Abstract
Background
There have been few studies that have evaluated the quality of end-of-life care (EOLC) for cancer patients in the ICU. The aim of this study was to explore the quality of transition to EOLC for cancer patients in ICU.
Methods
The study was undertaken on medical patients admitted to a specialist cancer hospital ICU over 6 months. Quantitative and qualitative methods were used to explore quality of transition to EOLC using documentary evidence. Clinical parameters on ICU admission were reviewed to determine if they could be used to identify patients who were likely to transition to EOLC during their ICU stay.
Results
Of 85 patients, 44.7% transitioned to EOLC during their ICU stay. Qualitative and quantitative analysis of the patients’ records demonstrated that there was collaborative decision-making between teams, patients and families during transition to EOLC. However, 51.4 and 40.5% of patients were too unwell to discuss transition to EOLC and DNACPR respectively. In the EOLC cohort, 76.3% died in ICU, but preferred place of death known in only 10%. Age, APACHE II score, and organ support, but not cancer diagnosis, were identified as associated with transition to EOLC (p = 0.017, p < 0.0001 and p = 0.001).
Conclusions
Advanced EOLC planning in patients with progressive disease prior to acute deterioration is warranted to enable patients’ wishes to be fulfilled and ceiling of treatments agreed. Better documentation and development of validated tools to measure the quality EOLC transition on the ICU are needed.
Similar content being viewed by others
Background
Outcomes for critically ill cancer patients have improved over the last decade, and intensivists are increasingly willing to initiate a ‘trial of intensive care unit (ICU) therapy’ in patients with advanced cancer [1, 2]. The aim of a trial of intensive care therapy is to support patients’ recovery, treat reversible causes of illness and help patients survive immediate threats to their lives. It is estimated that 18–30% of cancer patients use of intensive care services [3, 4]. Advances in cancer diagnosis, treatment and general ICU care have led to better outcomes in patients with cancer admitted to the ICU [1, 2]. However, morbidity and mortality still remains high, with cancer patients admitted to ICU having a mortality of 27–43% [5–7]. Recent studies have shown similar ICU mortality rates for patients with solid tumours and haematological malignancies [2, 8, 9].
The transition from intensive care with the goal to save or prolong life to end-of-life care (EOLC) can often be a difficult decision to make for clinicians, patients and families [10]. Clinical parameters to help clinicians identify patients who are likely to transition to EOLC during their ICU stay are often not easily defined [9, 11]. The ability to identify clinical parameters early in their ICU admission that could help predict likelihood of transition to EOLC could improve the quality of care for cancer patients.
Previous studies have demonstrated that care pathways and communication during transition to EOLC for critically ill patients and their families in the ICU setting is not always optimal [12–15]. Problems reported have been poor communication; discordance about treatment plans and goals; high prevalence of pain and distressing symptoms [16–19]. Healthcare providers also highlight a lack of knowledge and training in EOLC [20]. Furthermore, it is unclear which, if any, palliative measures are effective, or increase satisfaction, in the ICU [21]. Clarke et al. and a subsequent consensus statement from the American College of Critical Care Medicine recommended seven quality indicators (Table 1) for improving the EOLC of ICU patients during the dying process [15, 22].
The primary aim of this study was to explore the quality of transition to EOLC for medical patients with cancer who died, or transitioned to end-of-life on the ICU. Medical patients only were sampled, as the predicted and actual mortality for surgical patients in the unit was historically very low, and few transitioned to end-of-life [2]. The literature attests to the high mortality in medical ICU cancer patients, and low surgical mortality [24, 25]. The secondary aim was to identify parameters on ICU admission that could help determine patients who were likely to transition to EOLC during their ICU stay.
Methods
Setting
This study was approved by the local Committee for Clinical Research. The study took place on the ICU in a 269-bedded specialist adult cancer hospital located across two sites in London. The hospital serves the local population and is also a tertiary referral centre for patients with cancer from across the United Kingdom and abroad. The 16-bedded mixed medical and surgical ICU admits approximately 1,400 patients per year and has capacity for both level 2 (single organ support, or extensively post-operative care) and 3 (two or more organ support, or advanced respiratory support) care [26]. All referrals and admissions to ICU are discussed with the ICU consultant. No formal EOLC protocol was in place in the ICU during the study period following the abolition of the Liverpool Care Pathway [27]. However, several of the recommendations for improving communication at the end-of-life by the American College of Critical Care Medicine had been adopted in the unit [15]. These included formal communication skills training of ICU and Palliative Care Consultants, interdisciplinary team rounds, palliative care team input at weekly ICU multidisciplinary team meetings and regular family meetings.
