Abstract
Tannases can catalyze the hydrolysis of galloyl ester and depside bonds of hydrolysable tannins to release gallic acid and glucose, but tannases from different species have different substrate specificities. Our prior studies found that tannase from Lactobacillus plantarum (LP-tan) performed a higher esterase activity, while the tannase from Streptomyces sviceus (SS-tan) performed a higher depsidase activity; but the molecular mechanism is not elucidated. Based on the crystal structure of LP-tan and the amino acid sequences alignment between LP-tan and SS-tan, we found that the sandwich structure formed by Ile206-substrate-Pro356 in LP-tan was replaced with Ile253-substrate-Gly384 in SS-tan, and the flap domain (amino acids: 225–247) formed in LP-tan was missed in SS-tan, while a flap-like domain (amino acids: 93–143) was found in SS-tan. In this study, we investigated the functional role of sandwich structure and the flap (flap-like) domain in the substrate specificity of tannase. Site-directed mutagenesis was used to disrupt the sandwich structure in LP-tan (P356G) and rebuilt it in SS-tan (G384P). The flap in LP-tan and the flap-like domain in SS-tan were deleted to construct the new variants. The activity assay results showed that the sandwich and the flap domain can help to catalytic the ester bonds, while the flap-like domain in SS-tan mainly worked on the depside bonds. Enzymatic characterization and kinetics data showed that the sandwich and the flap domain can help to catalytic the ester bonds, while the flap-like domain in SS-tan may worked on the depside bonds.
Similar content being viewed by others
Introduction
Tannins, the fourth abundant plant constituent, existing as water soluble, poly-phenolic compounds is widely distributed in plant kingdom, especially in roots, leaves, fruits, and seeds. Tannins are toxic to many fungi, bacteria, and viruses (Aguilar et al. 2007). However, many microorganisms have developed the ability to grow in the presence of tannins through the induction of secreted enzymes that utilize these compounds as carbon and energy sources (Aguilar et al. 2007). These enzymes are serine hydrolases, including tannin acyl hydrolases (EC 3.1.1.20), commonly referred as tannases. They can catalyze the hydrolysis of ester and depside bonds of hydrolysable tannins to release gallic acid and glucose (Lopes et al. 2018; Rodríguez-Durán et al. 2011). Gallic acid was found to exert an anticancer effect against a variety of cancer cells (Tsai et al. 2018). Tannases also have application potential in the clarification of wine and soft drinks as well as de-tannification of food and animal feed for nutritional improvement (Chamorro et al. 2017; Martins et al. 2016; Li et al. 2018).
At present, the production of secreted tannase for industrial applications involves the utility of either crude or semi-purified enzyme prepared from submerged or solid-state cultures of Aspergillus niger or Aspergillus oryzae fermented in the presence of tannic acid (Aguilar et al. 2007; Varadharajan et al. 2017; Wu et al. 2018). However, low yield and purity, batch variability and a poor understanding of catalytic mechanism of tannase have limited its utility. In the post genomic era, with the identification of new and novel tannase genes from a range of different species, potential opportunities are available to engineer enzymes with high productivity, purity and activities (Curiel et al. 2009; Iwamoto et al. 2008; Noguchi et al. 2007; Sharma and John 2011; Wu et al. 2013, 2015).
In previous works (Ren et al. 2013), we reported the first 3D crystal structure of LP-tan (PDB no: 4JU1, 4J0C, 4J0D, 4J0G, 4J0H, 4J0I, 4J0J, 4J0K). The structure revealed the spatial details of the protein molecule, including its catalytic and substrate binding sites, and reaction mechanism of the tannase. From the complex structures of LP-tan, we found that the esterase and depsidase activities shared the same active center and catalytic mechanisms (Ren et al. 2013). However, LP-tan showed a higher esterase activity and substrate specificity. In another study, we successfully codon-optimized and chemically synthesized the SS-tan encoding gene from Streptomyces sviceus and cloned it into a recombinant prokaryotic heterologous expression system for high-yield tannase production (Wu et al. 2015). Compared to the LP-tan, the SS-tan showed a higher depsidase activity and a lower esterase activity. In order to further investigate the substrate specificity of these two tannases, the amino acid sequences alignment between SS-tan and LP-tan were performed, the results only showed 35% sequence similarity, but all the substrate binding and catalytic triad were conserved.
