Abstract
Autophagy is a cell self-digestion pathway through lysosome and plays a critical role in maintaining cellular homeostasis and cytoprotection. Characterization of autophagy related genes in cell and animal models reveals diverse physiological functions of autophagy in various cell types and tissues. In central nervous system, by recycling injured organelles and misfolded protein complexes or aggregates, autophagy is integrated into synaptic functions of neurons and subjected to distinct regulation in presynaptic and postsynaptic neuronal compartments. A plethora of studies have shown the neuroprotective function of autophagy in major neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS). Recent human genetic and genomic evidence has demonstrated an emerging, significant role of autophagy in human brain development and prevention of spectrum of neurodevelopmental disorders. Here we will review the evidence demonstrating the causal link of autophagy deficiency to congenital brain diseases, the mechanism whereby autophagy functions in neurodevelopment, and therapeutic potential of autophagy.
Similar content being viewed by others
Introduction
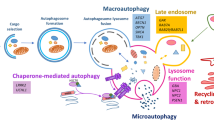
Macroautophagy (hereafter referred to as autophagy) is a fundamental degradation pathway by which cellular components are degraded and recycled through the lysosome. The post-mitotic, long-living neurons of the brain rely on autophagy in removing dysfunctional protein aggregates and organelles to maintain neuronal homeostasis. In neurons, autophagy deficiency causes accumulation of ubiquitinated proteins, axon dystrophy, abnormal synaptic transmission, and subsequent neurodegeneration. Aberrant autophagic activity is associated with various human central nervous system (CNS) diseases including neurodegenerative and neurodevelopmental disorders [1, 2].
To date, a highly conserved set of autophagy related genes (ATGs) have been characterized along with their roles in the autophagy process [3]. Autophagy begins with the formation of UNC-51-like kinase (ULK1) complex which consists of ULK1, ATG13L, FIP200 and ATG101 [4]. In response to nutrition status of cells, the ULK1 complex can be phosphorylated by mammalian target of AMP-activated protein kinase (AMPK) or rapamycin complex 1 (mTORC1) to initiate or terminate autophagy, respectively [5]. In nutrient depletion condition, ULK1 is released from mTORC1 inhibition, and subsequently phosphorylates the components of Beclin1-VPS34 complex to regulate VPS34 kinase activity, which is required for phagophore nucleation [6, 7]. The expansion of phagophore depends on two ubiquitin-like conjugation systems which are mediated by ATG7 [8]. As an E1 like enzyme, ATG7 can conjugate ATG5 and ATG12 to form ATG5-12 complex which further binds to ATG16L1. With the assist of ATG16L1/ATG5-12 complex, ATG7 can also promote the conjugation of protein LC3-I to phosphoethanolamine to generate LC3-II, which can bind autophagosomal membranes and aid in cargo recruitment [9, 10]. Eventually, the autophagosome fuses with a lysosome to form an autolysosome, where the degradation of the cytoplasmic material and organelles happens in the presence of the lysosomal hydrolytic enzymes.
Growing works have demonstrates a link of aberrant signaling pathways including autophagy to neurodevelopmental disorders. Neurodevelopmental disorders are a multifaceted group of mental diseases, which are characterized by cognitive deficits and behavioral impairment. The most prevalent developmental disorders are autism spectrum disorders (ASDs) which are diagnosed primarily by deficits in social communication and interaction, as well as restricted and repetitive behaviors [11]. ASDs are often accompanied by other comorbidities including intellectual disability, motor deficits, and sensory processing abnormalities [11]. Numerous studies have revealed the etiology of ASDs which is attributed to genetic factors and non-genetic factors, such as environmental factors affecting the risk of ASDs development in a complementary manner [12, 13]. A subset of ASDs includes autism, tuberous sclerosis, fragile X syndrome, and others [14]. The causal link of autophagy impairment to neurodevelopmental disorders is highlighted by a recent report of deleterious, recessive variants of ATG7 in human [15]. Here, we summarize the evidence linking autophagy dysregulation to neurodevelopmental disorders and review the concise roles of autophagy in neural development and synaptic function.
Evidence for autophagy dysregulation in neurodevelopmental disorders
Genetic mutations of autophagy-related genes in neurodevelopmental disorders
ASD
ASD is caused by the combination of genetic and environmental factors. The heritability of ASD is estimated at 60–90% by meta-analyses [16]. Human genetics studies from large cohorts of ASD patients and controls have identified many rare genetic variants including copy number variants (CNVs) and single nucleotide variants (SNVs) associated with ASD [17, 18]. By applying eXome Hidden Markov Model (XHMM) to an ancestry-matched sample of ASD cases and controls, Buxbaum and his colleagues previously identified an increase in small CNV in ASD cases [19]. By performing enrichment and pathway analyses of the genes disrupted by deletions in ASD cases, they observed significant enrichment of five autophagy related genes including GABARAPL2, GABARAPL1, MAP1LC3A, GABARAP, and MAP1LC3B, which are mammalian orthologs to yeast autophagy gene Atg8 [19]. The study implicates dysregulation of autophagy in ASD. In support of the notion, a recent study has reported that aggregation of GABARAPs is increased in the postmortem brain of ASD patients and that depletion of autophagy in forebrain GABAergic interneurons in adolescent mice causes the formation of high-molecular weight species of GABARAPs [20]. These mice with autophagy deficiency display an overlapping set of ASD-like social behavioral impairment [20].
Genetic leukoencephalopathies
Genetic leukoencephalopathies are a set of heterogeneous disorders characterized by brain white matter defects in central nervous system (CNS), motor impairment, ataxia, and impaired cognitive development [21]. VPS11 protein, encoded by VPS11 gene, is a core component of CORVET (class C core vacuole/endosome tethering) protein complexes which is involved in membrane trafficking and lysosome-endosome fusion. By using whole exome sequencing, our recent study reported a homozygosity for a missense variant of VPS11 (C846G) in five individuals with leukoencephalopathy syndrome [14]. The study further indicates that C846G mutation in VPS11 displays a loss of function in autophagy pathway in human cells and that zebrafish carrying a VPS11 mutation shows a significant reduction in CNS myelination [14]. The study reveals a defect in VPS11-mediated autophagy-lysosome trafficking pathway as an underlying mechanism for some forms of leukoencephalopathy.
