Abstract
Background
Acute Kidney Injury (AKI) is a frequent complication of severe SARS-CoV-2 infection. Multiple mechanisms are involved in COVID-19-associated AKI, from direct viral infection and secondary inflammation to complement activation and microthrombosis. However, data are limited in critically-ill patients. In this study, we sought to describe the prevalence, risk factors and prognostic impact of AKI in this setting.
Methods
Retrospective monocenter study including adult patients with laboratory confirmed SARS-CoV-2 infection admitted to the ICU of our university Hospital. AKI was defined according to both urinary output and creatinine KDIGO criteria.
Results
Overall, 100 COVID-19 patients were admitted. AKI occurred in 81 patients (81%), including 44, 10 and 27 patients with AKI stage 1, 2 and 3 respectively. The severity of AKI was associated with mortality at day 28 (p = 0.013). Before adjustment, the third fraction of complement (C3), interleukin-6 (IL-6) and ferritin levels were higher in AKI patients. After adjustment for confounders, both severity (modified SOFA score per point) and AKI were associated with outcome. When forced in the final model, C3 (OR per log 0.25; 95% CI 0.01–4.66), IL-6 (OR per log 0.83; 95% CI 0.51–1.34), or ferritin (OR per log 1.63; 95% CI 0.84–3.32) were not associated with AKI and did not change the model.
Conclusion
In conclusion, we did not find any association between complement activation or inflammatory markers and AKI. Proportion of patients with AKI during severe SARS-CoV-2 infection is higher than previously reported and associated with outcome.
Similar content being viewed by others
Background
Since December 2019, severe acute respiratory coronavirus 2 (SARS-CoV-2) has spread worldwide, causing more than 6.6 million cases and 390 000 deaths [1]. This pandemic has put unprecedented pressure on healthcare systems and especially on intensive care units (ICUs).
Acute Kidney Injury (AKI) is a frequent complication of severe SARS-CoV-2 infection but data are scarce in ICUs. AKI has been previously reported with an average incidence of 11% (8–17%) overall, with highest ranges in the critically ill (23%; 14–35%) [2,3,4]. Different applications of the Kidney Disease Improving Global Outcomes (KDIGO) criteria for AKI, in particular different methods to estimate missing baseline creatinine and handling urinary output, can cause important variations of estimated incidence [5, 6] and may contribute to the discrepancies among these studies.
Multiple mechanisms are involved in COVID-19-associated AKI, ranging from direct viral infection of the kidney and secondary inflammation to complement activation and microthrombosis [7]. In particular, severe COVID-19 is associated with uncontrolled systemic inflammatory response with high levels of IL-6 [8] that could potentially lead to intrarenal inflammation and increased vascular permeability and share several features with hyperferritinemic syndromes such as macrophage activation syndrome [9]. Furthermore, unrestrained activation of complement leads to endothelial cell dysfunction and intravascular coagulation that could participate in COVID-19-associated AKI [10]. Both IL-6 [11] and complement [12] have been proposed as therapeutic targets and understanding their role in COVID-19-associated AKI is therefore a priority.
However, most of the studies performed to date gave little data regarding definition of AKI or influence of inflammation and complement markers on AKI.
In this study, we sought to describe the prevalence, risk factors and prognostic impact of AKI during COVID-19 in the ICU.
Methods
Study design and cohort
We conducted a retrospective monocenter study including adult patients with laboratory confirmed SARS-CoV-2 infection admitted to the ICU of our university Hospital. All adult patients (age ≥ 18 years) who tested positive by polymerase chain reaction testing of a nasopharyngeal sample for COVID-19 and were hospitalized from March 1, 2020 to June 1, 2020 were eligible.
This study was approved by an institutional review board (French Intensive Care Society—CE SRLF n°20–32). Need for informed consent was waived as regard to the study observational design and in accordance with the French law. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Data collection, definitions and measurements
All data were obtained from medical records and patients’ charts. Baseline patients’ characteristics were collected, including demographics and comorbidities before ICU admission. The variables recorded regarding ICU admission and treatments were relative to clinical presentation, reason for ICU admission, diagnosis, therapies implemented and outcomes.
Blood sampling and routine biological testing were performed on the day of admission according to the standard laboratory protocols.
All samples were immediately centrifuged at 3000 rpm at 4 °C for 10 min, separated from the cells and stored at −80 °C until biochemical assays of complement proteins C3, C4 (nephelometry) and sC5B9 (ELISA), IL-6 (ELISA) were performed. The primary outcome was mortality at day 28.
