Abstract
Epitranscriptomics, also known as “RNA epigenetics”, is a chemical modification for RNA regulation. Ribonucleic acid (RNA) methylation is considered to be a major discovery following the deoxyribonucleic acid (DNA) and histone methylation. Messenger RNA (mRNA) methylation modification accounts for more than 60% of all RNA modifications and N6-methyladenosine (m6A) is known as one of the most common type of eukaryotic mRNA methylation modifications in current. The m6A modification is a dynamic reversible modification, which can directly or indirectly affect biological processes, such as RNA degradation, translation and splicing, and can play important biological roles in vivo. This article introduces the mRNA m6A methylation modification enzymes and binding proteins, and reviews the research progress and related mechanisms of the role of mRNA m6A methylation in the nervous system from the aspects of neural stem cells, learning and memory, brain development, axon growth and glioblastoma.
Similar content being viewed by others
Background
Epitranscriptomics, also known as “RNA epigenetics”, is a chemical modification for RNA regulation [1]. According to its function, RNA can be divided into two broad categories, including encoding protein mRNA and non-coding RNA. With the deep research of epitranscriptomics, the researchers found methylation modification on mRNA, which is involved in the regulation of eukaryotic gene expression [2,3,4].
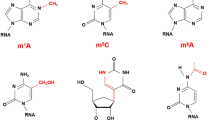
The mRNA is a type of RNA with genetic information synthesized by DNA transcription, which acts as a template in protein synthesis and determines the amino acid sequence of the peptide chain [5]. It is an important RNA in the human body. The methylation is the process of catalytically transferring a methyl group from an active methyl compound such as S-adenosylmethionine (SAM) to another compound, which can chemically modify certain proteins or nucleic acids to form a methylated product [6]. In biological systems, methylation influences heavy metal modification, regulation of gene expression, regulation of protein function, RNA processing, etc. [7]. At the early 1970s, scientists discovered the presence of the methylation modification in mRNA [8, 9]. The mRNA methylation modification mainly located in the nitrogen atom of the base group to form m6A, which is enriched in long exons and overrepresented in transcripts with alternative splicing variants [10]. The mRNA methylation modifications also include 5-methylcytosine (m5C), N1-methyladenosine (m1A), 5-hydroxymethylcytosine (5hmC), N6, 2′-O-dimethyladenosine (m6Am), 7-methylguanine (m7G) (Fig. 1). These modifications can affect regulation of various biological processes, such as RNA stability and mRNA translation, and abnormal mRNA methylation is linked to many diseases [11].
Main text
Discovery and distribution of m6A
The m6A is the most common and abundant methylation modification in mRNA [12, 13]. In 1974, Desrosie used the polyadenosinic acid (PolyA) structure in eukaryotes, to discover the methylation status of mRNA in hepatoma cells, and found that the main methylation modification in mRNA was m6A (approximately 80%) [8]. In addition, the presence of m6A was also detected in a variety of eukaryotes and viral mRNA [14].
In mammals, m6A is widely distributed in multiple tissues. Studies by Meyer showed that m6A expression was higher in liver, kidney and brain than in other tissues. It has also been found that the content of m6A is very different in various cancer cell lines [15]. With the help of high-throughput sequencing technology, a rough m6A modification map has been obtained. Meyer studied the m6A modification in mouse brain and found that it was mainly distributed inside the gene (94.8%), where the proportions in the protein coding region (CDS), untranslated regions (UTRs) and introns are 50.9%, 41.9%, and 2.0% respectively [16]. The m6A in the UTRs region tends to be enriched in the 3′UTR, while in the CDS region it is mainly enriched near the stop codon [17]. The m6A modification occurs mainly on the adenine in the RRACH sequence, where R is guanine or adenine, and H is uracil, adenine or cytosine [18] (Fig. 2).
mRNA m6A methylation modification enzyme
The methylation modification of m6A has been proved to be reversible, as it involves both methyltransferase and demethylase. The main role of methyltransferases is to catalyze the m6A modification of mRNA, while demethylases act on demethylation of bases that have had m6A modification [19, 20].
m6A methyltransferase
The m6A methyltransferase, also known as “Writers”, is an important kind of catalytic enzymes [21]. Methyltransferase like 3/14 (METTL3/14), Wilms’ tumour 1-associating protein (WTAP), KIAA1429 and RNA binding motifs protein 15/15B (RBM15/15B) are core components of the m6A methyltransferase, which form complexes that work together to perform catalytic functions. Besides, E3 ubiquitin-protein ligase Hakai (HAKAI) and zinc finger CCCH-type containing 13 (ZC3H13) are also the part of the mRNA methyltransferase complex.
The METTL3 is identified as a SAM-binding component of the complex and has its own catalytic ability, which is highly conserved in eukaryotes [22]. METTL14 is closely homologous to METTL3. It does not bind to the SAM domain and does not with independently m6A methyltransferase function. Biochemical characterization has shown that METTL3 and METTL14 proteins form a stable complex with a stoichiometric ratio of 1:1, and the methylation activity of the complex is higher than that of METTL3 alone. Among them, METTL3 is a catalytically active subunit, and METTL14 plays a key role in substrate identification [23, 24].
The WTAP is a regulatory subunit of the m6A methyltransferase complex, which can interact with METTL3 and METTL14. Knocking out WTAP can significantly reduce the m6A peak in cellular mRNA, even more effective than knocking down METTL3 or METTL14. The WTAP-bound gene has a change in alternative splicing patterns [25].
The KIAA1429, also known as vir-like m6A methyltransferase associated (VIRMA), is a homologous protein of the Virilizer protein in Drosophila, which is closely related to the methyltransferase complex. The N-terminus of KIAA1429 has the ability to gather methyltransferase-catalyzed core METTL3/METTL14/WTAP that can achieve the regulation of fixed-point m6A levels on mRNA [26].
It was identified by co-immunoprecipitation that the binding of RBM15/15B at the RRACH sequence site is three to fourfold o higher than that at the non-methylation site. Knocking down the RBM15 or RBM15B alone can reduce the m6A levels in cellular mRNA, and knocking down both RBM15 and RBM15B can result in a significant decrease of the m6A levels in mRNA [27].