Childhood ataxia
Childhood ataxia is a rare disease leading to difficulties in coordination and movements, as well as cognitive problems and developmental delay in children [22]. A recent study has identified a homozygous missense mutation in ATG5, a core gene in autophagy, in two siblings with congenital ataxia [22]. The cells derived from the subjects display defects in autophagy activity [22]. The yeast harboring homozygous mutation of ATG5 show lower levels of autophagy compared to normal cells [22]. Further experiments demonstrates that introducing wild-type human ATG5, but not mutated form, into fruit flies lacking fly ATG5 can restore normal movement [22]. The findings implicate that the mutation in ATG5 is responsible for the symptoms of childhood ataxia.
Primary microcephaly
Primary microcephaly is a congenital neurodevelopmental disorder characterized by reduced head circumference and brain volume [23]. By using the whole exome sequencing analysis, a recent study has identified a dominant mutation in ALFY, encoding an autophagy scaffold protein, as a causative mutation to primary microcephaly [23]. The results show that transgenic flies with overexpression of the mutant human ALFY recapitulate the phenotype of microcephaly in human patients [23]. Further experiments demonstrate that wild-type ALFY, but not mutant human ALFY, controls the removal of DVL3 aggregates to regulate Wnt signaling [23]. Recently, Mason et al. have reported that eliminate the expression of ALFY in mice brain causes developmental axonal connectivity and impairs the formation of the major forebrain commissures [24]. Collectively, these findings suggest ALFY-mediated autophagy plays a critical role in the development of human brain and microcephaly.
Complex developmental disorders
WIPI2, the mammalian homologue of the yeast Atg18, is a key regulator of autophagy. WIPI2 interacts with ATG16L1 and recruits the ATG12-ATG5-ATG16L1 complex to the phagophore and therefore promotes LC3 lipidation and subsequent autophagosome formation [25]. By performing whole exome sequencing on affected individuals with a complex developmental disorders including mental retardation, speech and language impairment, as well as other neurological and psychiatric abnormalities, a recent study identified a novel nonsynonymous homozygous mutation (V249M) in WIPI2 gene [26]. The same study reported that V231M mutation on WIPI2b (corresponding to V249M in WIPI2a) significantly reduced its interaction with ATG16L1 and ATG5-12 complex [26]. Compared to the controls, the fibroblasts derived from the patients carrying the V249M mutation show reduced LC3 lipidation, which is correlated to the reduced WIPI2 puncta, and subsequent reduced level of autophagy flux [26]. The results imply that the impairment of autophasosome formation may cause the neurodevelopmental disorders. In line with this notion, by performing whole exome sequencing on a family in which one of four children displays severe cortical atrophy, intellectual impairment, ataxia, and other neurological symptoms, Keays et al. identified a single homozygous coding mutation (L1224R) in VPS15 in an affected case [27]. VPS15 is a key component in VPS15-VPS34-Beclin1 complex which plays a critical role in autophagosome formation. The experiments performed by Keays et al. demonstrate that compared to controls, the dermal fibroblasts derived from affected individual show reduced protein levels of VPS15, VPS34 and Beclin1, decreased LysoTracker staining, and increased protein level of p62, an autophagy cargo receptor [27]. Further study indicates that ectopic expression of wild-type VPS15 in L1224R patient cells increases the protein levels of VPS15, stabilizes VPS34 and Beclin1, and decreases the protein level of p62 [27]. These findings indicate that L1224R mutation in VPS15 is associated with human neurodevelopmental disorders through compromising the function of VPS15-VPS34-Beclin1 complex in autophagy.
ATG7 is an essential effector enzyme for canonical autophagy. Most recently, by performing genetic and clinical analysis, Taylor et al. identified recessive and loss-of-function mutations in both ATG7 alleles in 12 individuals from five unrelated families, which exhibit complex neurodevelopmental disorders including ataxia and developmental delay [15]. Experiments conducted in the fibroblasts and skeletal muscles derived from the patients indicate that the expression of ATG7 is diminished or absent in patients derived cells, resulting in impaired LC3 lipidation and autophagy flux [15]. The functional complementation experiments in mice and yeast confirmed the functional deficiencies induced by the missense variants in ATG7 [15]. Taken together, the study reveals the critical role of basal autophagy in human neural development and integrity.
Autophagy dysregulation in neurodevelopmental disorders
Growing evidence indicates the dysregulation of mTOR in ASD [28,29,30]. mTOR is a central regulator of diverse cellular processes including autophagy. mTOR is negatively regulated by tuberous sclerosis complex 1/2 (TSC1/2) [16, 31, 32]. Previous study reported that the TSC2 ± mice display constitutive hyperactivity of mTOR, blockade of autophagy, and consequent spine pruning defects [33]. Moreover, an mTOR inhibitor rapamycin can correct the spine pruning defects and ASD-relevant behaviors in TSC2 ± mice, but not in TSC2±:ATG7 cKO mice [33]. A most recent study has reported the similar results in parvalbumin (PV) cell-restricted TSC1 conditional haploinsufficient and knockout mice, which show transient autophagy dysfunctions, a loss of perisomatic innervation and social behavior deficits [34]. Moreover, treatment with rapamycin in a sensitive period rescues PV cell connectivity and social behavior in TSC1 conditional haploinsufficient mice [34]. Apart from TSC1/2 models of ASD, recent studies have reported the impaired expression of autophagy related protein Beclin1 in animal models of ASD including Cc2d1a ± and ADNP ± mice [35, 36]. These studies indicate that dysregulation of autophagy may contribute to neuronal pathology and aberrant social behaviors in ASD.