Definition of AKI
AKI was defined according to both urinary output and serum creatinine KDIGO criteria [13] as follows: stage 1—increase in serum creatinine by 0.3 mg/dl within 48 h or a 1.5–1.9 times increase in serum creatinine from baseline or urinary output < 0.5 ml/kg/h for 6–12 h within 7 days; stage 2—2.9 times increase in serum creatinine or urinary output < 0.5 ml/kg/h for ≥ 12 h within 7 days; stage 3—3 times or more increase in serum creatinine or to ≥ 4.0 mg/dl or initiation of RRT or urinary output < 0.3 ml/kg/h for ≥ 24 h or anuria for ≥ 12 h within 7 days. Patients were stratified according to the highest AKI stage attained during the first 7 days of ICU stay.
Baseline creatinine was defined as the best value in the 3 preceding months or if unavailable as the lowest value during ICU stay or was back calculated based on a glomerular filtration rate of 60 mL/min/1.73m2 with MDRD equation in patients without known chronic kidney disease. Chronic kidney disease (CKD) was defined according to the KDIGO definition.
Modified SOFA was defined as SOFA score [14] excluding the renal component.
Statistical analysis
Continuous variables were described as median (interquartile range [IQR]) and compared between groups using the non-parametric Wilcoxon rank-sum test. Categorical variables were described as frequency (percentages) and compared between groups using Fisher’s exact test. Mortality was assessed using survival analysis.
Independent risk factors of day 28 mortality were assessed using Cox model. Conditional stepwise variable selection was performed with 0.2 as the critical p-value for entry into the model, and 0.1 as the p-value for removal. Interactions and correlations between the explanatory variables were carefully checked. Validity of proportional hazards assumption, influence of outliers, and linearity in relationship between the log hazard and the covariates were carefully checked.
Independent risk factors of AKI were assessed using logistic regression. Conditional backward stepwise variable selection was performed with 0.2 as the critical p-value for entry into the model, and 0.1 as the p-value for removal. Interactions and correlations between the explanatory variables were carefully checked. Influence of outliers, and linearity in relationship between the log hazard and the covariates were carefully checked. It was preplanned to force, one by one, in the final model inflammation biomarker, ferritin, complement pathway dosage and PEEP level at admission should these variable not be selected.
Kaplan–Meier graphs were used to express the probability of death from inclusion to day 28. Comparisons were performed using the log-rank test.
Overall, rate of missing data was 6.6% with rate of missing data among major outcome or covariates was < 5%. As regard to completeness of the dataset, no imputation of missing data was performed.
Statistical analyses were performed with R statistical software, version 3.6.2 (available online at https://www.r-project.org/) and the ‘Survival’ package was used. A p value < 0.05 was considered significant.
Results
Patients’ characteristics
Overall, 100 COVID-19 patients were admitted in our ICU between March and May 2020 and included in this study. Patients’ characteristics are described in Table 1. Median age was 59 years [53–67] and 70 patients (70%) were of male gender. Only 15 patients (15%) had no underlying disease. Hypertension (n = 56, 56%), diabetes (n = 30, 30%) and chronic kidney disease (n = 29, 29%) were the main comorbidities. Thirty-six patients were obese (n = 21, 21%) or overweight (n = 25, 25%) and 30 (30%) were treated with angiotensin converting enzyme inhibitors or angiotensin-receptor blockers. Median SOFA score at admission was 4 [2,3,4,5,6,7]. Median modified SOFA score [without the renal component] was 3 [2,3,4,5,6,7]. Fifty-six patients (55%) required mechanical ventilation and 51 (51%) vasopressor therapy.
Risk factors of AKI in COVID-19 patients
Rate of patients with missing baseline serum creatinine was 67% (n = 67) and did not differ between AKI and patients without AKI (74 vs. 65% respectively; p = 0.67).
AKI occurred in 81 patients (81%), including 44 patients, 10 patients, 27 patients with AKI stage 1, 2 and 3 respectively. Among patients with AKI, 33 (41%) met only urinary output KDIGO criteria, 28 (35%) met only creatinine criteria and 20 (25%) met both. Urinary output criteria alone was more frequently involved in diagnosis of milder stage of AKI (AKI stage 1 (n = 20, 45%) and stage 2 (n = 6, 60%) compared to 26% in stage 3 (n = 7, p = 0.007). Thirteen (13%) required renal replacement therapy during the first 7 days in ICU.
