, Fiorino KA, Quintana V, Kaplan CA, Vines DL. The effect of nebulizer position on aerosolized epoprostenol delivery in an adult lung model. Respir Care. 2017;62(11):1387–95.
Dugernier J, Wittebole X, Roeseler J, Michotte JB, Sottiaux T, Dugernier T, et al. Influence of inspiratory flow pattern and nebulizer position on aerosol delivery with a vibrating-mesh nebulizer during invasive mechanical ventilation: an in vitro analysis. J Aerosol Med Pulm Drug Deliv. 2015;28(3):229–36.
Ge HQ, Wang JM, Lin HL, Fink JB, Luo R, Xu P, et al. Effect of nebulizer location and spontaneous breathing on aerosol delivery during airway pressure release ventilation in bench testing. J Aerosol Med Pulm Drug Deliv. 2019;32(1):34–9.
Boukhettala N, Poree T, Diot P, Vecellio L. In vitro performance of spacers for aerosol delivery during adult mechanical ventilation. J Aerosol Med Pulm Drug Deliv. 2015;28(2):130–6.
Rau JL, Harwood RJ, Groff JL. Evaluation of a reservoir device for metered-dose bronchodilator delivery to intubated adults. An in vitro study. Chest. 1992;102(3):924–30.
Harwood R, Rau JL, Thomas-Goodfellow L. A comparison of three methods of metered dose bronchodilator delivery to a mechanically ventilated adult lung model. Respir Care. 1994;39(9):886–91.
Rau JL, Dunlevy CL, Hill RL. A comparison of inline MDI actuators for delivery of a beta agonist and a corticosteroid with a mechanically ventilated lung model. Respir Care. 1998;43(9):705–12.
Seif SM, Elnady MA, Rabea H, Saeed H, Abdelrahim MEA. Effect of different connection adapters on aerosol delivery in invasive ventilation setting; an in-vitro study. J Drug Delivery Sci Technol. 2021;61:102177.
Fuller HD, Dolovich MB, Turpie FH, Newhouse MT. Efficiency of bronchodilator aerosol delivery to the lungs from the metered dose inhaler in mechanically ventilated patients. A study comparing four different actuator devices. Chest. 1994;105(1):214–8.
Ke WR, Wang WJ, Lin TH, Wu CL, Huang SH, Wu HD, et al. In vitro evaluation of aerosol performance and delivery efficiency during mechanical ventilation between soft mist inhaler and pressurized metered-dose inhaler. Respir Care. 2020;65(7):1001–10.
Diot P, Morra L, Smaldone GC. Albuterol delivery in a model of mechanical ventilation. Comparison of metered-dose inhaler and nebulizer efficiency. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1391–4.
Fink JB, Dhand R, Grychowski J, Fahey PJ, Tobin MJ. Reconciling in vitro and in vivo measurements of aerosol delivery from a metered-dose inhaler during mechanical ventilation and defining efficiency-enhancing factors. Am J Respir Crit Care Med. 1999;159(1):63–8.
Fuller HD, Dolovich MB, Chambers C, Newhouse MT. Aerosol delivery during mechanical ventilation: a predictive in-vitro lung model. J Aerosol Med. 1992;5(4):251–9.
Fink JB, Dhand R, Duarte AG, Jenne JW, Tobin MJ. Aerosol delivery from a metered-dose inhaler during mechanical ventilation. An in vitro model. Am J Respir Crit Care Med. 1996;154(2 Pt 1):382–7.
Goode ML, Fink JB, Dhand R, Tobin MJ. Improvement in aerosol delivery with helium-oxygen mixtures during mechanical ventilation. Am J Respir Crit Care Med. 2001;163(1):109–14.
Miller DD, Amin MM, Palmer LB, Shah AR, Smaldone GC. Aerosol delivery and modern mechanical ventilation: In vitro/in vivo evaluation. Am J Respir Crit Care Med. 2003;168(10):1205–9.
Mitchell JP, Nagel MW, Wiersema KJ, Doyle CC, Migounov VA. The delivery of chlorofluorocarbon-propelled versus hydrofluoroalkane-propelled beclomethasone dipropionate aerosol to the mechanically ventilated patient: a laboratory study. Respir Care. 2003;48(11):1025–32.
Martin AR, Finlay WH. The effect of humidity on the size of particles delivered from metered-dose inhalers. Aerosol Sci Technol. 2005;39(4):283–9.