Fragile X syndrome (FXS), a leading genetic cause of autism, is a heritable form of intellectual disabilities including autistic behaviors, attentional deficits, emotional lability, impaired cognition and other neurological disabilities [37,38,39]. Fragile X mental retardation (Fmr1) is a causative gene to FXS [40]. Fragile X mental retardation protein (FMRP), encoded by Fmr1 gene, is an RNA-binding protein that tightly regulates the function of multiple neuronal mRNA critical to neuronal development and synaptic plasticity [41, 42]. Fmr1-KO mice is a well-characterized model of FXS [40]. Previous studies have reported the dysregulation of mTOR signaling in FXS mice and in humans with FXS [43, 44]. A recent study demonstrates that the biochemical markers of autophagy such as LC3II puncta, the active form of p-ULK1 and p-Beclin1, and consequent autophagy flux are significantly reduced, while p62 is accumulated, in the hippocampal neurons of Fmr1-KO mice, perhaps as a result of deregulated mTOR signaling [45]. Mechanistic investigations indicate that the mTORC1 activity is enhanced and Raptor, a defining component of mTORC1, translocates to lysosome [45]. And specific knockdown of Raptor in the hippocampal neurons activates autophagy and rescues the impaired synaptic plasticity and cognition in Fmr1-KO mice [45]. The findings indicate that the mTOR-dependent autophagy is impaired in FXS and activation of autophagy through mTOR inhibition prevents the neuronal deficits in FXS.
Recent studies have reported dysregulation of mTOR-dependent autophagy in other neurodevelopmental disorders including Schaaf-Yang syndrome (SHFYNG) and Koolen-de Vries syndrome (KdVS) [46, 47]. SHFYNG is a neurodevelopmental disorder caused by MAGEL2 mutations and the patients with SHFYNG show feeding difficulties, intellectual disability and cognitive impairment, and increased prevalence of ASD [48,49,50]. Schaaf et al. reported that the mTOR activity is increased, accompanied by decreased autophagy flux in MAGEL2 null mice and fibroblasts derived from SHFYNG patients [46]. The induced pluripotent stem cell (iPSC)-derived neurons from SHFYNG patients show impaired dendrite formation which can be rescued by treatment with rapamycin [46]. KdVS is neurodevelopmental disorder caused by mutations with loss-of-function in KANSL1 gene and patients with KdVS manifest epilepsy, congenital malformations and developmental delay [51,52,53]. Most recently, Kasri et al. have reported that iPSC-derived neurons from KdVS patients display accumulated autophagosome at excitatory synapses, resulting in reduced synaptic density and impaired neuronal network activity [47]. Mechanistically, they found that in these iPSC-derived neurons, the mTOR activity is enhanced and the lysosome function is decreased, thus preventing the clearance of autophagosome [47]. Taken together, these findings indicate that the mTOR-dependent autophagy is disrupted in these neurodevelopmental disorders.
Autophagy controls neurogenesis
The evidence that loss-of-function mutations in essential autophagy genes causes the neurodevelopmental disorders demonstrate a crucial role of autophagy in neurodevelopment. What is the mechanism underlying the function of autophagy in controlling neurodevelopment? Autophagy is constitutively active in the development of CNS [54]. Through digesting the toxic proteins or aggregates and damaged organelles, autophagy critically regulates neuronal plasticity during neuronal development. Given the growing interest in the role of autophagy in neural proliferation and in maintenance of neuronal stem cells (NSC), here we review the evidence linking autophagy to neurogenesis.
By using knockout strategies, previous studies have investigated the role of autophagy in embryonic neurogenesis [55]. Jiao et al. have shown a crucial role of autophagy in cortical neurogenesis during early brain development [56]. They found that the ATG5 expression increased during cortical development and differentiation [56]. Suppression of ATG5 by using electroporation of short hairpin shRNAs causes reduced neural progenitor cells (NPCs) differentiation, and consequent impaired morphology of cortical neurons [56]. Mechanistic investigations indicate that ATG5-mediated autophagy regulates β-Catenin signaling pathway, which is critical for NPCs proliferation and differentiation in neurodevelopment. They showed that autophagy cooperates with β-Catenin to modulate the proliferation and differentiation of cortical NPCs in embryonic neurogenesis during brain development [56]. Furthermore, another study reported that depletion of ATG5 represses astrocyte differentiation in vitro and in the developing mouse cortex, whereas overexpression of ATG5 enhances astrocyte differentiation [57]. Additional evidence indicated that through promoting the degradation of SOCS2, ATG5-mediated autophagy activates the JAK2-STAT3 signaling, which regulates the differentiation of astrocyte, while the impaired astrocyte differentiation caused by ATG5 deficiency can be rescued by SOCS2 knockdown [57]. These studies indicate that ATG5-mediated autophagy regulates both neurogenesis and gliogenesis during early brain development.
FIP200 (also known as Rb1cc1) is an essential gene for autophagy induction. Guan’s group previously reported that depletion of FIP200 causes a progressive loss of NSCs and impairs neuronal differentiation in postnatal brain of mice, which can be rescued by treatment with the antioxidant N-acetylcysteine [58]. Recently, the same group found evidence that microglial dysregulation contributes to the impairment of neurogenesis in FIP200-null NSCs from FIP200;p53hGFAP 2cKO mice [59]. Mechanistically, the study reveals that ablation of FIP200 leads to increased infiltration of microglia into the subventricular zone and subsequent microglia activation in FIP200; p53hGFAP 2cKO mice [59]. Inhibition of microglia infiltration and activation can rescue the defective neurogenesis in these 2cKO mice [59]. These findings demonstrate that FIP200-mediated autophagy plays a critical role in regulating neurogenesis in postnatal NSCs through the control of microglia migration and activation.