The HAKAI, also known as CBL proto-oncogene like 1(CBLL1), is an E3 ubiquitin ligase. Down-regulation of the HAKAI in Arabidopsis can result in a decrease in m6A level [28]. ZC3H13 is also an important component of the methyltransferase complex and is key to anchor the complex in the nucleus [29]. Methyltransferase like 16 (METTL16) is a m6A methyltransferase of the mRNA precursor that maintains SAM homeostasis by regulating alternative splicing of methionine adenosyltransferase II alpha (MAT2a) [30,31,32].
m6A demethylase
The m6A demethylase, also known as the “Erasers”. In eukaryotes, m6A demethylases are fat mass and obesity-associated protein (FTO) and alkB homolog 5 (alkB homolog 5, ALKHB5). In Arabidopsis, the alkB homolog 10B (ALKHB10B) has also been found as a m6A demethylase of mRNA.
The FTO also known as alkB homolog 9 (ALKBH9), which is a member of the Alkb protein family and associated with obesity. FTO is the first-discovered RNA demethylase. The long stem loop domain at the C-terminus of FTO enables the FTO proteins demethylate [33, 34].
The ALKBH5 is another protein of the AlkB family and plays an important regulatory role in biological processes, such as mRNA processing. The ALKBH5 is similar to FTO and is also a Fe2+ and α-Ketoglutaric acid dependent non-heme oxygenase. The ALKBH5 has an alanine-rich region at the N-terminus and a unique coiled-coil structure. It only demethylates the m6A modification on single-stranded RNA/DNA, and the catalytic reaction removes methyl groups directly from m6A-methylated adenosine instead of oxidative demethylation [35, 36].
The ALKBH10B is an m6A demethylase of mRNA in Arabidopsis, which regulates mRNA stability and affects the transformation of Arabidopsis from vegetative growth to reproductive growth [37].
mRNA m6A methylation binding protein
The m6A-modified mRNA that performs a specific biological function requires a specific RNA-binding protein-readers. Binding assays of RNA protein in vitro have identified a variety of binding proteins, including YTH domain containing RNA binding protein (YTP), heterogeneous nuclear ribonucleoprotein (hnRNP), eukaryotic initiation factor 3 (eIF3), Insulin-like growth factor 2 mRNA-binding protein (IGF2BP) and Proline rich coiled-coil 2A (Prrc2a). The functions of these binding proteins mainly include specific binding to the m6A methylation region, weakening the homologous binding to RNA reading proteins, and altering the secondary structure of RNA to alter protein–RNA interaction [38, 39].
YTH domain containing RNA binding protein include YTH domain-containing family protein 1/2/3 (YTHDF1/2/3) and YTH domain-containing protein 1/2 (YTHDC1/2). YTHDF1/2/3 and YTHDC2 specifically recognize the m6A-modified mRNA in the cytoplasm, while the recognizing sites of YTHDC1 are mainly in the nucleus. These proteins all have a YTH domain at the C-terminus. They are capable of overlapping with the m6A RRACH fragment to mediate RNA-specific binding, while its proline/glutamine/asparagine enrichment (P/Q/N-rich) domain is related to subcellular localization [40, 41].
YTHDF1 is combined with translation initiation factors and ribosomes, improving the translation efficiency. YTHDF2 is the first-discovered binding protein. Specifically, it recognizes and binds m6A-containing RNAs, and regulates mRNA stability [42, 43]. YTHDF3 promotes the translation of mRNA and regulates the mRNA stability. YTHDF3 and YTHDF1 coordinately control during translation [44, 45]. YTHDC1 regulates the mRNA cleavage by recruiting splicing factors [46,47,48]. YTHDC2 accelerates the degradation of the modified mRNA and enhances the translation of the corresponding protein by recognizing m6A [49].
The hnRNP is a group of RNA-binding proteins that contain nearly 30 nucleic acid-binding proteins with molecular weights ranging from 30 to 120 kDa, which can interact with each other to form the complex, where A1, A2, B1, B2, C1 and C2 are the main core components. Heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) is capable of specifically recognizing the m6A modifications on transcripts, activating downstream variable shear events of partial genes [50, 51]. Heterogeneous nuclear ribonucleoprotein C (HNRNPC) is responsible for recognizing the m6A modifying group and mediating the processing of the mRNA precursor in the nucleus, affecting the abundance and alternative splicing of target transcripts. The m6A can increase the accessibility of its surrounding RNA sequences binding to heterogeneous nuclear ribonucleoprotein G (HNRNPG). In the transcriptome, the m6A site regulates RNA-HNRNPG interactions to alter target mRNA expression and alternative splicing patterns [52].
In mammalian cells, eIF3 is the largest eukaryotic initiation factor and plays a key role in the initiation of eukaryotic translation. It is able to directly bind to the 5′UTR m6A of mRNA, thereby facilitating translation of mRNA. IGF2BP protein is a unique m6A reader. The family mainly includes IGF2BP1/2/3. IGF2BP can make the target gene and corresponding translation more stable [53]. Prrc2a is a new m6A reader that stabilizes mRNA expression by binding to a consensus GGACU motif in the CDS region in an m6A-dependent manner [54] (Fig. 3).
Neurobiological function of mRNA m6A methylation
Sequencing results have showed that the level of RNA m6A modification increase significantly during embryogenesis [55,56,57,58]. Compared to other organs or tissues, the overall level of m6A in the head is significantly higher. This suggests that the mRNA m6A modification has potential neurobiological functions in the nervous system and is worthy of further study [59,60,61,62,63].
Effect of m6A on neural stem cells
Neural stem cells maintain cell populations through self-renewal, and can differentiate into various nerve cells such as neurons, astrocytes, and oligodendrocytes [64,65,66,67]. A number of studies have shown that the mRNA m6A modification can affect the self-renewal and differentiation of neural stem cells [68,69,70]. These new findings will promote stem cell therapy and gene-targeted therapy for neurological diseases.