In this study, our aim is to investigate the substrates specificity of LP-tan and SS-tan. Based on the structures and biochemical data of LP-tan, we have proposed the substrate specificity between these two tannases, which maybe benefit the further industrial applications and modifications of tannase.
Materials and methods
Protein expression and purification
Cloning, expression, and purification of tannases from Lactobacillus plantarum (GeneBank: AB379685.1) and S. sviceus (GeneBank: LK985323.1) have been described elsewhere (Wu et al. 2013, 2015). In brief, the tannase gene was inserted into a C-terminal hexa-histidine tagged protein expression vector pET-43b, and the recombinant vector was transformed into E. coli BL21-DE3 cells (Life Technologies, USA) for protein expression. The cells were grown at 37 °C, 200 rpm/min in 2YT medium until a cell density of 1.0 (OD600nm) reached. Protein expression was induced with the addition of 0.5 mM IPTG at 20 °C, 200 rpm/min, for 20 h. Then the cells were harvested and lysed. The lysate was centrifuged at 20,000 rpm/min for 30 min at 4 °C, and the supernatant was loaded onto a 5 ml HisTrap column (GE Healthcare) equilibrated with the loading buffer containing 20 mM Tris–HCl, 150 mM NaCl, 10 mM imidazole, pH 8.0. The column was subsequently washed and eluted with a similar buffer containing 30 and 300 mM imidazole, respectively. The collected protein was further purified by a gel filtration column (HiLoad 16/60 Superdex 200, GE Healthcare) equilibrated with 20 mM Tris–HCl, 150 mM NaCl, pH 8.0. Purity of the protein was monitored by 12% SDS-PAGE under reducing conditions.
Site-directed mutagenesis
Site-directed mutagenesis was performed using Quick-Change Lightning Site-directed Mutagenesis Kit (Agilent Technologies) with PCR method. Plasmid pET-43b with LP-tan gene and SS-tan gene were used as templates (primer sequences were listed in Table 1). The single point mutated proteins were expressed and purified with the same procedures as the wild-type protein.
New variants construct
In order to investigate the substrate specificity of tannases, two new variants were developed. The variants without the flap domain (amino acid 225–247) in LP-tan and the flap-like domain (amino acid 93–144) in SS-tan were chemically synthesized. The nucleotide sequence GGAGGATCC (amino acids sequence: Gly–Gly-Ser) was used as the linker to replace the flap and flap-like domain. The variants were expressed and purified as mentioned previously.
Enzyme activity assay
Rhodanine reacts only with gallic acid but not with galloyl esters or other phenolics. So the tannase activity was assayed by a method based on chromogen formation between gallic acid and rhodanine (Curiel et al. 2009). The single point mutants and variants were assayed by a method based on chromogen formation between gallic acid and rhodanine. In brief, a standard curve using gallic acid concentrations ranging from 0.125 to 1 mM was prepared. Activities of recombinant tannase were measured using 25 mM methyl gallate (Sigma, USA) and 3 mM tannic acid (Sigma, USA) as substrates.
The reaction conditions with variants were optimized regarding temperature and pH. Activities of recombinant tannase were measured at pH 8.0, temperature ranged from 10 to 70 °C to determine the optimal temperature. The optimum pH value for enzymatic activity was determined at 37 °C by studying its pH-dependence within the pH range from 3 to 10. All the activity assays were performed at the optimum temperature and pH. One unit of activity was defined as the amount of enzyme required to release 1 μM of gallic acid per minute under standard reaction conditions.
Kinetics analysis
The substrates methyl gallate (0.1–5 mM) and tannic acid (0.005–0.04 mM) were incubated with appropriate amount of enzyme to calculate the kinetics (mentioned previously). The amount of gallic acid which was formed by the catalysis of tannase was calibrated using the absorbance at 520 nm. Kinetic parameters were obtained according to the Line-weaver and Burk (double reciprocal) method.
Results
Amino acid sequences alignment and analysis
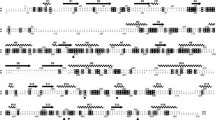
The amino acid sequences alignment only showed 35% sequence similarity between SS-tan and LP-tan, but the substrate binding and catalytic triad were conserved (Fig. 1). Based on the structure analysis of LP-tan, we found that the sandwich structure formed by Ile206-Pro356 in LP-tan was replaced with Ile253-Gly384 in SS-tan, and the flap domain formed in LP-tan was missed in the SS-tan, while an additional flap-like sequence (93–144) was found in the SS-tan.