In addition to embryonic neurogenesis, adult neurogenesis can also be regulated by autophagy [60]. The Notch signaling pathway has an important role in adult neurogenesis [61]. Previous studies have shown that activation of Notch signaling inhibits proliferation and differentiation of NSCs in adult brain [62, 63]. Rubinsztein’s group has recently reported that Notch1, a plasma membrane-resident receptor in Notch signaling, is degraded by autophagy through ATG16L1, a crucial autophagy protein [64]. They demonstrated that ATG16L1 protein level was reduced, whereas the levels of Notch signaling proteins Notch1, NICD and Hes1 are significantly increased in the brain of ATG16L1-hypomorph mice [64]. They further showed decreased differentiation of NSCs and smaller cortical plate in ATG16L1-hypomorph compared with the control mice [64]. Using BrdU labeling, they showed that the number of BrdU-positive cells is significantly reduced in ATG16L1-hypomorph mice compared with the controls at 9–11 months old [64]. The results indicate that ATG16L1-mediated autophagy controls both embryonic and adult neurogenesis through regulating the degradation of proteins in Notch signaling pathway.
The Forkhead Box O (FOXO) proteins are a class of conserved transcription factors that control gene expression programs involved in multiple cell signaling pathways [65]. Previous studies have reported the roles of FOXO proteins in the regulation of adult NSCs homeostasis and autophagy induction [66,67,68]. A recent study has demonstrated that FOXO3 directly regulates autophagy pathway to maintain the proteostasis in adults NSCs [69]. Wurst et al. recently reported that conditional knockout of FOXO1/3/4 in adults NSCs by using GLAST::CreERT2 impairs autophagy flux both in in vitro and in vivo [70]. FOXO1/3/4 deficiency leads to altered dendrite and spine development of adult-generated neurons and impaired long-term survival eventually [70]. Further evidence indicates that autophagy inducer rapamycin not only rescues the impaired autophagy flux in NSCs, but also reverses the impaired phenotypes of dendrite and spine of adult-generated neurons with FOXO1/3/4 deficiency [70]. These findings indicate that FOXO proteins regulate neuronal morphogenesis via the maintenance of autophagy flux during adult neurogenesis.
Autophagy regulates presynaptic and postsynaptic development and synaptic activity
Neurons are postmitotic cells which are maintained for the lifetime of the organism. However, the synapses of neurons are highly dynamic, especially during early lifetime as the growing rate of synapses experiences an initial increase and subsequent decrease before an eventual stabilization of synapse development. During the synapse development the properties of synapses can be changed and reshaped, while new neural circuits are developed under circumstances such as learning and stress. Available evidence showed that autophagy plays a role in synapse formation and pruning, a process facilitating the removal of exuberant neuronal connections [71]. In addition, autophagy may also regulate synaptic activity, the function of which depends on the synapse transmission and plasticity.
Autophagy regulates presynaptic and postsynaptic development
During early development, autophagy is required for axon pathfinding and synaptic vesicle clustering formation during early synaptogenesis [72,73,74]. Previous studies showed that loss of autophagic scaffolding protein ALFY leads to a failure in axon guidance and outgrowth in the development of mouse brain [75]. Neural-specific depletion of ATG9 results in abnormal development of axon tracts in mouse brain regions including the corpus callosum and anterior commissure [73]. In C. elegans interneuron, autophagy controls presynaptic assembly and axon outgrowth dynamics, which is spatially regulated through the coordination of ATG9 and synaptic vesicle kinesin, KIF1A/UNC-104 [76]. Consistent with this notion, a previous study conducted in Drosophila indicated that Atg1 (an ortholog of ULK1 in S. cerevisiae) mutant causes reduced total neuromuscular junction (NMJ) area and decreased number of synapses [77]. However, overexpression of wildtype Atg1 increases NMJ synaptic bouton number in an autophagy-dependent manner [77].
In the postsynaptic site, autophagy was shown to be involved in synaptic pruning. Deficiencies in autophagy result in an overabundance of dendritic spines, ultimately manifesting as autism-like phenotypes [33]. For example, the primary hippocampal neuron cultures with reduced Atg7 expression showed increased PSD95 density, a marker for postsynaptic abundance [33]. The similar results were shown in mouse models with Atg7 knockdown [33]. Another study showed that NMJs from autophagy deficient neurons exhibited postsynaptic folds without presynaptic axon terminal opposed to them, suggesting an abnormal postsynaptic pruning [78]. These results indicate that basal autophagy may play an important role in specific postsynaptic receptor degradation and postnatal spine pruning.
Autophagy regulates synaptic activity
Multiple lines of evidence show that autophagy modulates neurotransmitter release and neural plasticity. Marijn Kuijpers et al. recently found that the neurotransmission and calcium sensitivity is significantly increased in primary hippocampus excitatory neurons in Atg5 knock-out mouse due to the deregulation of ER turnover [79]. Moreover, a previous study found that depletion of autophagy by using a dopaminergic neuron-specific Atg7 knock-out mouse model significantly affects dopamine release and reuptake [80]. The study further showed that striatal slices from Atg7 DAT::Cre mice had increased evoked dopamine release in cyclic voltammetry experiments and enhanced presynaptic recovery following paired-pulse stimulation. Moreover, rapamycin treatment reduced stimulus-evoked dopamine release in slices from Atg7 DAT::Cre mice [80]. These studies provide evidence for the role of presynaptic autophagy in the regulation of neurotransmission.
A recent study showed that autophagy regulates development-related synaptic plasticity and memory [81]. NMDA receptor-dependent long-term depression (NMDAR-LTD) is a long-lasting form of synaptic plasticity [82]. The induction of NMDAR-LTD is mediated by the removal of AMPA receptors from postsynaptic membranes to late endosomes for degradation [83]. It is induced in early stage of neuronal development while greatly reduced in adulthood during CNS development. Down-regulation of NMDAR-LTD in adults is physiologically significant for memory formation. Shen et al. revealed that autophagic flux in CA1 neurons was transiently decreased during the induction phase of NMDAR-LTD. Autophagy inhibition caused a reduction of endocytic recycling and is required for AMPA receptor internalization and synaptic depression in mouse CA1 neurons. In adulthood, autophagy is up-regulated to decrease the inducibility of LTD, thereby preventing the adverse effect of excessive LTD on memory consolidation [81]. Additionally, Compans et al. found that autophagy is required for the degradation of T19-phosphorylated form of PSD95 in NMDAR-LTD induction, which triggers a depletion of PSD95 from synapses and eventually increases short-term plasticity to improve neuronal responsiveness of depressed synapses [84]. A most recent study has shown that constitutive induction of mTOR-dependent autophagy rescues the prevention of NMDAR-LTD induced by disrupting synergistic action of CREB and CRTC1, two essential transcriptional factors for late-phase long-term synaptic potentiation [85]. These findings reveal the previously unrecognized functions of autophagy in the regulation of synaptic plasticity and memory (Fig. 1).