Lin HL, Fink JB, Zhou Y, Cheng YS. Influence of moisture accumulation in inline spacer on delivery of aerosol using metered-dose inhaler during mechanical ventilation. Respir Care. 2009;54(10):1336–41.
Ari A, Harwood R, Sheard M, Alquaimi MM, Alhamad B, Fink JB. Quantifying aerosol delivery in simulated spontaneously breathing patients with tracheostomy using different humidification systems with or without exhaled humidity. Respir Care. 2016;61(5):600–6.
American Association for Respiratory Care. AARC clinical practice guidelines. Endotracheal suctioning of mechanically ventilated patients with artificial airways 2010. Respir Care. 2010;55(6):758–64.
Craven DE, Goularte TA, Make BJ. Contaminated condensate in mechanical ventilator circuits. A risk factor for nosocomial pneumonia? Am Rev Respir Dis. 1984;129(4):625–8.
Ari A, Alwadeai KS, Fink JB. Effects of heat and moisture exchangers and exhaled humidity on aerosol deposition in a simulated ventilator-dependent adult lung model. Respir Care. 2017;62(5):538–43.
Bihan K, Zahr N, Becquemin MH, Lu X, Bertholon JF, Vezinet C, et al. Influence of diluent volume of colistimethate sodium on aerosol characteristics and pharmacokinetics in ventilator-associated pneumonia caused by MDR bacteria. J Antimicrob Chemother. 2018;73(6):1639–46.
Kacmarek RM, Stoller JK, Heuer AJ. Egan’s fundamental of respiratory care 11 ed. Elsevier, 2016.
O’Doherty MJ, Thomas SH, Page CJ, Treacher DF, Nunan TO. Delivery of a nebulized aerosol to a lung model during mechanical ventilation. Effect of ventilator settings and nebulizer type, position, and volume of fill. Am Rev Respir Dis. 1992;146(2):383–8.
Thomas SH, O’Doherty MJ, Page CJ, Treacher DF, Nunan TO. Delivery of ultrasonic nebulized aerosols to a lung model during mechanical ventilation. Am Rev Respir Dis. 1993;148(4):872–7.
Dhanani JA, Tang P, Wallis SC, Parker SL, Pandey P, Fraser JF, et al. Characterisation of 40mg/ml and 100mg/ml tobramycin formulations for aerosol therapy with adult mechanical ventilation. Pulm Pharmacol Ther. 2018;50:93–9.
Ari A, Harwood RJ, Sheard MM, Fink JB. Pressurized metered-dose inhalers versus nebulizers in the treatment of mechanically ventilated subjects with artificial airways: an in vitro study. Respir Care. 2015;60(11):1570–4.
Leung SSY, Parumasivam T, Tang P, Chan HK. A proof-of-principle setup for delivery of relenza((r)) (zanamivir) inhalation powder to intubated patients. J Aerosol Med Pulm Drug Deliv. 2016;29(1):30–5.
O’Riordan TG, Greco MJ, Perry RJ, Smaldone GC. Nebulizer function during mechanical ventilation. Am Rev Respir Dis. 1992;145(5):1117–22.
Tonnellier M, Ferrari F, Goldstein I, Sartorius A, Marquette CH, Rouby JJ. Intravenous versus nebulized ceftazidime in ventilated piglets with and without experimental bronchopneumonia: comparative effects of helium and nitrogen. Anesthesiology. 2005;102(5):995–1000.
Mojoli F, Iotti GA, Imberti R, Braschi A. The importance of protecting the mechanical ventilator during colistin methanesulfonate nebulization. Intensive Care Med. 2013;39(3):535–6.
Tonnelier A, Lellouche F, Bouchard PA, L’Her E. Impact of humidification and nebulization during expiratory limb protection: an experimental bench study. Respir Care. 2013;58(8):1315–22.
Malliotakis P, Mouloudi E, Prinianakis G, Kondili E, Georgopoulos D. Influence of respiratory efforts on b2-agonist induced bronchodilation in mechanically ventilated copd patients: a prospective clinical study. Respir Med. 2007;101(2):300–7.
Dugernier J, Reychler G, Wittebole X, Roeseler J, Depoortere V, Sottiaux T, et al. Aerosol delivery with two ventilation modes during mechanical ventilation: a randomized study. Ann Intensive Care. 2016;6(1):73.
Williams L, Fletcher GC, Daniel M, Kinsella J. A simple in vitro method for the evaluation of an ultrasonic nebulizer for drug delivery to intubated, ventilated patients and the effect of nebulizer and ventilator settings on the uptake of fluid from the nebulizer chamber. Eur J Anaesthesiol. 1999;16(7):479–84.