All medical patients with cancer admitted to the ICU over a 6-month period from 01.03.2013 to 01.08.2013 were included in this study. Patients <16 years, without a cancer diagnosis, or who had undergone elective or emergency surgery were excluded as the focus on this study was primarily on medical cancer patients. For those patients who had more than one admission during the study period, only the patient’s last admission was included in the analysis. Sepsis was defined according to the Surviving Sepsis Guidelines [28].
Study design
A retrospective review of patient records was carried out to explore patients’ demographics and co-morbidities; cancer type and stage; reason for hospital and ICU admission; documented communication between teams, patients and relatives (detailed from care records, team meetings and daily ward round annotations in the electronic patient records); documented palliative care team involvement; documented evidence of resuscitation and ceiling of care decisions; documented quality of EOLC provision, and ICU mortality.
To determine the care process and quality of EOLC transitions on the ICU, quality indicators recommended by American College of Critical Care Medicine were used in the qualitative documentary analysis (Table 1) [15]. The quantitative variables were categorised into process and outcome domains. The first five points (Domains 1–5) were used in the analysis on processes of EOL transition and provision of EOLC in our ICU. Communication not only included documented presence of patient, family and team members, but also in some cases, a qualitative analysis of the documented discussion. At the time of this study, standardised symptom assessment tools were not routinely used or integrated into the electronic documentation. Symptom control (Domain 5) was defined, for simplicity, as documented evidence of pharmacological and non-pharmacological control of symptoms such as pain, nausea, vomiting, diarrhoea, and anxiety. Assessments were made prior and after intervention to determine if patients’ had achieved adequate symptom control. Domains 6 and 7 were excluded as they relate to organisational processes that could not be determined through patient records review. Palliative or non-curative treatment intent was defined as patients with no further active treatment options available, but those that could be on phase 1 trials or be receiving treatment to control or maintain disease stability but without curative intent. In contrast, curative intent was defined as patients who were receiving active treatment for their cancer with an intention of cure [29].
Patient records were examined using the Electronic Patient Record (EPR), IntelliView Clinical Information Portfolio (ICIP), chaplaincy database, and written notes. All data was double checked for accuracy by two investigators. All data were entered onto an excel database and collated into 46 variables under the aforementioned process and outcome categories. These variables were then categorised and tabulated into the five domains (Domains 1–5). Data were pseudo-anonymised, and dealt with Good Clinical Practice guidelines and United Kingdom Data Protection Act.
Identification of cohorts: ‘EOLC’ vs ‘full active ICU care’
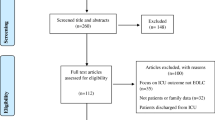
Following data collection, patients were divided into two cohorts: patients who received full active management with organ support throughout their ICU stay and whose main focus of care remained prolongation of survival with curative intent (‘full active ICU care’ cohort); and those patients who had transitioned from full active management to EOLC during their ICU stay or died on the ICU (‘EOLC’ cohort) (Fig. 1). The transition to EOLC was ascertained on an individual patient basis and decisions were made when the teams (parent medical team, ICU team and sometimes the palliative care team) jointly concluded that continuation of life-prolonging organ support would be futile and not in the patients’ best interests. Families (and patients, if they had capacity) were involved in these discussions. It is recognised however, that some of EOLC cohort may have received life-prolonging organ support during the early part of their ICU stay before transitioning to EOL care. Equally, symptom control was given in the full active care cohort where needed.
Data analysis
Quantitative data were analysed using SPSS statistics software (version 20, Chicago, IL, USA). Comparison was made between the two cohorts using standard descriptive statistics. For categorical variables such as organ support required in ICU and performance status, Fisher’s exact test and Chi squared tests were performed to compare data between the two groups. Continuous data was analysed using the Mann–Whitney U (for non-parametric data including age) and student t test for parametric data. A statistical significance p < 0.05 was used when comparing the two cohorts.