Inactivation of Mettl3 in mouse and human embryonic stem cells leads to a decrease in m6A, and severely impairs the transition of neurons from self-renewal to differentiation. The knockout of Mettl3 can cause early embryonic lethality and impair the formation of mature neurons in the embryoid body [58, 71]. Wang et al. found that when knocking out Mettl14 in embryonic neural stem cells in a mouse model, the proliferation of neural stem cells was significantly reduced and differentiated prematurely. It indicates that m6A can promote the proliferation of neural stem cells and prevent premature differentiation of cells, thus ensuring the reserve of neural stem cell bank [72, 73]. Mettl14 and Mettl3 can participate in neurogenesis by regulating the cell cycle progression of cortical neural stem cells, which acts in a m6A-dependent way [74]. The SMAD2/3 protein binds to the METTL3-METTL14-WTAP complex and promotes the differentiation of embryonic stem cells into neuroendodermal cells [75].
The Ythdf2-mediated m6A mRNA clearance has a regulatory effect on neurodevelopment in mice. Proliferation and differentiation of neural stem cells are seriously affected by the deletion of embryonic Ythdf2 [76].
Effect of m6A on learning and memory
In the emerging field of epitranscriptomic mechanisms, mRNA m6A modification has potential role in learning and memory [77]. It regulates physiological and stress-induced behavior in the adult mammalian brain, and augments the strength of weak memories [78,79,80]. As a newly identified element in the region-specific gene regulatory network in the mouse brain, mRNA m6A modification plays a vital role in the death of dopaminergic neuron [81, 82].
Mettl3-mediated RNA m6A modification has the direct effect on regulating hippocampal-dependent long-term memory formation. The decrease of Mettl3 in the mice hippocampus may reduce its memory consolidation, and adequate training or restoration would restore the ability of learn and memory. The abundance of Mettl3 in the hippocampus of wild-type mice is positively correlated with learning efficiency, and the overexpression of Mettl3 can significantly enhance the long-term memory consolidation [83]. METTL14 is critical for striatum function and transcriptional regulation of learning epitopes. In cell experiments, the deletion of METTL14 reduces striatum m6A levels without altering cell number or morphology, increases neuronal excitability and severely impaired striatal-mediated behavior [84].
Fto can regulate the activity of dopaminergic midbrain circuits. Inactivation of the Fto gene weaken neuronal activity and behavioral responses that are dependent on dopamine receptor type 2 (D2R) and type 3 (D3R) (collectively D2-like receptors) [85]. FTO also regulates dopaminergic neurotransmission deficits caused by arsenite [86]. Walters [87] has found that Fto plays an important role in the formation of mouse hippocampal-dependent memory. The decrease in Fto protein observed shortly after the situational fear reflex indicates that Fto typically limits memory formation. The m6A is regulated in the activity-dependent way in the adult brain, and may fine-tune mRNA turnover during memory-related processes [88]. When knocking out the Fto gene in the prefrontal cortex of mice, the intensity of m6A on several fear-related genes in neurons increases significantly, and the knockdown of Fto further enhances the consolidation of fear memory [89]. FTO plays important roles in learning and memory. The loss of FTO led to the altered expression of several key components of the brain derived neurotrophic factor pathway that were marked by m6A [90].
In the adult mouse hippocampus, the m6A binding protein Ythdf1 can promote neuronal stimulation of protein translation of target transcripts, thereby facilitating learning and memory. Mice with a genetic deletion of Ythdf1 have showed the deficits of learning and memory, impaired hippocampal synaptic transmission and long-term potentiation [91]. Prrc2a controls the specification and myelination of oligodendrocyte, and Prrc2a knockout induces cognitive defects in a mouse model [54].
Effect of m6A on brain development
Widespread and dynamic m6A methylation were identified in the developing mouse cerebellum. RNA m6A methylation is controlled in a precise spatiotemporal manner and participates in the regulation of postnatal development of the mouse cerebellum [92, 93].
Specific inactivation of Mettl3 in mouse nervous system causes severe developmental defects in the brain. Mettl3-mediated m6A participates in cerebellar development by controlling mRNA stability of genes involved in cerebellar development and apoptosis [94].
Under the low pressure and hypoxia, the level of RNA m6A methylation in the cerebellar of Alkbh5-deficient mouse is imbalanced, which leads to an increase in the efficiency of extranuclear RNA excretion and a significant change in cerebellar phenotype, including neuronal structural disorder, abnormal cell proliferation and differentiation, and other phenotypes [92].
Effect of m6A on synaptic growth
The m6A modification plays a key role in synaptic regeneration of mature mouse neurons. Increased m6A in somatic neurons alters the transcriptome response to synaptic plasticity [77, 89]. The m6A methylation of neurological function-related genes in the hippocampus of human immunodeficiency virus transgenic rats is significantly different, suggesting synaptic damage and neurodegeneration [95]. The m6A methylation of synaptic mRNAs critically contribute to synaptic function in healthy adult mouse forebrains [96].
Deletion of Mettl14 reduces functional axonal regeneration in the peripheral nervous system of the body. After knockdown of Mettl14, the axonal regeneration of retinal ganglion neurons in the central nervous system is also diminished [97].
The m6A modification can affect axon growth by regulating local translation of mRNA in neuronal axons. FTO is highly expressed in axons of neurons. Local translation in axons plays an important role in neurodevelopment, including axon guidance, axon growth, and neuronal specifications [90, 98].
The mRNA m6A modification of synaptic plays a key role in synaptic function. After knocking out the dendritic positioning readers Ythdf1 and Ythdf3 in cultured hippocampal neurons, m6A-reader-deficient neurons have abnormal spine morphology and the spines are reduced. Knocking out the Ythdf1 gene of mouse, in the peripheral and peripheral nervous system, functional axon regeneration is reduced [97, 99]. The neurons of YTHDF2−/− could not produce normal synapses [76].
Effect of m6A on glioblastoma
Several studies have revealed the role of m6A witers and erasers in glioblastoma. Changes of the m6A level in glioblastoma stem cell-like cells (GSC) severely affect the growth, self-renewal and development of tumor. The mRNA m6A methylation is expected to be a new target for the treatment of glioblastoma [100].