Autophagy regulates synaptic development and activity. In the presynaptic site, autophagy genes, such as ALFY, Atg9 and Atg1, regulate exon guidance and outgrowth, neuromuscular junction (NMJ) and synapse formation. Atg5 and Atg7 regulate neurotransmission. Atg5 mediated autophagy regulates neurotransmission through regulation of ER turnover. In the postsynaptic site, autophagy mediates synaptic pruning through the degradation of PSD95 and phosphorylated PSD95. Besides, autophagy regulates NMDAR-LTD by the removal of AMPA receptors from postsynaptic membranes to late endosomes for degradation to control synaptic plasticity
Conclusive remarks
Growing evidence has highlighted the important role of autophagy in regulating neurodevelopment and synaptic plasticity. Alteration of autophagy may lead to the abnormal neurodevelopment and malfunction of synapses in the brain. Recent human genetic and clinical studies have identified the link of congenital mutations in key autophagy related genes to neurodevelopmental disorders (Table 1). However, the causality of the autophagy deficiency to the disease awaits further clarification due to non-autophagy functions associated with many autophagy related genes. It is also extremely challenging to address the specific role of autophagy because of the difficulty of monitoring autophagy flux directly in human brain. While rodent models are valuable tools to dissect the role for autophagy in neurodevelopment and differentiation (e.g., engineering autophagy gene deletion in CNS), the possibly differential functions of autophagy homologous genes between rodent and human were noticed and may complicate the interpretation of the results [86].
A most recent study has identified recessive and loss-of-function mutations in both ATG7 alleles in patients with neurodevelopmental disorders in five unrelated families [15]. Despite the complete absence of ATG7 protein, the patients carrying the missense variants of ATG7 had approached population life expectancy [15, 87]. In contrast, mice lacking Atg7 gene die early postnatally. The data suggests that humans are much more tolerant to the loss of ATG7 or ATG7-mediated autophagy. Alternative explanation is that cellular functions that can compensate for the loss of ATG7 function in survival are more robust in humans than rodents. To dissect directly ATG7-mediated autophagy in neurodevelopment in humans, future experiment should use human neurons carrying corresponding mutants. The human neurons derived from induced pluripotent stem cells (iPSCs) would provide an important model to investigate the mechanism whereby autophagy deficiency leads to neurodevelopmental disease, and to test therapeutic strategy by restoring autophagy function.
Availability of data and materials
Not applicable.
References
Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–97.
Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42.
Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, Ohsumi Y. A unified nomenclature for yeast autophagy-related genes. Dev Cell. 2003;5:539–45.
Suzuki H, Kaizuka T, Mizushima N, Noda NN. Structure of the Atg101-Atg13 complex reveals essential roles of Atg101 in autophagy initiation. Nat Struct Mol Biol. 2015;22:572–80.
Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011;7:643–4.
Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–50.
Wold MS, Lim J, Lachance V, Deng Z, Yue Z. ULK1-mediated phosphorylation of ATG14 promotes autophagy and is impaired in Huntington’s disease models. Mol Neurodegener. 2016;11:76.
Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–9.
Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395:395–8.
Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–92.
Lazaro MT, Golshani P. The utility of rodent models of autism spectrum disorders. Curr Opin Neurol. 2015;28:103–9.
Hertz-Picciotto I, Schmidt RJ, Krakowiak P. Understanding environmental contributions to autism: causal concepts and the state of science. Autism Res. 2018;11:554–86.
Mehta SQ, Golshani P. Clinical neurogenetics: autism spectrum disorders. Neurol Clin. 2013;31:951–68.
Lee KM, Hwang SK, Lee JA. Neuronal autophagy and neurodevelopmental disorders. Exp Neurobiol. 2013;22:133–42.
Collier JJ, Guissart C, Olahova M, Sasorith S, Piron-Prunier F, Suomi F, Zhang D, Martinez-Lopez N, Leboucq N, Bahr A, Azzarello-Burri S, Reich S, Schols L, Polvikoski TM, Meyer P, Larrieu L, Schaefer AM, Alsaif HS, Alyamani S, Zuchner S, Barbosa IA, Deshpande C, Pyle A, Rauch A, Synofzik M, Alkuraya FS, Rivier F, Ryten M, McFarland R, Delahodde A, McWilliams TG, Koenig M, Taylor RW. Developmental consequences of defective ATG7-mediated autophagy in humans. N Engl J Med. 2021;384:2406–17.
Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. The heritability of autism spectrum disorder. JAMA. 2017;318:1182–4.
Geschwind DH, State MW. Gene hunting in autism spectrum disorder: on the path to precision medicine. Lancet Neurol. 2015;14:1109–20.
Schaaf CP, Zoghbi HY. Solving the autism puzzle a few pieces at a time. Neuron. 2011;70:806–8.
Poultney CS, Goldberg AP, Drapeau E, Kou Y, Harony-Nicolas H, Kajiwara Y, De Rubeis S, Durand S, Stevens C, Rehnstrom K, Palotie A, Daly MJ, Ma’ayan A, Fromer M, Buxbaum JD. Identification of small exonic CNV from whole-exome sequence data and application to autism spectrum disorder. Am J Hum Genet. 2013;93:607–19.