Mouloudi E, Katsanoulas K, Anastasaki M, Hoing S, Georgopoulos D. Bronchodilator delivery by metered-dose inhaler in mechanically ventilated copd patients: influence of tidal volume. Intensive Care Med. 1999;25(11):1215–21.
Mouloudi E, Prinianakis G, Kondili E, Georgopoulos D. Bronchodilator delivery by metered-dose inhaler in mechanically ventilated copd patients: influence of flow pattern. Eur Respir J. 2000;16(2):263–8.
Hess DR, Dillman C, Kacmarek RM. In vitro evaluation of aerosol bronchodilator delivery during mechanical ventilation: pressure-control vs. volume control ventilation. Intensive Care Med. 2003;29(7):1145–50.
Vecellio L, Guerin C, Grimbert D, De Monte M, Diot P. In vitro study and semiempirical model for aerosol delivery control during mechanical ventilation. Intensive Care Med. 2005;31(6):871–6.
Guerin C, Durand PG, Pereira C, Richard JC, Poupelin JC, Lemasson S, et al. Effects of inhaled fenoterol and positive end-expiratory pressure on the respiratory mechanics of patients with chronic obstructive pulmonary disease. Can Respir J. 2005;12(6):329–35.
Mouloudi E, Katsanoulas K, Anastasaki M, Askitopoulou E, Georgopoulos D. Bronchodilator delivery by metered-dose inhaler in mechanically ventilated copd patients: influence of end-inspiratory pause. Eur Respir J. 1998;12(1):165–9.
Franca EE, Dornelasde Andrade AF, Cabral G, Almeida Filho P, Silva KC, Galindo Filho VC, et al. Nebulization associated with bi-level noninvasive ventilation: analysis of pulmonary radioaerosol deposition. Respir Med. 2006;100(4):721–8.
Reychler G, Leal T, Roeseler J, Thys F, Delvau N, Liistro G. Effect of continuous positive airway pressure combined to nebulization on lung deposition measured by urinary excretion of amikacin. Respir Med. 2007;101(10):2051–5.
Calvert LD, Jackson JM, White JA, Barry PW, Kinnear WJ, O’Callaghan C. Enhanced delivery of nebulised salbutamol during non-invasive ventilation. J Pharm Pharmacol. 2006;58(11):1553–7.
Parkes SN, Bersten AD. Aerosol kinetics and bronchodilator efficacy during continuous positive airway pressure delivered by face mask. Thorax. 1997;52(2):171–5.
Pollack CV Jr, Fleisch KB, Dowsey K. Treatment of acute bronchospasm with beta-adrenergic agonist aerosols delivered by a nasal bilevel positive airway pressure circuit. Ann Emerg Med. 1995;26(5):552–7.
Brandao DC, Lima VM, Filho VG, Silva TS, Campos TF, Dean E, et al. Reversal of bronchial obstruction with bi-level positive airway pressure and nebulization in patients with acute asthma. J Asthma. 2009;46(4):356–61.
Galindo-Filho VC, Brandao DC, Ferreira Rde C, Menezes MJ, Almeida-Filho P, Parreira VF, et al. Noninvasive ventilation coupled with nebulization during asthma crises: a randomized controlled trial. Respir Care. 2013;58(2):241–9.
Nava S, Karakurt S, Rampulla C, Braschi A, Fanfulla F. Salbutamol delivery during non-invasive mechanical ventilation in patients with chronic obstructive pulmonary disease: a randomized, controlled study. Intensive Care Med. 2001;27(10):1627–35.
Branconnier MP, Hess DR. Albuterol delivery during noninvasive ventilation. Respir Care. 2005;50(12):1649–53.
Abdelrahim ME, Plant P, Chrystyn H. In-vitro characterisation of the nebulised dose during non-invasive ventilation. J Pharm Pharmacol. 2010;62(8):966–72.
Haw A, McPeck M, Cuccia AD, Smaldone GC. Face mask leak determines aerosol delivery in noninvasive ventilation. Respir Care. 2021;66(1):95–103.
Michotte JB, Jossen E, Roeseler J, Liistro G, Reychler G. In vitro comparison of five nebulizers during noninvasive ventilation: analysis of inhaled and lost doses. J Aerosol Med Pulm Drug Deliv. 2014;27(6):430–40.