Decreasing the m6A levels by knocking down METTL3 and/or METTL14 enhance growth and self-renewal of GSCs in vitro, and promote the ability of GSCs to form brain tumors in vivo. The Mettl3-mediated m6A modification plays a key role in neurosphere maintenance and glioma cell dedifferentiation [101,102,103]. Ethyl form of methylbenzoic acid (MA2) is a selective inhibitor of FTO, which can significantly inhibit tumor progression and prolong the lifespan of GSC mice. Therefore, The Fto may play a key carcinogenic role in GSC self-renewal and is required for the development of glioblastoma [101]. ALKBH5 is able to maintain stem cell in malignant glioma cells, and ALKBH5-mediated m6A modification on forkhead box M1 (FOXM1) mRNA is involved in the maintenance of tumor stem cell. High expression of ALKBH5 predicts poor prognosis in glioblastoma patients [104, 105] (Table 1).
Conclusion
In summary, the mRNA methylation is an important epitranscriptomic modification and the m6A is highly expressed in the brain. The mRNA m6A methylation has a wide range of effects on the nervous system, and plays an important part in self-renewal of neural stem cells, learning memory, brain development, synaptic growth and proliferation of glioma cells. This new regulatory system will promote targeted therapy for neurological diseases.
However, mRNA m6A methylation is a relatively new field and many problems remain unknown. Up till now, all of the demethylases found belong to the AlkB family, and whether other proteins in the AlkB family are also involved in mRNA demethylation is worthy for further study. HNRNPA1, HNRNPG and HNRNPM play a key role in the methylation of protein arginine. These proteins are similar to HNRNPA2B1 and HNRNPC, and belong to the hnRNP binding protein family. It is worth exploring its role in mRNA m6A methylation.
Variations in the FTO gene can not only regulate D2R-dependent reward learning [106,107,108], but also affect nerve adjust food visual, produce more frequent rewards [109,110,111], affect the control of mood and impulse [112,113,114], and affect obesity by regulating brain signaling pathways [115, 116]. The homozygous mutation of FTO gene can reduce the brain capacity of healthy elderly people, increase the susceptibility to brain atrophy during aging, and even affect the brain volume of adolescents [117, 118]. The genetic polymorphism of FTO is related to attention-deficit/hyperactivity disorder (ADHD), Alzheimer’s disease and depression [119,120,121,122,123,124]. Whether it is as demethylase that affects these diseases, is worthy of further study.
The genetic polymorphism of ZC3H13 is associated with schizophrenia. Nito, another member of the m6A methyltransferase complex in Drosophila, called RBM15 in human, controls the axonal growth and differentiation and regulates the synapse formation through neuronal activity. Whether human ZC3H13 and RBM15 genes have the effect on synaptic growth, is worthy of further study.
To study the methylation mechanism of mRNA m6A and find potential targets for treatment, it is hopeful to develop inhibitors or agonists of related proteins for clinical treatment in the future.
Availability of data and materials
Not applicable.
Abbreviations
- 5hmC:
-
5-hydroxymethylcytosine
- ADHD:
-
attention-deficit/hyperactivity disorder
- ALKHB5/9/10B:
-
alkB homolog 5/9/10B
- CBLL1:
-
CBL proto-oncogene like 1
- CDS:
-
coding region
- D2/3R:
-
dopamine receptor type 2/3
- DNA:
-
deoxyribonucleic acid
- eIF3:
-
eukaryotic initiation factor 3
- FOXM1:
-
forkhead box M1
- FTO:
-
fat mass and obesity-associated protein
- GSC:
-
glioblastoma stem cell-like cells
- HAKAI:
-
E3 ubiquitin-protein ligase Hakai
- hnRNP:
-
heterogeneous nuclear ribonucleoprotein
- HNRNPA2B1/C/G:
-
heterogeneous nuclear ribonucleoprotein A2B1/C/G
- IGF2BP:
-
insulin-like growth factor 2 mRNA-binding protein
- m1A:
-
N1-methyladenosine
- m5C:
-
5-methylcytosine
- m6A:
-
N6-methyladenosine
- m6Am:
-
N6, 2′-O-dimethyladenosine
- m7G:
-
7-methylguanine
- MA2:
-
methylbenzoic acid
- MAT2a:
-
methionine adenosyltransferase II Alpha
- METTL3/14/16:
-
methyltransferase like 3/14/16
- mRNA:
-
messenger RNA
- P/Q/N-rich:
-
proline/glutamine/asparagine enrichment
- PolyA:
-
polyadenosinic acid
- Prrc2a:
-
proline rich coiled-coil 2A
- RBM15/15B:
-
RNA binding motifs protein 15/15B
- RNA:
-
ribonucleic acid
- SAM:
-
S-adenosylmethionine
- UTRs:
-
untranslated regions
- VIRMA:
-
vir-like m6A methyltransferase associated
- WTAP:
-
wilms’ tumour 1-associating protein
- YTHDC1/2:
-
YTH domain-containing protein 1/2
- YTHDF1/2/3:
-
YTH domain-containing family protein 1/2/3
- YTP:
-
YTH domain containing RNA binding protein
- ZC3H13:
-
zinc finger CCCH-type containing 13
References
Roundtree IA, He C. RNA epigenetics–chemical messages for posttranscriptional gene regulation. Curr Opin Chem Biol. 2016;30:46–51. https://doi.org/10.1016/j.cbpa.2015.10.024.
Zheng G, Dahl JA, Niu Y, Fu Y, Klungland A, Yang YG, et al. Sprouts of RNA epigenetics. RNA Biol. 2013;10:915–8. https://doi.org/10.4161/rna.24711.
Liu N, Pan T. RNA epigenetics. Transl Res. 2015;165:28–35. https://doi.org/10.1016/j.trsl.2014.04.003.
Nachtergaele S, He C. Chemical modifications in the life of an mRNA transcript. Annu Rev Genet. 2018;52:349–72. https://doi.org/10.1146/annurev-genet-120417-031522.
Roignant JY, Soller M. m6A in mRNA: an ancient mechanism for fine-tuning gene expression. Trends Genet. 2015;33:380–90. https://doi.org/10.1016/j.tig.2017.04.003.
Tuck MT. The formation of internal 6-methyladenine residues in eucaryotic messenger RNA. Int J Biochem. 1992;24:379–86. https://doi.org/10.1016/0020-711x(92)90028-y.
Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, et al. N6-methyladenosine modulates messenger rna translation efficiency. Cell. 2015;161:1388–99. https://doi.org/10.1016/j.cell.2015.05.014.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger-RNA from Novikoff hepatoma-cells. Proc Natl Acad Sci USA. 1974;71:3971–5. https://doi.org/10.1073/pnas.71.10.3971.
Canaani D, Kahana C, Lavi S, Groner Y. Identification and mapping of N6-methyladenosine containing sequences in simian virus 40 RNA. Nucleic Acids Res. 1979;6:2879–99. https://doi.org/10.1093/nar/6.8.2879.
Zhao BS, Nachtergaele S, Roundtree IA, He C. Our views of dynamic N6-methyladenosine RNA methylation. RNA. 2018;24:268–72. https://doi.org/10.1261/rna.064295.117.
Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537:369–73. https://doi.org/10.1038/nature19342.
Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–55. https://doi.org/10.1101/gad.262766.115.
Shi H, Wei J, He C. Where, when, and how: context-dependent functions of rna methylation writers, readers, and erasers. Mol Cell. 2019;74:640–50. https://doi.org/10.1016/j.molcel.2019.04.025.
Cao G, Li HB, Yin Z, Flavell RA. Recent advances in dynamic m6A RNA modification. Open Biol. 2014;6:160003. https://doi.org/10.1098/rsob.160003.
Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol. 2014;15(5):313–26. https://doi.org/10.1038/nrm3785.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–46. https://doi.org/10.1016/j.cell.2012.05.003.
Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 2015;29:2037–53. https://doi.org/10.1101/gad.269415.115.
Deng X, Su R, Weng H, Huang H, Li Z, Chen J. RNA N6-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–17. https://doi.org/10.1038/s41422-018-0034-6.
Yang Y, Hsu PJ, Chen YS, Yang YG. Dynamic transcriptomic m6A decoration: writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018;28:616–24. https://doi.org/10.1038/s41422-018-0040-8.
Meyer KD, Jaffrey SR. Rethinking m6A readers, writers, and erasers. Annu Rev Cell Dev Biol. 2017;33:319–42. https://doi.org/10.1146/annurev-cellbio-100616-060758.
Rana AK, Ankri S. Reviving the RNA world: an insight into the appearance of rna methyltransferases. Front Genet. 2016;7:99. https://doi.org/10.3389/fgene.2016.00099.
Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–45. https://doi.org/10.1016/j.molcel.2016.03.021.
Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–5. https://doi.org/10.1038/nchembio.1432.
Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, et al. Corrigendum: structural basis of N6-adenosine methylation by the METTL3-METTL14 complex. Nature. 2017;542:260. https://doi.org/10.1038/nature21073.
Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–89. https://doi.org/10.1038/cr.2014.3.
Yue Y, Liu J, Cui X, Cao J, Luo G, Zhang Z, et al. VIRMA mediates preferential m6A mRNA methylation in 3′UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2014;4:10. https://doi.org/10.1038/s41421-018-0019-0.
Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 2018;32:415–29. https://doi.org/10.1101/gad.309146.117.
Růžička K, Zhang M, Campilho A, Bodi Z, Kashif M, Saleh M, et al. Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017;215:157–72. https://doi.org/10.1111/nph.14586.
Wen J, Lv R, Ma H, Shen H, He C, Wang J, et al. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69:1028–38. https://doi.org/10.1016/j.molcel.2018.02.015.
Shima H, Matsumoto M, Ishigami Y, Ebina M, Muto A, Sato Y, et al. S-Adenosylmethionine synthesis is regulated by selective N6-adenosine methylation and mRNA degradation involving METTL16 and YTHDC1. Cell Rep. 2017;21:3354–63. https://doi.org/10.1016/j.celrep.2017.11.092.
Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Höbartner C, et al. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18:2004–14. https://doi.org/10.15252/embr.201744940.
Ruszkowska A, Ruszkowski M, Dauter Z, Brown JA. Structural insights into the RNA methyltransferase domain of METTL16. Sci Rep. 2018;8:5311. https://doi.org/10.1038/s41598-018-23608-8.
Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7. https://doi.org/10.1038/nchembio.687.
Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3′-end processing. Nucleic Acids Res. 2017;45:11356–70. https://doi.org/10.1093/nar/gkx778.
Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. https://doi.org/10.1016/j.molcel.2012.10.015.
Feng C, Liu Y, Wang G, Deng Z, Zhang Q, Wu W, et al. Crystal structures of the human RNA demethylase Alkbh5 reveal basis for substrate recognition. J Biol Chem. 2014;289:11571–83. https://doi.org/10.1074/jbc.M113.546168.
Duan HC, Wei LH, Zhang C, Wang Y, Chen L, Lu Z, et al. ALKBH10B is an RNA N6-methyladenosine demethylase affecting arabidopsis floral transition. Plant Cell. 2017;29:2995–3011. https://doi.org/10.1105/tpc.16.00912.
Wu B, Li L, Huang Y, Ma J, Min J. Readers, writers and erasers of N6-methylated adenosine modification. Curr Opin Struct Biol. 2017;47:67–76. https://doi.org/10.1016/j.sbi.2017.05.011.
Adhikari S, Xiao W, Zhao YL, Yang YG. m(6)A: signaling for mRNA splicing. RNA Biol. 2016;13:756–9. https://doi.org/10.1080/15476286.2016.1201628.
Liao S, Sun H, Xu C. YTH domain: a family of N6-methyladenosine (m6A) readers. Genomics Proteom Bioinform. 2018;16:99–107. https://doi.org/10.1016/j.gpb.2018.04.002.
Patil DP, Pickering BF, Jaffrey SR. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 2018;28:113–27. https://doi.org/10.1016/j.tcb.2017.10.001.
Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, et al. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR44–NOT deadenylase complex. Nat Commun. 2016;7:12626. https://doi.org/10.1038/ncomms12626.
Zhu T, Roundtree IA, Wang P, Wang X, Wang L, Sun C, et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 2014;24:1493–6. https://doi.org/10.1038/cr.2014.152.
Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, et al. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–7. https://doi.org/10.1038/cr.2017.10.
Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27:315–28. https://doi.org/10.1038/cr.2017.15.
Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–19. https://doi.org/10.1016/j.molcel.2016.01.012.
Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927–9. https://doi.org/10.1038/nchembio.1654.
Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, et al. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife. 2017;6:e31311. https://doi.org/10.7554/eLife.31311.
Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–27. https://doi.org/10.1038/cr.2017.99.
Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–308. https://doi.org/10.1016/j.cell.2015.08.011.
Geissler R, Simkin A, Floss D, Patel R, Fogarty EA, Scheller J, et al. A widespread sequence-specific mRNA decay pathway mediated by hnRNPs A1 and A2/B1. Genes Dev. 2016;30:1070–85. https://doi.org/10.1101/gad.277392.116.
Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–63. https://doi.org/10.1093/nar/gkx141.
Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–95. https://doi.org/10.1038/s41556-018-0045-z.
Wu R, Li A, Sun B, Sun JG, Zhang J, Zhang T, et al. A novel m6A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2018;29:23–41. https://doi.org/10.1038/s41422-018-0113-8.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6. https://doi.org/10.1038/nature11112.
Chang M, Lv H, Zhang W, Ma C, He X, Zhao S, et al. Region-specific RNA m6A methylation represents a new layer of control in the gene regulatory network in the mouse brain. Open Biol. 2017. https://doi.org/10.1098/rsob.170166.
Noack F, Calegari F. Epitranscriptomics: a new regulatory mechanism of brain development and function. Front Neurosci. 2018;12:85. https://doi.org/10.3389/fnins.2018.00085.
Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–19. https://doi.org/10.1016/j.stem.2014.09.019.
Liu J, Harada BT, He C. Regulation of gene expression by N6-methyladenosine in cancer. Trends Cell Biol. 2019;29:487–99. https://doi.org/10.1016/j.tcb.2019.02.008.
Maity A, Das B. N6-methyladenosine modification in mRNA: machinery, function and implications for health and diseases. FEBS J. 2016;283:1607–30. https://doi.org/10.1111/febs.13614.
Nainar S, Marshall PR, Tyler CR, Spitale RC, Bredy TW. Evolving insights into RNA modifications and their functional diversity in the brain. Nat Neurosci. 2016;19:1292–8. https://doi.org/10.1038/nn.4378.
Widagdo J, Anggono V. The m6A-epitranscriptomic signature in neurobiology:from neurodevelopment to brain plasticity. J Neurochem. 2018;147:137–52. https://doi.org/10.1111/jnc.14481.
Engel M, Chen A. The emerging role of mRNA methylation in normal and pathological behavior. Genes Brain Behav. 2018;17:e12428. https://doi.org/10.1111/gbb.12428.
Ji P, Wang X, Xie N, Li Y. N6-methyladenosine in RNA and DNA: an epitranscriptomic and epigenetic player implicated in determination of stem cells fate. Stem Cells Int. 2018;2018:3256524. https://doi.org/10.1155/2018/3256524.
Morena F, Argentati C, Bazzucchi M, Emiliani C, Martino S. Above the epitranscriptome: RNA modifications and stem cell identity. Genes. 2018. https://doi.org/10.3390/genes9070329.
Chen J, Wang C, Fei W, Fang X, Hu X. Epitranscriptomic m6A modification in the stem cell field and its effects on cell death and survival. Am J Cancer Res. 2019;9:752–64.
Chen J, Fang X, Zhong P, Song Z, Hu X. N6-methyladenosine modifications: interactions with novel RNA-binding proteins and roles in signal transduction. RNA Biol. 2019;16:991–1000. https://doi.org/10.1080/15476286.2019.1620060.
Yao B, Christian KM, He C, Jin P, Ming GL, Song H. Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci. 2016;17:537–49. https://doi.org/10.1038/nrn.2016.70.
Zhou H, Wang B, Sun H, Xu X, Wang Y. Epigenetic regulations in neural stem cells and neurological diseases. Stem Cells Int. 2018;2018:6087143. https://doi.org/10.1155/2018/6087143.
Boles NC, Temple S. Epimetronomics: m6A marks the tempo of corticogenesis. Neuron. 2017;96:718–20. https://doi.org/10.1016/j.neuron.2017.11.002.
Angelova MT, Dimitrova DG, Dinges N, Lence T, Worpenberg L, Carré C, et al. The emerging field of epitranscriptomics in neurodevelopmental and neuronal disorders. Front Bioeng Biotechnol. 2014;6:46. https://doi.org/10.3389/fbioe.2018.00046.
Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–6. https://doi.org/10.1126/science.1261417.
Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D, et al. Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell. 2017;171:877–89. https://doi.org/10.1016/j.cell.2017.09.003.
Yoon KJ, Vissers C, Ming GL, Song H. Epigenetics and epitranscriptomics in temporal patterning of cortical neural progenitor competence. J Cell Biol. 2018;217:1901–14. https://doi.org/10.1083/jcb.201802117.
Bertero A, Brown S, Madrigal P, Osnato A, Ortmann D, Yiangou L, et al. The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature. 2018;555:256–9. https://doi.org/10.1038/nature25784.
Li M, Zhao X, Wang W, Shi H, Pan Q, Lu Z, et al. Ythdf2-mediated m6A mRNA clearance modulates neural development in mice. Genome Biol. 2018;19:69. https://doi.org/10.1186/s13059-018-1436-y.
Leighton LJ, Ke K, Zajaczkowski EL, Edmunds J, Spitale RC, Bredy TW. Experience-dependent neural plasticity, learning, and memory in the era of epitranscriptomics. Genes Brain Behav. 2018;17:e12426. https://doi.org/10.1111/gbb.12426.
Yoon KJ, Ming GL, Song H. Epitranscriptomes in the adult mammalian brain: dynamic changes regulate behavior. Neuron. 2018;99:243–5. https://doi.org/10.1016/j.neuron.2018.07.019.
Jung Y, Goldman D. Role of RNA modifications in brain and behavior. Genes Brain Behav. 2017;17:e12444. https://doi.org/10.1111/gbb.12444.
Kruttner S, Caroni P. m6A-epitranscriptome modulates memory strength. Cell Res. 2019;29:4–5. https://doi.org/10.1038/s41422-018-0121-8.
Chang M, Lv H, Zhang W, Ma C, He X, Zhao S, et al. Region-specific RNA m6A methylation represents a new layer of control in the gene regulatory network in the mouse brain. Open Biol. 2017. https://doi.org/10.1098/rsob.170166.