Multiple logistic regression was used to investigate the joint effect of clinical variables on the whether or not patients transitioned to the EOLC cohort or not. Factors with p < 0.1 on univariate analysis were included in the multivariate modelling. Variables with p > 0.1 were excluded in order to reduce the number of predictor variables. Variable selection was carried out using a stepwise method, with p < 0.05 and p > 0.1 as criteria for entry and removal, respectively. Only factors with p < 0.05 were retained in the final model.
Data from the EOLC cohort were further analysed using descriptive statistics to explore the quality of EOLC being delivered on the ICU.
Text data from all medical records (ICIP and hospital EPR) was analysed using qualitative thematic analysis. Undertaking textual analysis enriched the quantitative data yielded [30, 31]. A mixed analysis allowed examination and exploration of unusual quantitative findings [32]. Thematic analysis provided a structure for identifying patterns in data and involved steps of data codification; testing and connecting codes, searching and establishing themes; reviewing and corroborating themes.
Results
There were 3,203 hospital in-patient admissions during the study period, and of these 669 were admitted it the ICU (20.9%). Of the ICU admissions, 85 were medical patients (12.2%), all of whom were included in the analysis.
Quantitative analysis comparing ‘Full active ICU care’ with ‘EOLC’ cohorts
Of the 85 patients analysed, 38 patients (44.7%) met the criteria for ‘EOLC’ and 47 patients (55.3%) remained for ‘full active ICU care’. Patient demographics are outlined in Table 2. Median age and APACHE II score was higher in the EOLC group compared to the full active ICU care group (66.5 vs. 59 years, p = 0.017) and (21 vs. 16, p < 0.0001), respectively. The EOLC cohort required a higher level of organ support (intubation 44.7 vs. 12.8%, p = 0.001; vasopressors 55.3 vs. 27.7%, p = 0.014 and RRT 28.9 vs. 8.5%, p = 0.021). There was no statistical difference in cancer type, disease stage or co-morbidities. On multivariate analysis, APACHE II score, the need for intubation and non-invasive ventilation remained significantly associated with a poor prognosis (Table 3).
Overall ICU mortality was 34% for all medical patients admitted to the ICU and 3.7% (118/3,203 patients) for all hospital in-patients during the study period.
Quantitative analysis of the quality of transition to EOL
Of the 38 patients who were included in the EOLC cohort, only one patient was admitted for symptom control alone. All other patients were initially admitted for full active ICU management.
Most patients in the EOLC cohort (37/38 patients; 97.4%) had clear documented evidence of transition from full active care to EOLC.
ICU and in-hospital mortality
Most patients (29/38 patients; 76.3%) in the EOLC cohort died on the ICU. Of the remaining 9 patients, 7 died on the ward; one died in hospice and one was transferred to their home country whilst remaining intubated. In-hospital mortality for the EOLC cohort was (36/38 patients; 94.7%).
Resuscitation decisions
On admission to ICU most patients in the EOLC cohort (34/38 patients; 89.5%) were for full resuscitation. In the EOLC cohort ‘do not attempt cardiopulmonary resuscitation’ (DNACPR) orders were completed for 47% patients within 48 h of ICU admission. Most patients (97.4%) in the EOLC cohort had documented evidence of DNACPR at some point during their ICU stay. Sixty-nine percent of patients died within 3 days of DNACPR decision. The time from DNACPR decision to death was a median of 1.5 days (range 0–93 days). In contrast, amongst the ‘full active ICU care’ cohort, just 2/45 patients (4.4%), had a DNACPR order documented during their ICU stay.
Documented quality markers for transition to EOLC
The quality of EOLC delivered during transition to end-of-life care was assessed using the domains adapted from the American College of Critical Care Medicine Consensus statement, is shown in Table 4 [15, 22, 23].
Qualitative thematic analysis
Data were analysed qualitatively for all patients under a priori framework analysis categories of prognostication, decision-making and EOLC based on the quantitative findings. These were modified according to the 19 sub-themes (Fig. 2) and resulted in five main themes (Table 5).
When initiating end-of-life transitions, where patients had capacity, decisions were made collaboratively with families and teams. When supportive care was deemed appropriate, rather than focusing on active treatment, palliative care input was quickly sought. The excerpt (1.1) exemplifies consensus building as well as highlighting the consensus between teams in the next theme.
Parent medical and critical care team decision-making referred to how critical care teams sought early joint decision-making with parent medical teams when there was doubt over the possibility of critical illness recovery (theme 3). This planning meant that some patients were transferred out of the unit and received EOLC on the ward.