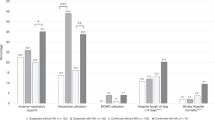
Before adjustment, C3 (p = 0.01), IL-6 (p = 0.03) and ferritin levels (p = 0.03) were associated with AKI severity, whereas soluble C5b9 fraction was not different (p = 0.42) (Fig. 1).
In a multivariate model incorporating baseline chronic kidney disease, only modified SOFA was significantly associated with the development of AKI (OR per point 1.29; 95% CI 1.04–1.70).
When forced in the final model, C3 (OR per log 0.25; 95% CI 0.01–4.66), IL-6 (OR per log 0.83; 95% CI 0.51–1.34), ferritin (OR per log 1.63; 95% CI 0.84–3.32), or PEEP level (OR per mmHg 1.04; 95% CI 0.91–1.22) were not associated with AKI and did not change the model.
However, after adjustment for modified SOFA and CKD, C3 value higher than median was significantly associated with a lower risk for AKI stage 2 or 3, compared to no AKI or AKI stage 1 (OR 0.17 95% CI [0.05–0.54], p = 0.004), while the association between AKI stage 2 or 3 and IL-6, Ferritin, sC5b9 and PEEP levels did not reach statistical significance (Additional file 1: Table S1 and Figure S1).
Outcome analysis
Twenty-nine (29%) patients had died by day 28, 28 (35%) patients with AKI and 1 (5%) patient without AKI (p = 0.02). More than half of the patients with AKI stage 2 (n = 5, 50%) and 3 (n = 15, 56%) died before day 28. The severity of AKI was associated with mortality at day 28 (p = 0.013) (Fig. 2 and Additional file 1: Figure S2).
Patients who died were older (66 versus 57 years, p = 0.001), suffered more frequently from chronic kidney disease (52 versus 20%, p = 0.003), had higher SOFA score (8 versus 2, p < 0.001), ferritin levels (1805 versus 1148 mg/L, p = 0.004) and IL-6 levels (204 versus 103 ng/mL, p = 0.02), and were less often immunocompetent (46 versus 18%, p = 0.01). Neither comorbidities nor BMI were associated with death at day 28. Patients who died more often required mechanical ventilation (90 versus 41%, p < 0.001), vasopressors (90 versus 36%, p < 0.001) and renal replacement therapy (31 versus 6%, p = 0.002).
After adjustment for confounders, both AKI and severity (modified SOFA score per point) were associated with survival (Table 2).
There was a similar trend towards poorer survival in patients with (n = 33) and without (n = 67) baseline serum creatinine (Additional file 1: Figure S3).
We also tested the interaction between missing baseline serum creatinine and reported results and did not find any significant interaction (Additional file 1: Table S2).
Treatments with lopinavir/ritonavir (n = 10), tocilizumab (n = 1), eculizumab (n = 2) or chloroquine (n = 3) were associated neither with mortality nor with the development of AKI.
Discussion
In this study, we describe the incidence of AKI in 100 COVID-19 patients admitted to the ICU, and the link between AKI, inflammation markers and complement levels. The main results of this case series are the higher than previously reported incidence of AKI and its lack of association with IL-6, ferritin or complement factors C3 and sC5B9. The importance of AKI in COVID-19 patients has been increasingly recognized. If initial reports from China reported rates of AKI as low as 2.9–8% in severe patients [2, 15, 16], incidences from later studies ranged between 15% [17] and 44% [18] in critically ill patients. Most reports lack a clear operational AKI definition [19,20,21], but even in studies that used KDIGO serum creatinine criteria, diuresis was inconsistently taken into account, and none of them reported AKI stages. Our study is the largest published to date reporting incidence of AKI specifically in critically ill patients. With 81% of patients diagnosed with AKI, the rate of AKI in our study is much higher than previously reported.
Interestingly, one of the largest cohort from the United States reported a prevalence of AKI in COVID-19 patients requiring mechanical ventilation of 80% [22], close to our finding of 90%.
One strength of our study is the rigorous use of KDIGO criteria, including urinary output. Furthermore, given that many patients will present with AKI without a reliable baseline serum creatinine on record, estimation of the latter is of tremendous importance. Several methods to estimate missing baseline creatinine can lead to variations in incidence of AKI up to 15% [6]. Using complete KDIGO definition we found a 80% incidence of AKI during the first 7 days of ICU stay and a 90% incidence in patients requiring mechanical ventilation.