Chen X, Yu C, Guo M, Zheng X, Ali S, Huang H, et al. Down-regulation of m6A mRNA methylation is involved in dopaminergic neuronal death. ACS Chem Neurosci. 2019;10:2355–63. https://doi.org/10.1021/acschemneuro.8b00657.
Zhang Z, Wang M, Xie D, Huang Z, Zhang L, Yang Y, et al. METTL3-mediated N6-methyladenosine mRNA modification enhances long-term memory consolidation. Cell Res. 2018;28:1050–61. https://doi.org/10.1038/s41422-018-0092-9.
Koranda JL, Dore L, Shi H, Patel MJ, Vaasjo LO, Rao MN, et al. Mettl14 is essential for epitranscriptomic regulation of striatal function and learning. Neuron. 2018;99:283–92. https://doi.org/10.1016/j.neuron.2018.06.007.
Hess ME, Hess S, Meyer KD, Verhagen LA, Koch L, Brönneke HS, et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci. 2013;16:1042–8. https://doi.org/10.1038/nn.3449.
Bai L, Tang Q, Zou Z, Meng P, Tu B, Xia Y, et al. m6A demethylase FTO regulates dopaminergic neurotransmission deficits caused by arsenite. Toxicol Sci. 2018;165:431–46. https://doi.org/10.1093/toxsci/kfy172.
Walters BJ, Mercaldo V, Gillon CJ, Yip M, Neve RL, Boyce FM, et al. The role of the rna demethylase FTO (fat mass and obesity-associated) and mRNA methylation in hippocampal memory formation. Neuropsychopharmacology. 2017;42:1502–10. https://doi.org/10.1038/npp.2017.31.
Widagdo J, Zhao QY, Kempen MJ, Tan MC, Ratnu VS, Wei W, et al. Experience-dependent accumulation of N6-methyladenosine in the prefrontal cortex is associated with memory processes in mice. J Neurosci. 2016;36:6771–7. https://doi.org/10.1523/JNEUROSCI.4053-15.2016.
Engel M, Eggert C, Kaplick PM, Eder M, Röh S, Tietze L, et al. The role of m6A/m-RNA methylation in stress response regulation. Neuron. 2018;99:389–403. https://doi.org/10.1016/j.neuron.2018.07.009.
Li L, Zang L, Zhang F, Chen J, Shen H, Shu L, et al. Fat mass and obesity-associated (FTO) protein regulates adult neurogenesis. Hum Mol Genet. 2017;26:2398–411. https://doi.org/10.1093/hmg/ddx128.
Shi H, Zhang X, Weng YL, Lu Z, Liu Y, Lu Z, et al. m6A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature. 2018;563:249–53. https://doi.org/10.1038/s41586-018-0666-1.
Ma C, Chang M, Lv H, Zhang ZW, Zhang W, He X, et al. RNA m 6 A methylation participates in regulation of postnatal development of the mouse cerebellum. Genome Biol. 2018;19:68. https://doi.org/10.1186/s13059-018-1435-z.
Frye M, Harada BT, Behm M, He C. RNA modifications modulate gene expression during development. Science. 2018;361:1346–9. https://doi.org/10.1126/science.aau1646.
Wang CX, Cui GS, Liu X, Xu K, Wang M, Zhang XX, et al. METTL3-mediated m6A modification is required for cerebellar development. PLoS Biol. 2018;16:e2004880. https://doi.org/10.1371/journal.pbio.2004880.
Fu Y, Zorman B, Sumazin P, Sanna PP, Repunte-Canonigo V. Epitranscriptomics: correlation of N6-methyladenosine RNA methylation and pathway dysregulation in the hippocampus of HIV transgenic rats. PLoS ONE. 2019;14:e0203566. https://doi.org/10.1371/journal.pone.0203566.
Merkurjev D, Hong WT, Iida K, Oomoto I, Goldie BJ, Yamaguti H, et al. Synaptic N6-methyladenosine (m6A) epitranscriptome reveals functional partitioning of localized transcripts. Nat Neurosci. 2018;21:1004–14. https://doi.org/10.1038/s41593-018-0173-6.
Weng YL, Wang X, An R, Cassin J, Vissers C, Liu Y, et al. Epitranscriptomic m6A regulation of axon regeneration in the adult mammalian nervous system. Neuron. 2018;97:313–25. https://doi.org/10.1016/j.neuron.2017.12.036.
Yu J, Chen M, Huang H, Zhu J, Song H, Zhu J, et al. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res. 2016;46:1412–23. https://doi.org/10.1093/nar/gkx1182.
Zhuang M, Li X, Zhu J, Zhang J, Niu F, Liang F, et al. The m6A reader YTHDF1 regulates axon guidance through translational control of Robo3.1 expression. Nucleic Acids Res. 2019;47:4765–77. https://doi.org/10.1093/nar/gkz157.
Chai RC, Wu F, Wang QX, Zhang S, Zhang KN, Liu YQ, et al. m6A RNA methylation regulators contribute to malignant progression and have clinical prognostic impact in gliomas. Aging. 2018;11:1204–25. https://doi.org/10.18632/aging.101829.
Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, et al. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–34. https://doi.org/10.1016/j.celrep.2017.02.059.
Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V, et al. Essential role of METTL3-mediated m6A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2018;37:522–33. https://doi.org/10.1038/onc.2017.351.
Visvanathan A, Patil V, Abdulla S, Hoheisel JD, Somasundaram K. N6-Methyladenosine landscape of glioma stem-like cells: METTL3 is essential for the expression of actively transcribed genes and sustenance of the oncogenic signaling. Genes. 2019. https://doi.org/10.3390/genes10020141.
Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606. https://doi.org/10.1016/j.ccell.2017.02.013.
Dixit D, Xie Q, Rich JN, Zhao JC. Messenger RNA methylation regulates glioblastoma tumorigenesis. Cancer Cell. 2017;31:474–5. https://doi.org/10.1016/j.ccell.2017.03.010.
Sevgi M, Rigoux L, Kühn AB, Mauer J, Schilbach L, Hess ME, et al. An obesity-predisposing variant of the FTO gene regulates D2R-dependent reward learning. J Neurosci. 2015;35:12584–92. https://doi.org/10.1523/JNEUROSCI.1589-15.2015.