Tan W, Dai B, Xu DY, Li LL, Li J. In-vitro comparison of single limb and dual limb circuit for aerosol delivery via noninvasive ventilation. Respir Care. 2022;67(7):807–13.
Hassan A, Salah Eldin R, Abdelrahman MM, Abdelrahim ME. In-vitro/in-vivo comparison of inhaled salbutamol dose delivered by jet nebulizer, vibrating mesh nebulizer and metered dose inhaler with spacer during non-invasive ventilation. Exp Lung Res. 2017;43(1):19–28.
Hassan A, Rabea H, Hussein RRS, Eldin RS, Abdelrahman MM, Said AS, et al. In-vitro characterization of the aerosolized dose during non-invasive automatic continuous positive airway pressure ventilation. Pulm Ther. 2016;21:15–26.
Saeed H, Elberry AA, Eldin AS, Rabea H, Abdelrahim ME. Effect of nebulizer designs on aerosol delivery during non-invasive mechanical ventilation: a modeling study of in vitro data. Pulm Ther. 2017;3(1):233–41.
Saeed H, Mohsen M, Fink JB, Dailey P, Eldin AS, Abdelrahman MM, et al. Fill volume, humidification and heat effects on aerosol delivery and fugitive emissions during noninvasive ventilation. J Drug Delivery Sci Technol. 2017;39:372–8.
AlQuaimi M, Fink J, Ari A. Efficiency of different aerosol devices and masks during noninvasive positive pressure ventilation in a simulated adult lung model. J Respir Med Lung Dis. 2017;1018.
Abdelrahim ME, Plant PK, Chrystyn H. The relative lung and systemic bioavailability of terbutaline following nebulisation in non-invasively ventilated patients. Int J Pharm. 2011;420(2):313–8.
Mohsen M, Elberry AA, Eldin AS, Hussein RRS, Abdelrahim MEA. Effects of heat and humidification on aerosol delivery during auto-cpap noninvasive ventilation. Arch Pulmonology Respir Care. 2017;3(1):011–5.
Saeed H, Ali AMA, Elberry AA, Eldin AS, Rabea H, Abdelrahim MEA. Modeling and optimization of nebulizers’ performance in non-invasive ventilation using different fill volumes: comparative study between vibrating mesh and jet nebulizers. Pulm Pharmacol Ther. 2018;50:62–71.
Chatmongkolchart S, Schettino GP, Dillman C, Kacmarek RM, Hess DR. In vitro evaluation of aerosol bronchodilator delivery during noninvasive positive pressure ventilation: effect of ventilator settings and nebulizer position. Crit Care Med. 2002;30(11):2515–9.
Dai B, Kang J, Sun LF, Tan W, Zhao HW. Influence of exhalation valve and nebulizer position on albuterol delivery during noninvasive positive pressure ventilation. J Aerosol Med Pulm Drug Deliv. 2014;27(2):125–32.
Sutherasan Y, Ball L, Raimondo P, Caratto V, Sanguineti E, Costantino F, et al. Effects of ventilator settings, nebulizer and exhalation port position on albuterol delivery during non-invasive ventilation: an in-vitro study. BMC Pulm Med. 2017;17(1):9.
Tan W, Dai B, Lu CL, Hou HJ, Zhao HW, Wang W, et al. The effect of different interfaces on the aerosol delivery with vibrating mesh nebulizer during noninvasive positive pressure ventilation. J Aerosol Med Pulm Drug Deliv. 2021;34(6):366–73.
Beuvon C, Coudroy R, Bardin J, Marjanovic N, Rault C, Bironneau V, et al. Beta agonist delivery by high-flow nasal cannula during copd exacerbation. Respir Care. 2022;67(1):9–15.
Reminiac F, Vecellio L, Bodet-Contentin L, Gissot V, Le Pennec D, Salmon Gandonniere C, et al. Nasal high-flow bronchodilator nebulization: a randomized cross-over study. Ann Intensive Care. 2018;8(1):128.
Braunlich J, Wirtz H. Oral versus nasal high-flow bronchodilator inhalation in chronic obstructive pulmonary disease. J Aerosol Med Pulm Drug Deliv. 2018;31(4):248–54.
Li J, Williams L, Fink JB. The impact of high-flow nasal cannula device, nebulizer type, and placement on trans-nasal aerosol drug delivery: an in vitro study. Respir Care. 2021;67:1–8.