Hui KK, Takashima N, Watanabe A, Chater TE, Matsukawa H, Nekooki-Machida Y, Nilsson P, Endo R, Goda Y, Saido TC, Yoshikawa T, Tanaka M. GABARAPs dysfunction by autophagy deficiency in adolescent brain impairs GABA(A) receptor trafficking and social behavior. Sci Adv. 2019;5: eaau8237.
Vanderver A, Prust M, Tonduti D, Mochel F, Hussey HM, Helman G, Garbern J, Eichler F, Labauge P, Aubourg P, Rodriguez D, Patterson MC, Van Hove JLK, Schmidt J, Wolf NI, Boespflug-Tanguy O, Schiffmann R, van der Knaap MS, Consortium G. Case definition and classification of leukodystrophies and leukoencephalopathies. Mol Genet Metab. 2015;114(494):500.
Kim M, Sandford E, Gatica D, Qiu Y, Liu X, Zheng Y, Schulman BA, Xu J, Semple I, Ro S-H, Kim B, Mavioglu RN, Tolun A, Jipa A, Takats S, Karpati M, Li JZ, Yapici Z, Juhasz G, Lee JH, Klionsky DJ, Burmeister M. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife. 2016;5: e12245.
Kadir R, Harel T, Markus B, Perez Y, Bakhrat A, Cohen I, Volodarsky M, Feintsein-Linial M, Chervinski E, Zlotogora J, Sivan S, Birnbaum RY, Abdu U, Shalev S, Birk OS. ALFY-controlled DVL3 autophagy regulates Wnt signaling, determining human brain size. PLoS Genet. 2016;12: e1005919.
Dragich JM, Kuwajima T, Hirose-Ikeda M, Yoon MS, Eenjes E, Bosco JR, Fox LM, Lystad AH, Oo TF, Yarygina O, Mita T, Waguri S, Ichimura Y, Komatsu M, Simonsen A, Burke RE, Mason CA, Yamamoto A. Autophagy linked FYVE (Alfy/WDFY3) is required for establishing neuronal connectivity in the mammalian brain. eLife. 2016;5: e14810.
Dooley HC, Razi M, Polson HEJ, Girardin SE, Wilson MI, Tooze SA. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol Cell. 2014;55:238–52.
Jelani M, Dooley HC, Gubas A, Mohamoud HSA, Khan MTM, Ali Z, Kang C, Rahim F, Jan A, Vadgama N, Khan MI, Al-Aama JY, Khan A, Tooze SA, Nasir J. A mutation in the major autophagy gene, WIPI2, associated with global developmental abnormalities. Brain. 2019;142:1242–54.
Gstrein T, Edwards A, Pristoupilova A, Leca I, Breuss M, Pilat-Carotta S, Hansen AH, Tripathy R, Traunbauer AK, Hochstoeger T, Rosoklija G, Repic M, Landler L, Stranecky V, Durnberger G, Keane TM, Zuber J, Adams DJ, Flint J, Honzik T, Gut M, Beltran S, Mechtler K, Sherr E, Kmoch S, Gut I, Keays DA. Mutations in Vps15 perturb neuronal migration in mice and are associated with neurodevelopmental disease in humans. Nat Neurosci. 2018;21:207–17.
Oguro-Ando A, Rosensweig C, Herman E, Nishimura Y, Werling D, Bill BR, Berg JM, Gao F, Coppola G, Abrahams BS, Geschwind DH. Increased CYFIP1 dosage alters cellular and dendritic morphology and dysregulates mTOR. Mol Psychiatry. 2015;20:1069–78.
Suzuki AM, Griesi-Oliveira K, de Oliveira Freitas Machado C, Vadasz E, Zachi EC, Passos-Bueno MR, Sertie AL. Altered mTORC1 signaling in multipotent stem cells from nearly 25% of patients with nonsyndromic autism spectrum disorders. Mol Psychiatry. 2015;20:551–2.
Huber KM, Klann E, Costa-Mattioli M, Zukin RS. Dysregulation of mammalian target of rapamycin signaling in mouse models of autism. J Neurosci. 2015;35:13836–42.
Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–8.
Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41.
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, Yue Z, Arancio O, Peterson BS, Champagne F, Dwork AJ, Goldman J, Sulzer D. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–43.
Amegandjin CA, Choudhury M, Jadhav V, Carrico JN, Quintal A, Berryer M, Snapyan M, Chattopadhyaya B, Saghatelyan A, Di Cristo G. Sensitive period for rescuing parvalbumin interneurons connectivity and social behavior deficits caused by TSC1 loss. Nat Commun. 2021;12:3653.
Dana H, Bayramov KK, Delibasi N, Tahtasakal R, Bayramov R, Hamurcu Z, Sener EF. Disregulation of autophagy in the transgenerational Cc2d1a mouse model of autism. NeuroMol Med. 2020;22:239–49.
Amram N, Hacohen-Kleiman G, Sragovich S, Malishkevich A, Katz J, Touloumi O, Lagoudaki R, Grigoriadis NC, Giladi E, Yeheskel A, Pasmanik-Chor M, Jouroukhin Y, Gozes I. Sexual divergence in microtubule function: the novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol Psychiatry. 2016;21:1467–76.
Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–55.
Bagni C, Tassone F, Neri G, Hagerman R. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Investig. 2012;122:4314–22.
McCary LM, Roberts JE. Early identification of autism in fragile X syndrome: a review. J Intellect Disabil Res. 2013;57:803–14.
Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile X syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–29.
Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for FXS. Nat Neurosci. 2013;16:1530–6.
Gross C, Chang C-W, Kelly SM, Bhattacharya A, McBride SMJ, Danielson SW, Jiang MQ, Chan CB, Ye K, Gibson JR, Klann E, Jongens TA, Moberg KH, Huber KM, Bassell GJ. Increased expression of the PI3K enhancer PIKE mediates deficits in synaptic plasticity and behavior in fragile X syndrome. Cell Rep. 2015;11:727–36.
Hoeffer CA, Sanchez E, Hagerman RJ, Mu Y, Nguyen DV, Wong H, Whelan AM, Zukin RS, Klann E, Tassone F. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 2012;11:332–41.
Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702.
Yan J, Porch MW, Court-Vazquez B, Bennett MVL, Zukin RS. Activation of autophagy rescues synaptic and cognitive deficits in fragile X mice. Proc Natl Acad Sci USA. 2018;115:E9707–16.
Crutcher E, Pal R, Naini F, Zhang P, Laugsch M, Kim J, Bajic A, Schaaf CP. mTOR and autophagy pathways are dysregulated in murine and human models of Schaaf-Yang syndrome. Sci Rep. 2019;9:15935.
Linda K, Lewerissa EI, Verboven AHA, Gabriele M, Frega M, Klein Gunnewiek TM, Devilee L, Ulferts E, Hommersom M, Oudakker A, Schoenmaker C, van Bokhoven H, Schubert D, Testa G, Koolen DA, de Vries BBA, Nadif Kasri N. Imbalanced autophagy causes synaptic deficits in a human model for neurodevelopmental disorders. Autophagy. 2021. https://doi.org/10.1080/15548627.2021.1936777.
Schaaf CP, Gonzalez-Garay ML, Xia F, Potocki L, Gripp KW, Zhang B, Peters BA, McElwain MA, Drmanac R, Beaudet AL, Caskey CT, Yang Y. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism. Nat Genet. 2013;45:1405–8.
Fountain MD, Schaaf CP. Prader-Willi syndrome and Schaaf-Yang syndrome: neurodevelopmental diseases intersecting at the MAGEL2 gene. Diseases. 2016;4:2.
McCarthy J, Lupo PJ, Kovar E, Rech M, Bostwick B, Scott D, Kraft K, Roscioli T, Charrow J, Schrier Vergano SA, Lose E, Smiegel R, Lacassie Y, Schaaf CP. Schaaf-Yang syndrome overview: report of 78 individuals. Am J Med Genet A. 2018;176:2564–74.
Zollino M, Orteschi D, Murdolo M, Lattante S, Battaglia D, Stefanini C, Mercuri E, Chiurazzi P, Neri G, Marangi G. Mutations in KANSL1 cause the 17q21.31 microdeletion syndrome phenotype. Nat Genet. 2012;44:636–8.
Koolen DA, Kramer JM, Neveling K, Nillesen WM, Moore-Barton HL, Elmslie FV, Toutain A, Amiel J, Malan V, Tsai AC-H, Cheung SW, Gilissen C, Verwiel ETP, Martens S, Feuth T, Bongers EMHF, de Vries P, Scheffer H, Vissers LELM, de Brouwer APM, Brunner HG, Veltman JA, Schenck A, Yntema HG, de Vries BBA. Mutations in the chromatin modifier gene KANSL1 cause the 17q21.31 microdeletion syndrome. Nat Genet. 2012;44:639–41.
Koolen DA, Pfundt R, Linda K, Beunders G, Veenstra-Knol HE, Conta JH, Fortuna AM, Gillessen-Kaesbach G, Dugan S, Halbach S, Abdul-Rahman OA, et al. The Koolen-de Vries syndrome: a phenotypic comparison of patients with a 17q2131 microdeletion versus a KANSL1 sequence variant. Eur J Hum Genet. 2016;24(652):659.
Wu X, Won H, Rubinsztein DC. Autophagy and mammalian development. Biochem Soc Trans. 2013;41:1489–94.
Kuma A, Komatsu M, Mizushima N. Autophagy-monitoring and autophagy-deficient mice. Autophagy. 2017;13:1619–28.
Lv X, Jiang H, Li B, Liang Q, Wang S, Zhao Q, Jiao J. The crucial role of Atg5 in cortical neurogenesis during early brain development. Sci Rep. 2014. https://doi.org/10.1038/srep06010.
Wang S, Li B, Qiao H, Lv X, Liang Q, Shi Z, Xia W, Ji F, Jiao J. Autophagy-related gene Atg5 is essential for astrocyte differentiation in the developing mouse cortex. EMBO Rep. 2014;15:1053–61.
Wang C, Liang CC, Bian ZC, Zhu Y, Guan JL. FIP200 is required for maintenance and differentiation of postnatal neural stem cells. Nat Neurosci. 2013;16:532–42.
Wang C, Yeo S, Haas MA, Guan JL. Autophagy gene FIP200 in neural progenitors non-cell autonomously controls differentiation by regulating microglia. J Cell Biol. 2017;216:2581–96.
Dhaliwal J, Trinkle-Mulcahy L, Lagace DC. Autophagy and adult neurogenesis: discoveries made half a century ago yet in their infancy of being connected. Brain plasticity. 2017;3:99–110.
Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci. 2011;12:269–83.
Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–98.
Zhang Z, Yan R, Zhang Q, Li J, Kang X, Wang H, Huan L, Zhang L, Li F, Yang S, Zhang J, Ren X, Yang X. Hes1, a Notch signaling downstream target, regulates adult hippocampal neurogenesis following traumatic brain injury. Brain Res. 2014;1583:65–78.
Wu X, Fleming A, Ricketts T, Pavel M, Virgin H, Menzies FM, Rubinsztein DC. Autophagy regulates Notch degradation and modulates stem cell development and neurogenesis. Nat Commun. 2016;7:10533.
Eijkelenboom A, Burgering BMT. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97.
Renault VM, Rafalski VA, Morgan AA, Salih DAM, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–39.
Paik J-H, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae S-S, Zheng H, Ying H, Mahoney J, Hiller D, Jiang S, Protopopov A, Wong WH, Chin L, Ligon KL, DePinho RA. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–53.
van der Vos KE, Eliasson P, Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen IJ, Mauthe M, Zellmer S, Pals C, Verhagen LP, Groot Koerkamp MJA, Braat AK, Dansen TB, Holstege FC, Gebhardt R, Burgering BM, Coffer PJ. Modulation of glutamine metabolism by the PI(3)K–PKB–FOXO network regulates autophagy. Nat Cell Biol. 2012;14:829–37.