Poor critical illness related outcomes and cancer prognosis made it straightforward to reach consensus and rationalise decisions. Prognosis was communicated where possible (2.1) and where there was uncertainty regarding outcome from the critical illness perspective, it was more likely that patients died with full intensive care treatments in situ (2.2, 2.3).
Furthermore, teams negotiated to try critical care treatment for a period of time, allowing opportunity for anti-cancer treatments to work, and allowing for a trial of ICU to reverse acute problems. If families were reluctant for EOLC transitions and wished for full active treatment to continue, the intensivists would often continue with the caveat that if there was no improvement in a specified time, they would initiate a transition to end-of-life (2.1).
If families (and patients, when able) were receptive to EOLC transition, then the critical care team acted swiftly to focus on EOLC, either placing a ceiling of care (limitation of treatment) (5.2), or withdrawing treatment (5.1, 5.3).
Integrative palliative care referred to how palliative care input was sought on the majority of patients who actually died in critical care, despite the short time to death from withdrawal or limitation of treatment (theme 4). This facilitated the focus on comfort measures, and away from high-intensity treatment at end-of-life, but depended on timely referral. Some intensivists advocated an integrative approach where palliative care became involved early, to assist in advanced care planning.
Discussion
In this study we found that APACHE II scores, age and levels organ support were found to be associated with patients who were likely to transition from full ICU active care to EOLC. These parameters have also been found to correlate with poor outcomes in other studies [2, 8, 33]. Cancer stage or diagnosis was not associated with transition to EOLC in keeping with findings from other studies [2, 9]. On multivariate analysis, APACHE II score, the need for intubation and non-invasive ventilation were significantly associated with transition to EOLC.
Our results indicate that teams and relatives communicated frequently during the period of transition to EOLC. These findings were supported by the qualitative analysis, which illustrated that families understood the appropriateness of transitions to EOLC, and were keen to avoid suffering when reversal of the underlying condition was not possible. Despite some qualitative documented cases of good communication with families, patients were often too unwell to engage in conversations around transition to EOLC and/or DNACPR once in ICU. Even in this group of patients with known cancer, few patients in the EOLC cohort had been asked and had documented evidence of a preferred place of death. Despite the recent initiatives and published reports that patients want to be involved in decision-making, use of advanced care planning and discussions about EOLC with patients and their families remains infrequent [34–36]. Research indicates that most people want to die at home [37, 38]. However, the majority of our patients in the EOLC cohort died in hospital, with over three-quarters of patients in the EOLC cohort dying in the ICU. For patients with advanced disease it is important that discussions and documentation about ceilings of treatment, prognosis and patient wishes, are done early in the disease course when patients are still well enough to engage, rather than in the ICU. Even in our institution, where the palliative care team forms an integral part of the service this issue remains challenging. In this study the number of patients in the EOLC cohort that we able to participate in discussions about their cancer prognosis (43.2%), DNACPR decision (40.5%) and preferred place of death (10.5%) during their ICU stay was low. Whilst this may just represent of the number of patients who presented with acute, unforeseen deterioration in their clinical condition unrelated to their cancer progression, it highlights the need for early palliative care involvement and advanced EOLC planning. There may be frequent opportunities for discussion during outpatient visits and in-patient admissions. A recent study indicated that patients having palliative chemotherapy towards the end-of-life are more likely to die in ICU [39]. When the focus of care is on survival, health professionals are often reluctant to discuss prognosis or plans in the event of deterioration as it may be considered unnecessarily distressing to patients [40]. Yet, for some patients, earlier discussion of prognosis, treatment options and EOLC wishes may mean they are better informed to make decisions about their care. Also, Rees et al. [41] did not find that these discussions caused distress for most of their patients.
Often the decision to make a patient DNACPR or to transition to EOLC was made within 48 h of ICU admission, following a ‘trial of ICU’. Tools have been developed for use in patients admitted to ICU to improve communication and palliative care [42]. These tools require further validation and review but may be used to support medical decision-making and prompt early discussions between clinicians, patients and their families about patients’ wishes, potential therapeutic options and anticipated outcomes.