Dugernier J, Hesse M, Jumetz T, Bialais E, Roeseler J, Depoortere V, et al. Aerosol delivery with two nebulizers through high-flow nasal cannula: A randomized cross-over single-photon emission computed tomography-computed tomography study. J Aerosol Med Pulm Drug Deliv. 2017;30(5):349–58.
Madney YM, Fathy M, Elberry AA, Rabea H, Abdelrahim ME. Aerosol delivery through an adult high-flow nasal cannula circuit using low-flow oxygen. Respir Care. 2019;64(4):453–61.
Li J, Wu W, Fink JB. In vitro comparison between inspiration synchronized and continuous vibrating mesh nebulizer during trans-nasal aerosol delivery. Intensive Care Med Exp. 2020;8(1):6.
Szychowiak P, Gensburger S, Bocar T, Landel C, Philippe M, Pennec DL, et al. Pressurized metered dose inhaler aerosol delivery within nasal high-flow circuits: a bench study. J Aerosol Med Pulm Drug Deliv. 2021;34(5):303–10.
Alcoforado L, Ari A, Barcelar JM, Brandao SCS, Fink JB, de Andrade AD. Impact of gas flow and humidity on trans-nasal aerosol deposition via nasal cannula in adults: a randomized cross-over study. Pharmaceutics. 2019;11(7):320.
Dhand R. Inhalation therapy in invasive and noninvasive mechanical ventilation. Curr Opin Crit Care. 2007;13(1):27–38.
Acknowledgments
We would like to appreciate the panelists who participated in the first and second rounds of review on the available evidence and recommendations for their diligent work and thoughtful comments. We deeply mourn the passing of Dr. Paolo Pelosi, whose dedication, expertise, and valuable contributions will forever remain an integral part of this work and and our understanding of critical care medicine.
Funding
This consensus has no funding support.
Author information
Authors and Affiliations
Contributions
JL, JBF, and SE conceived this project. JL, KL, SL, GJ, and BD performed the literature search, extracted the data, summarized the study findings, drafted the recommendations, and summarized the scores and comments for three rounds of review, and attended the monthly working committee meetings. JL invited and communicated with the panelist, organized the monthly working committee meetings and the online meetings, and drafted the manuscript. JBF and SE supervised the project, communicated with panelist, and provided critical revisions to the manuscript. The panel (JL, BD, RD, HL, PP, AB, JR, AT, CEL, JM, QL, GR, LV, AD, JJR, JBF, and SE) evaluated the recommendation in light of available evidence, suggested missing literature, scored and commented on the recommendations, and revised the manuscript. JL, KL, SL, GJ, and BD equally contributed to the overall project described in this article. SE, JBF, and JL were responsible for the decision to submit the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JL discloses research funding from Fisher & Paykel Healthcare Ltd, Aerogen Ltd, American Association for Respiratory Care (AARC), and Rice Foundation, and speaker fees from AARC, Aerogen Ltd, Heyer Ltd, and Fisher & Paykel Healthcare Ltd. HL discloses research funding from Chang Fung Memorial Foundation. AT discloses research funding from Bayer, and Cardeas. AB discloses research funding from Trudell Medical International, participation in clinical advisory board of Hollo Medical Inc, and participation as science advisor to International Pharmaceutical Aerosol Consortium on Regulation and Science. JR discloses grants or consultancies from ESCMID, European Respiratory Society, Bayer, and Genentech, speaker’s bureau for Norma Hellas. CEL discloses consultancies from Aerogen Ltd, AdvanzPharma, Merck and Pfizer. RD reports personal fees from Astra-Zeneca, Boehringer-Ingelheim, Mylan, UptoDate, and Teva. He is the recipient of research grants form Mylan, Viatris and GSK. AT discloses research funding from Bayer, and Cardeas. LV was employed by DTF Medical from 2001 to 2018 and by Nemera from 2018 to 2020. JBF is Chief Science Officer for Aerogen Pharma Corp 2016 to present and previously Chief Clinical Officer at Aerogen Ltd. SE discloses consultancies from Aerogen Ltd, research support, speaker fees, and travel support from Aerogen Ltd and Fisher & Paykel healthcare. Others do not have any conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Paolo Pelosi: Deceased.
Supplementary Information
Additional file 1.
Appendix 1–11.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Liu, K., Lyu, S. et al. Aerosol therapy in adult critically ill patients: a consensus statement regarding aerosol administration strategies during various modes of respiratory support. Ann. Intensive Care 13, 63 (2023). https://doi.org/10.1186/s13613-023-01147-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-023-01147-4