Audesse AJ, Dhakal S, Hassell L-A, Gardell Z, Nemtsova Y, Webb AE. FOXO3 directly regulates an autophagy network to functionally regulate proteostasis in adult neural stem cells. PLoS Genet. 2019;15: e1008097.
Schaffner I, Minakaki G, Khan MA, Balta EA, Schlotzer-Schrehardt U, Schwarz TJ, Beckervordersandforth R, Winner B, Webb AE, DePinho RA, Paik J, Wurst W, Klucken J, Lie DC. FoxO function is essential for maintenance of autophagic flux and neuronal morphogenesis in adult neurogenesis. Neuron. 2018;99:1188-1203 e1186.
Riccomagno MM, Kolodkin AL. Sculpting neural circuits by axon and dendrite pruning. Annu Rev Cell Dev Biol. 2015;31:779–805.
De Pace R, Skirzewski M, Damme M, Mattera R, Mercurio J, Foster AM, Cuitino L, Jarnik M, Hoffmann V, Morris HD, Han TU, Mancini GMS, Buonanno A, Bonifacino JS. Altered distribution of ATG9A and accumulation of axonal aggregates in neurons from a mouse model of AP-4 deficiency syndrome. PLoS Genet. 2018;14: e1007363.
Yamaguchi J, Suzuki C, Nanao T, Kakuta S, Ozawa K, Tanida I, Saitoh T, Sunabori T, Komatsu M, Tanaka K, Aoki S, Sakimura K, Uchiyama Y. Atg9a deficiency causes axon-specific lesions including neuronal circuit dysgenesis. Autophagy. 2018;14:764–77.
Binotti B, Pavlos NJ, Riedel D, Wenzel D, Vorbrüggen G, Schalk AM, Kühnel K, Boyken J, Erck C, Martens H, Chua JJ, Jahn R. The GTPase Rab26 links synaptic vesicles to the autophagy pathway. Elife. 2015;4: e05597.
Dragich JM, Kuwajima T, Hirose-Ikeda M, Yoon MS, Eenjes E, Bosco JR, Fox LM, Lystad AH, Oo TF, Yarygina O, Mita T, Waguri S, Ichimura Y, Komatsu M, Simonsen A, Burke RE, Mason CA, Yamamoto A. Autophagy linked FYVE (Alfy/WDFY3) is required for establishing neuronal connectivity in the mammalian brain. Elife. 2016. https://doi.org/10.7554/eLife.14810.
Stavoe AK, Hill SE, Hall DH, Colón-Ramos DA. KIF1A/UNC-104 transports ATG-9 to regulate neurodevelopment and autophagy at synapses. Dev Cell. 2016;38:171–85.
Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J Cell Biol. 2009;187:71–9.
Rudnick ND, Griffey CJ, Guarnieri P, Gerbino V, Wang X, Piersaint JA, Tapia JC, Rich MM, Maniatis T. Distinct roles for motor neuron autophagy early and late in the SOD1(G93A) mouse model of ALS. Proc Natl Acad Sci USA. 2017;114:E8294-e8303.
Kuijpers M, Kochlamazashvili G, Stumpf A, Puchkov D, Swaminathan A, Lucht MT, Krause E, Maritzen T, Schmitz D, Haucke V. Neuronal autophagy regulates presynaptic neurotransmission by controlling the axonal endoplasmic reticulum. Neuron. 2021;109:299-313.e299.
Hernandez D, Torres CA, Setlik W, Cebrián C, Mosharov EV, Tang G, Cheng HC, Kholodilov N, Yarygina O, Burke RE, Gershon M, Sulzer D. Regulation of presynaptic neurotransmission by macroautophagy. Neuron. 2012;74:277–84.
Shen H, Zhu H, Panja D, Gu Q, Li Z. Autophagy controls the induction and developmental decline of NMDAR-LTD through endocytic recycling. Nat Commun. 2020;11:2979.
Lüscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 2012. https://doi.org/10.1101/cshperspect.a005710.
Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26.
Compans B, Camus C, Kallergi E, Sposini S, Martineau M, Butler C, Kechkar A, Klaassen RV, Retailleau N, Sejnowski TJ, Smit AB, Sibarita JB, Bartol TM Jr, Perrais D, Nikoletopoulou V, Choquet D, Hosy E. NMDAR-dependent long-term depression is associated with increased short term plasticity through autophagy mediated loss of PSD-95. Nat Commun. 2021;12:2849.
Pan Y, He X, Li C, Li Y, Li W, Zhang H, Wang Y, Zhou G, Yang J, Li J, Qu J, Wang H, Gao Z, Shen Y, Li T, Hu H, Ma H. Neuronal activity recruits the CRTC1/CREB axis to drive transcription-dependent autophagy for maintaining late-phase LTD. Cell Rep. 2021;36: 109398.
Lin S, Lin Y, Nery JR, Urich MA, Breschi A, Davis CA, Dobin A, Zaleski C, Beer MA, Chapman WC, Gingeras TR, Ecker JR, Snyder MP. Comparison of the transcriptional landscapes between human and mouse tissues. Proc Natl Acad Sci. 2014;111:17224.
Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–34.
Acknowledgements
We thank members of Yue lab and Lu lab for their suggestions.
Funding
This study was supported by China minister of Science and Technology grant MoST-2017YFE0120100, the Science and Technology Development Fund, Macau SAR (No. 0110/2018/A3, 0128/2019/A3, China), the University of Macau grants (No. MYRG2019-00129-ICMS, China) awarded to Jia-Hong Lu, and NIH/R01NS060123 and R01 R01AG072520 awarded to Zhenyu Yue.
Author information
Authors and Affiliations
Contributions
ZY, JL and ZD conceived the project. ZD and XZ searched the literatures and drafted the manuscript. ZY, JL, ZD, and XZ edited and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, Z., Zhou, X., Lu, JH. et al. Autophagy deficiency in neurodevelopmental disorders. Cell Biosci 11, 214 (2021). https://doi.org/10.1186/s13578-021-00726-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-021-00726-x