Few patients (10%) who transitioned to EOLC during their ICU stay were already known to the palliative care team prior to ICU admission. This may be due to the fact that many patients were treated with curative intent, and had not required palliative care intervention prior to their critical illness. Our qualitative data suggests that intensivists had the skills to manage symptoms and support families. Even when the patient was admitted to the ICU, the palliative care team were only involved in 53% of patients in the EOLC cohort. Early palliative care involvement has been associated with a lower proportion of deaths occurring in the ICU [43].
Based on clinical documentation, spiritual, psychological and welfare support was offered to patients/families at end-of-life in only a third of cases. It may have been that support was offered and declined, that the ICU team was already giving adequate support to patients and their families, or that these needs were not consistently considered. Family satisfaction with EOLC decision-making has been closely associated with documented discussion of patient wishes and spiritual needs [44]. In the United Kingdom, one of the useful aspects of the Liverpool Care Pathway (LCP) was that it acted as a prompt for staff to consider emotional, spiritual and psychological support at the end-of-life [27, 45]. In the post-Liverpool Care Pathway era, we need to ensure these vital quality indicators of good EOLC are considered, offered if appropriate and clearly documented. There are local and national initiatives underway to develop such tools [46].
One of the limitations of this study was that the data were collected retrospectively therefore the results not only reflect the processes of care, but also were reliant on the quality of documentation. For example, only specific aspects of the documented content of communication were evaluated and the quality of communication could not always be ascertained from the documented data. Also, the binary assessment of some of the quality markers may have been, in some circumstances, insufficient to assess quality of the care process provided but in some cases could be further explored in the qualitative analysis. It could be also argued that the findings are limited to the results of a single, tertiary centre; however, the authors believe that the findings may have wider applicability, as the themes identified are common to findings from other studies which have included general ICUs [14, 15]. The small sample size and possible existence of unmeasured confounding factors may have impacted on some of the conclusions drawn. Finally, only a limited assessment of end-of-life symptoms, and subsequent management, was undertaken; given the retrospective nature of the study. The use of defined assessment or outcome scales may have provided more robust information about the quality symptom management.
Conclusions
Larger studies are required to identify and validate clinical factors that may help identify patients with cancer who are likely to transition to EOLC during their ICU stay. Although qualitative and quantitative analysis of the records demonstrated that there was communication and collaborative decision-making between medical teams, patients and families during transition to EOLC, 40% of patients were too unwell to engage in conversations about preferences for care. This highlights the need for early advanced care planning, ideally prior to ICU admission. Our findings, although arguably those from a single centre, do have widely applicability, and demonstrate that there is a clear need for the development and validation of tools to support high-quality patient-centred EOLC in a critical care setting, across all domains.
References
Azoulay E, Soares M, Darmon M, Benoit D, Pastores S, Afessa B (2011) Intensive care of the cancer patient: recent achievements and remaining challenges. Ann Intensive Care. 1(1):5. doi:10.1186/2110-5820-1-5
Bird GT, Farquhar-Smith P, Wigmore T, Potter M, Gruber PC (2012) Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: a 5 yr study. Br J Anaesth 108(3):452–459. doi:10.1093/bja/aer449
Soares M, Caruso P, Silva E, Teles JM, Lobo SM, Friedman G et al (2010) Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med 38(1):9–15. doi:10.1097/CCM.0b013e3181c0349e
De Jonge E, Bos MM (2009) Patients with cancer on the ICU: the times they are changing. Crit Care 13(2):122. doi:10.1186/cc7721
Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E (2007) The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med 35(3):808–814. doi:10.1097/01.CCM.0000256846.27192.7A
Hampshire PA, Welch CA, McCrossan LA, Francis K, Harrison DA (2009) Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care 13(4):R137. doi:10.1186/cc8016
McGrath S, Chatterjee F, Whiteley C, Ostermann M (2010) ICU and 6-month outcome of oncology patients in the intensive care unit. QJM 103(6):397–403. doi:10.1093/qjmed/hcq032
Aygencel G, Turkoglu M, Turkoz Sucak G, Benekli M (2014) Prognostic factors in critically ill cancer patients admitted to the intensive care unit. J Crit Care 29(4):618–626. doi:10.1016/j.jcrc.2014.01.014
Massion PB, Dive AM, Doyen C, Bulpa P, Jamart J, Bosly A et al (2002) Prognosis of hematologic malignancies does not predict intensive care unit mortality. Crit Care Med 30(10):2260–2270. doi:10.1097/01.CCM.0000030456.11264.EF
Truog RD, White DB (2013) Futile treatments in intensive care units. JAMA Intern Med 173(20):1894–1895. doi:10.1001/jamainternmed.2013.7098
Staudinger T, Stoiser B, Müllner M, Locker GJ, Laczika K, Knapp S et al (2000) Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med 28(5):1322–1328
Nelson JE, Angus DC, Weissfeld LA, Puntillo KA, Danis M, Deal D et al (2006) End-of-life care for the critically ill: a national intensive care unit survey. Crit Care Med 34(10):2547–2553. doi:10.1097/01.CCM.0000239233.63425.1D
Desbiens NA, Wu AW, Broste SK, Wenger NS, Connors AF Jr, Lynn J et al (1996) Pain and satisfaction with pain control in seriously ill hospitalized adults: findings from the SUPPORT research investigations. For the SUPPORT investigators. Study to understand prognoses and preferences for outcomes and risks of treatment. Crit Care Med 24(12):1953–1961
Azoulay E, Timsit JF, Sprung CL, Soares M, Rusinová K, Lafabrie A et al (2009) Prevalence and factors of intensive care unit conflicts: the conflicus study. Am J Respir Crit Care Med 180(9):853–860. doi:10.1164/rccm.200810-1614OC
Truog RD, Campbell ML, Curtis JR, Haas CE, Luce JM, Rubenfeld GD et al (2008) Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med 36(3):953–963. doi:10.1097/CCM.0B013E3181659096
Azoulay E, Chevret S, Leleu G, Pochard F, Barboteu M, Adrie C et al (2000) Half the families of intensive care unit patients experience inadequate communication with physicians. Crit Care Med 28(8):3044–3049
Breen CM, Abernethy AP, Abbott KH, Tulsky JA (2001) Conflict associated with decisions to limit life-sustaining treatment in intensive care units. J Gen Intern Med 16(5):283–289
Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL et al (2001) Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med 29(2):277–282
Visser M, Deliens L, Houttekier D (2014) Physician-related barriers to communication and patient- and family-centred decision-making towards the end of life in intensive care: a systematic review. Crit Care 18(6):604. doi:10.1186/s13054-014-0604-z
Vincent JL (1990) European attitudes towards ethical problems in intensive care medicine: results of an ethical questionnaire. Intensive Care Med 16(4):256–264. http://www.ncbi.nlm.nih.gov/pubmed/2358559. Accessed June 16 2015
Aslakson R, Cheng J, Vollenweider D, Galusca D, Smith TJ, Pronovost PJ (2014) Evidence-based palliative care in the intensive care unit: a systematic review of interventions. J Palliat Med 17(2):219–235. doi:10.1089/jpm.2013.0409
Clarke EB, Curtis JR, Luce JM, Levy M, Danis M, Nelson J et al (2003) Quality indicators for end-of-life care in the intensive care unit. Crit Care Med 31(9):2255–2262. doi:10.1097/01.CCM.0000084849.96385.85
Mularski RA, Curtis JR, Billings JA, Burt R, Byock I, Fuhrman C et al (2006) Proposed quality measures for palliative care in the critically ill: a consensus from the Robert Wood Johnson Foundation Critical Care Workgroup. Crit Care Med 34(11 Suppl):S404–S411. doi:10.1097/01.CCM.0000242910.00801.53
Bos MMEM, Bakhshi-Raiez F, Dekker JWT, de Keizer NF, de Jonge E (2013) Outcomes of intensive care unit admissions after elective cancer surgery. Eur J Surg Oncol 39(6):584–592. doi:10.1016/j.ejso.2013.02.014
Bos MMEM, de Keizer NF, Meynaar IA, Bakhshi-Raiez F, de Jonge E (2012) Outcomes of cancer patients after unplanned admission to general intensive care units. Acta Oncol 51(7):897–905. doi:10.3109/0284186X.2012.679311
Intensive Care Society (2009) Levels of critical care for adult patients. http://www.ics.ac.uk/ics-homepage/guidelines-and-standards/. Accessed 10 May 2015