We also tested the hypotheses that COVID-19-associated-AKI is linked to the elevation of cytokines induced by SARS-CoV-2 infection [23], complement dysregulation [12] induced by SARS-CoV-2 infection or mechanical ventilation settings. High levels of IL-6 have been associated with the development of severe disease [24, 25] and acute respiratory distress syndrome [8] during COVID-19 infection, but the role of inflammation markers in COVID-19-induced-AKI remains speculative [7]. The deleterious role of IL-6 has been demonstrated in different models of AKI, including ischemic AKI, nephrotoxin-induced AKI and sepsis-induced AKI [26, 27]. In our study, IL-6 and ferritin levels correlated with severity but were not independently associated with AKI. The complement system represents the first response of the host immune system. It participates in the development of AKI [28] and has also been suspected to play a role in AKI in the context of SARS-CoV-2 infection [10, 12]. Recent studies showed a strong immunohistochemical staining of complement cascade components in the lungs [29] and kidneys [30] of severe COVID-19 patients. However, even if C3 levels were associated with AKI severity in univariate analysis, the association did not persist when forced into a multivariate model, and soluble C5b9 showed no association with AKI. C3 level was only independently associated with AKI stage 2 and 3 compared to no AKI or AKI stage 1. Overall, our results do not support a role for complement dysregulation in COVID-19-induced-AKI, even though complement dysregulation may be involved in the most severe forms of AKI.
Although our study did not include extensive coagulation explorations [31], the lack of difference in fibrinogen levels in patients with AKI when compared to patients without AKI does not support the evidence of a role of hypercoagulability in COVID-19-induced-AKI, suggested by the presence of thrombi in glomerular loops described by others [32, 33]. Last, high levels of PEEP in COVID-19 patients [34, 35] have also been suggested as a potential factor for increased AKI in severe COVID-19 patients [36]. In our study, PEEP levels were not independently associated with the development of AKI, but the lack of statistical power and longitudinal data on PEEP levels does not allow for any definite conclusion.
Our study also has limitations. First, despite being the largest focusing on COVID-19-induced-AKI in the ICU, the limited number of patients can translate into a lack of statistical power. Nevertheless, rate of AKI and sample size allow confirming a high AKI incidence with a reasonable level of confidence (81%; 95% CI 72–89). In addition, the monocenter design may have limited external validity of our findings. The proportion of missing baseline serum creatinine value (67%) may also be an issue. Missing baseline creatinine value is a common problem in research focusing on AKI, and although most studies do not report the proportion of missing baseline values, rates as high as 85% are common [22]. We provide sensitivity analyses (Additional file 1: Figure S3 and Table S2) to show that back calculation of missing baseline creatinine values unlikely results in a significant bias. Last, lack of association between inflammation biomarker, IL-6 or complement component do not preclude participation of these mechanisms to AKI. They only underline that in this setting and when adjusted to severity, these factors have little influence on AKI rate. Additional studies, assessing levels of proteinuria and hematuria [22, 37], pathological findings and translational research are needed to further explore different mechanisms that may participate to AKI during severe SARS-CoV-2 infection.
In conclusion, we did not find any association between complement activation or inflammatory markers and AKI. Our study suggests a tremendously high incidence of AKI in our cohort of critically ill COVID-19 patients, along with an independent association between AKI and outcome. Because of its marked influence on outcome, AKI should be identified promptly during SARS-CoV-2 infection.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- AKI:
-
Acute kidney injury
- COVID-19:
-
Coronavirus disease 2019
- ICU:
-
Intensive care unit
- KDIGO:
-
Kidney disease improving global outcome
- PEEP:
-
Positive end expiratory pressure
- SARS-COV-2:
-
Severe acute respiratory coronavirus 2
References
COVID-19 Map. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html.
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020. https://www-nejm-org.proxy.insermbiblio.inist.fr/doi/10.1056/NEJMoa2002032.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497–506.
Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339.
Koeze J, Keus F, Dieperink W, van der Horst ICC, Zijlstra JG, van Meurs M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017;18(1):70.
Wiersema R, Jukarainen S, Eck RJ, Kaufmann T, Koeze J, Keus F, et al. Different applications of the KDIGO criteria for AKI lead to different incidences in critically ill patients: a post hoc analysis from the prospective observational SICS-II study. Crit Care Lond Engl. 2020;24(1):164.
Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31:1380. https://jasn-asnjournals-org.proxy.insermbiblio.inist.fr/content/early/2020/05/04/ASN.2020040419
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan China. JAMA Intern Med. 2020;18:1.