Olivo G, Wiemerslage L, Nilsson EK, Solstrand Dahlberg L, Larsen AL, Olaya Búcaro M, et al. Resting-state brain and the fto obesity risk allele: default mode, sensorimotor, and salience network connectivity underlying different somatosensory integration and reward processing between genotypes. Front Hum Neurosci. 2016;10:52. https://doi.org/10.3389/fnhum.2016.00052.
Ruud J, Alber J, Tokarska A, Engström Ruud L, Nolte H, Biglari N, et al. The fat mass and obesity-associated protein (FTO) regulates locomotor responses to novelty via D2R medium spiny neurons. Cell Rep. 2019;27:3182–98. https://doi.org/10.1016/j.celrep.2019.05.037.
Kühn AB, Feis DL, Schilbach L, Kracht L, Hess ME, Mauer J, et al. FTO gene variant modulates the neural correlates of visual food perception. Neuroimage. 2016;128:21–31. https://doi.org/10.1016/j.neuroimage.2015.12.049.
Dang LC, Samanez-Larkin GR, Smith CT, Castrellon JJ, Perkins SF, Cowan RL, et al. FTO affects food cravings and interacts with age to influence age-related decline in food cravings. Physiol Behav. 2018;192:188–93. https://doi.org/10.1016/j.physbeh.2017.12.013.
Melhorn SJ, Askren MK, Chung WK, Kratz M, Bosch TA, Tyagi V, et al. FTO genotype impacts food intake and corticolimbic activation. Am J Clin Nutr. 2018;107:145–54. https://doi.org/10.1093/ajcn/nqx029.
Wiemerslage L, Nilsson EK, Solstrand Dahlberg L, Ence-Eriksson F, Castillo S, Larsen AL, et al. An obesity-associated risk allele within the FTO gene affects human brain activity for areas important for emotion, impulse control and reward in response to food images. Eur J Neurosci. 2016;43:1173–80. https://doi.org/10.1111/ejn.13177.
Castellini G, Franzago M, Bagnoli S, Lelli L, Balsamo M, Mancini M, et al. Fat mass and obesity-associated gene (FTO) is associated to eating disorders susceptibility and moderates the expression of psychopathological traits. PLoS ONE. 2017;12:e0173560. https://doi.org/10.1371/journal.pone.0173560.
Chuang YF, Tanaka T, Beason-Held LL, An Y, Terracciano A, Sutin AR, et al. FTO genotype and aging: pleiotropic longitudinal effects on adiposity, brain function, impulsivity and diet. Mol Psychiatry. 2015;20:133–9. https://doi.org/10.1038/mp.2014.49.
Lin L, Hales CM, Garber K, Jin P. Fat mass and obesity-associated (FTO) protein interacts with CaMKII and modulates the activity of CREB signaling pathway. Hum Mol Genet. 2014;23:3299–306. https://doi.org/10.1093/hmg/ddu043.
Rosskopf D, Schwahn C, Neumann F, Bornhorst A, Rimmbach C, Mischke M, et al. The growth hormone-IGF-I axis as a mediator for the association between FTO variants and body mass index: results of the Study of Health in Pomerania. Int J Obes. 2011;35:364–72. https://doi.org/10.1038/ijo.2010.158.
Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, et al. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci USA. 2010;107:8404–9. https://doi.org/10.1073/pnas.0910878107.
Melka MG, Gillis J, Bernard M, Abrahamowicz M, Chakravarty MM, Leonard GT, et al. FTO, obesity and the adolescent brain. Hum Mol Genet. 2010;22:1050–8. https://doi.org/10.1093/hmg/dds504.
Choudhry Z, Sengupta SM, Grizenko N, Thakur GA, Fortier ME, Schmitz N, et al. Association between obesity-related gene FTO and ADHD. Obesity. 2013;21:e738–44. https://doi.org/10.1002/oby.20444.
Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. J Alzheimers Dis. 2011;23:461–9. https://doi.org/10.3233/JAD-2010-101068.
Reitz C, Tosto G, Mayeux R, Luchsinger JA, et al. Genetic variants in the fat and obesity associated (FTO) gene and risk of Alzheimer’s disease. PLoS ONE. 2012;7:e50354. https://doi.org/10.1371/journal.pone.0050354.
Li H, Ren Y, Mao K, Hua F, Yang Y, Wei N, et al. FTO is involved in Alzheimer’s disease by targeting TSC1-mTOR-Tau signaling. Biochem Biophys Res Commun. 2018;498:234–9. https://doi.org/10.1016/j.bbrc.2018.02.201.
Samaan Z, Anand SS, Zhang X, Desai D, Rivera M, Pare G, et al. The protective effect of the obesity-associated rs9939609 A variant in fat mass- and obesity-associated gene on depression. Mol Psychiatry. 2013;18:1281–6. https://doi.org/10.1038/mp.2012.160.
Rivera M, Locke AE, Corre T, Czamara D, Wolf C, Ching-Lopez A, et al. Interaction between the FTO gene, body mass index and depression: meta-analysis of 13701 individuals. Br J Psychiatry. 2017;211:70–6. https://doi.org/10.1192/bjp.bp.116.183475.
Acknowledgements
We would like to acknowledge Qixue Li, Fengdi Wu, Minghui Li, Xianchao Du, Bingchen Liu and Haiying Wang for their advice.
Funding
We gratefully acknowledge funding from the Basic Research Fund of Young Program of Higher Education of Liaoning Province (Grant No. QNK201735), the National Natural Science Foundation of China (Grant No. 81302406) and the Funds for Distinguished Young Scientists in School of Public Health, China Medical University.
Author information
Authors and Affiliations
Contributions
Each author substantially contributed to the review. JL: conception and design, drafting the review; XY, ZQ, YS, YL, BX, WL and ZX: revising the manuscript; YD: conception and design, revising it critically for important intellectual content, and final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, J., Yang, X., Qi, Z. et al. The role of mRNA m6A methylation in the nervous system. Cell Biosci 9, 66 (2019). https://doi.org/10.1186/s13578-019-0330-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-019-0330-y