Neuberger J (2013) More care less pathway: a review of the Liverpool Care Pathway. https://www.gov.uk/government/…data/…/Liverpool_Care_Pathway.pdf. Accessed 10 May 2015
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39(2):165–228. doi:10.1007/s00134-012-2769-8
Pattison N, O’Gara G, Wigmore T (2015) Negotiating transitions: involvement of critical care outreach teams in end-of-life decision making. Am J Crit Care 24(3):232–240. doi:10.4037/ajcc2015715
Creswell JW, Fetters MD, Ivankova NV (2004) Designing a mixed methods study in primary care. Ann Fam Med. 2(1):7–12. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1466635&tool=pmcentrez&rendertype=abstract. Accessed May 18 2015
Bradley EH, Curry LA, Devers KJ (2007) Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 42(4):1758–1772. doi:10.1111/j.1475-6773.2006.00684.x
Klassen AC, Creswell J, Plano Clark VL, Smith KC, Meissner HI (2012) Best practices in mixed methods for quality of life research. Qual Life Res 21(3):377–380. doi:10.1007/s11136-012-0122-x
Azevedo LCP, Caruso P, Silva UVA et al (2014) Outcomes for patients with cancer admitted to the ICU requiring ventilatory support: results from a prospective multicenter study. Chest 146(2):257–266. doi:10.1378/chest.13-1870
Smith SW (2013) Aintree University Hospital NHS Foundation Trust v James, [2013] EWCA CIV 65. Med Law Rev 21(4):622–631. doi:10.1093/medlaw/fwt028
Coulter A (2010) Do patients want a choice and does it work? BMJ 341:c4989. http://www.ncbi.nlm.nih.gov/pubmed/20947576. Accessed June 16 2015
Liberating the NHS (2014) No decision without me, about me. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216980/Liberating-the-NHS-No-decision-about-me-without-me-Government-response.pdf. Accessed May 15 2014
Higginson IJ, Sen-Gupta GJ (2000) Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med. 3(3):287–300. doi:10.1089/jpm.2000.3.287
Gomes B, Calanzani N, Gysels M, Hall S, Higginson IJ (2013) Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care 12:7. doi:10.1186/1472-684X-12-7
Wright AA, Zhang B, Keating NL, Weeks JC, Prigerson HG (2010) Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: prospective cohort study. BMJ 348:g1219. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3942564&tool=pmcentrez&rendertype=abstract. Accessed June 16 2015
Mack JW, Smith TJ (2012) Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol 30(22):2715–2717. doi:10.1200/JCO.2012.42.4564
Rees E, Hardy J (2003) Novel consent process for research in dying patients unable to give consent. BMJ 327(7408):198. doi:10.1136/bmj.327.7408.198
Higginson IJ, Koffman J, Hopkins P, Prentice W, Burman R, Leonard S et al (2013) Development and evaluation of the feasibility and effects on staff, patients, and families of a new tool, the Psychosocial Assessment and Communication Evaluation (PACE), to improve communication and palliative care in intensive care and during clinical uncertainty. BMC Med 11:213. doi:10.1186/1741-7015-11-213
Alsirafy SA, Mohammed AA, Al-Zahrani AS, Raheem AA, El-Kashif AT (2014) The relation between the timing of palliative care and the frequency and timing of do-not-resuscitate orders among cancer deaths in a Tertiary Care Hospital. Am J Hosp Palliat Care. doi:10.1177/1049909114529014
Gries CJ, Curtis JR, Wall RJ, Engelberg RA (2008) Family member satisfaction with end-of-life decision making in the ICU. Chest 133(3):704–712. doi:10.1378/chest.07-1773
McCrossan L, Harper J (2013) Correspondence regarding: the future of the Liverpool care pathway in caring for the dying in intensive care. J Intensive Care Soc. 14(3):274–275
London cancer alliance principles of caring for dying patients (2014). http://www.londoncanceralliance.nhs.uk/news. Accessed May 15 2014
Authors’ contributions
All authors have contributed to conception and design of the study, acquisition of data, analysis and interpretation of data, drafting of the manuscript, revising it critically for important intellectual content. All authors read and approved the final manuscript.
Acknowledgements
Kabir Mohammad, Trust Statistician at The Royal Marsden Hospital.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sophie J Miller and Nishita Desai contributed equally to this paper
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Miller, S.J., Desai, N., Pattison, N. et al. Quality of transition to end-of-life care for cancer patients in the intensive care unit. Ann. Intensive Care 5, 18 (2015). https://doi.org/10.1186/s13613-015-0059-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-015-0059-7