Colafrancesco S, Alessandri C, Conti F, Priori R. COVID-19 gone bad: a new character in the spectrum of the hyperferritinemic syndrome? Autoimmun Rev. 2020;5:102573.
Noris M, Benigni A, Remuzzi G. The case of Complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;98:314.
Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci. 2020;117(20):10970–5.
Risitano AM, Mastellos DC, Huber-Lang M, Yancopoulou D, Garlanda C, Ciceri F, et al. Complement as a target in COVID-19? Nat Rev Immunol. 2020;20(6):343–4.
Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
Vincent J-L, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: on behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine (see contributors to the project in the appendix). Intensive Care Med. 1996;22(7):707–10.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061.
Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343–8.
Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;1(127):104364.
Cao J, Hu X, Cheng W, Yu L, Tu W-J, Liu Q. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020;46(5):851–3.
Xu S, Fu L, Fei J, Xiang H-X, Xiang Y, Tan Z-X, et al. Acute kidney injury at early stage as a negative prognostic indicator of patients with COVID-19: a hospital-based retrospective analysis. medRxiv. 2020. https://doi.org/10.1101/2020.03.24.20042408.
Luo X, Xia H, Yang W, Wang B, Guo T, Xiong J, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020. https://doi.org/10.1101/2020.03.19.20033175.
Hu L, Chen S, Fu Y, Gao Z, Long H, Wang J-M, et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa539.
Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209. https://www.kidney-international.org/article/S0085–2538(20)30532–9/abstract
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl. 2020;395(10229):1033–4.
Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370.
Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature [Internet]. 2020 May 20 [cited 2020 Jun 6]; Available from: https://www.nature.com/articles/s41586–020–2355–0
Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47(4):693–705.
Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–10.
McCullough JW, Renner B, Thurman JM. The role of the complement system in acute kidney injury. Semin Nephrol. 2013;33(6):543–56.
Gao T, Hu M, Zhang X, Li H, Zhu L, Liu H, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. Infect Dis. 2020. https://doi.org/10.1101/2020.03.29.20041962.
Diao B, Feng Z, Wang C, Wang H, Liu L, Wang C, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020. https://doi.org/10.1101/2020.03.04.20031120.
Mortus JR, Manek SE, Brubaker LS, Loor M, Cruz MA, Trautner BW, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open. 2020;3(6):e2011192–e20111922011192.
Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219. https://linkinghub.elsevier.com/retrieve/pii/S0085253820303690
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089.
Beloncle FM, Pavlovsky B, Desprez C, Fage N, Olivier P-Y, Asfar P, et al. Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care. 2020;10(1):55.
Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099.
Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med. 2016;194(4):402–14.
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–38.
Author information
Authors and Affiliations
Contributions
MD, LZ and EA contributed to the study conception and design. AJ, MD, AM and LZ performed the data collection and the initial data analysis. AJ, LZ and MD prepared the first draft of the manuscript. All authors contributed to the data analysis and to the critical revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and competing interests
MD declares having received grant from MSD, speaker fees from MSD, Astelas and Gilead-Kite and having attended an advisory board for Gilead-Kite. LZ declares having received research grant from Jazz Pharmaceuticals. The other authors have no conflict of interest to declare.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Boxplots depicting relationship between AKI stage 2 and 3 and C3 [ng/mL] (A), IL-6 [ng/mL] (B), sC5b9 [ng/mL] (C) and ferritin levels [mg/L] (D) levels, and predicted probabilities of severe AKI according to ferritin (E) and C3 (F) deciles (per log). Figure S2. Kaplan-Meier curve for day-28 survival in patients without AKI or with AKI stage 1 (n=63) compared to AKI stage 2 or 3 (n= 37). Figure S3. Kaplan-Meier curves for day-28 survival according to AKI (A and B) and AKI stages (C and D) in patients with (n=33, B and D) and without (n= 67, A and C) baseline creatinine value. Table S1. Factors associated with risk of AKI stage 2 and 3, compared to no AKI or AKI stage 1, after adjustment for modified SOFA and chronic kidney disease (logistic regression). Table S2. Interaction between missing baseline serum creatinine and reported results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joseph, A., Zafrani, L., Mabrouki, A. et al. Acute kidney injury in patients with SARS-CoV-2 infection. Ann. Intensive Care 10, 117 (2020). https://doi.org/10.1186/s13613-020-00734-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-020-00734-z