Amino acid sequence alignment of tannase from L. plantarum and S. sviceus. The secondary structure elements of LP-tan are indicated. The conserved residues of the catalytic triad are indicated by red rectangle. The flap part of LP-tan is indicated by black rectangle. The residues of the catalytic triad are indicated by red triangles and those involved in substrates binging are indicated by black triangles. The flap-like domain of SS-tan is indicated by red rectangle. The black dot indicated the mutation of sandwich structure
Enzymatic characterization of the variants and mutants
The single point mutants and the variants were well expressed in E. coli BL21-DE3 cells and expressed at high levels. Including the C-terminal hexa-histidine tag, SDS-page showed that SS-tan variant showed a molecular mass of about 51 kDa, and LP-tan variant showed a molecular mass of about 49 kDa (Fig. 2a). SS-tan variant and LP-tan variant also displayed maximum activity at pH 8.0, and the optimum temperature for SS-tan variant was 50 °C, LP-variant was 30 °C, which is similar with the wild type (Fig. 3).
a The purified tannases were analyzed with 12% SDS-PAGE. M: molecular mass of standards; (1) LP-tan; (2) LP-tan variant; (3) SS-tan; (4) SS-tan variant. b Activities of site-directed mutagenesis of SS-tan relative to wild-type of SS-tan. Tannic acid was used as the substrate and each measurement was performed in quintuplicate
The sandwich structure was dismissed or rebuilt by site-directed mutagenesis in LP-tan and SS-tan. The single point mutant LP-tan P356G only had 48.2% esterase activity left comparing with LP-tan, while the single point mutant LP-tan P356G had a higher depsidase activity (131.7%). Comparing with SS-tan, SS-tan G384P had a higher esterase activity (138.1%) and a lower depsidase activity (61.1%) (Table 2). The SS-tan variant showed a lower (55.3%) depsidase activity and a lower (91.2%) esterase activity comparing with the wild type. In addition, the variant from LP-tan had a higher (244.7%) depsidase activity and a lower (67.6%) esterase activity (Table 3).
Kinetic analysis on substrate preference
The Kcat and Km value were always used to compare the substrate specificity of enzymes. SS-tan showed a higher substrate affinity (lower Km value) and catalytic efficiency (high Kcat/Km value) for depside bonds than ester bonds, while the LP-tan showed a higher substrate affinity and catalytic efficiency for ester bonds than depside bonds (Tables 2, 3).
The Km values of LP-tan P356G for methyl gallate and tannic acid was about twofold higher and 1.8-fold smaller than the corresponding Km values of LP-tan, respectively. Compared with the wild type, LP-tan P356G had a smaller kcat/Km value (about 2.4-fold) when methyl gallate was used as the substrate and a higher kcat/Km value (about 1.4-fold) when tannic acid was used as the substrate. In opposite, the Km values of SS-tan G384P for methyl gallate and tannic acid were about 1.4-fold smaller and twofold higher than those values of the wild type, respectively (Table 2). When methyl gallate was used as the substrate, the corresponding kcat/Km value of SS-tan G384P was about 1.4-fold higher than SS-tan. While the corresponding kcat/Km value of SS-tan G384P for tannic acid was about 1.6-fold smaller than SS-tan. Therefore, the sandwich structure could help to bind and catalyse the hydrolysis of ester bond.
Compared with SS-tan, the SS-tan variants showed higher Km value and lower kcat/Km value (about 1.6-fold) when using tannic acid as the substrate, but the Km and kcat/Km values for methyl gallate had no significant changes (only about 1.1 fold) (Table 3). The Km value of LP-tan variants for tannic acid and methyl gallate was about 2.4-fold smaller and 1.7-fold higher than the corresponding Km values of LP-tan, respectively. The kcat/Km values of LP-tan variants for methyl gallate and tannic acid were about 1.7-fold lower and 2.6-fold higher than the wild type, respectively (Table 3).
Discussion
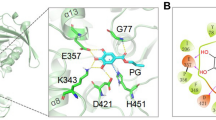
Tannase can catalyse the hydrolysis of ester and depside bond in hydrolysable tannins to release glucose and gallic acid. Thus, tannase was crucial in the industries of foods, drinks and pharmaceutics (Chamorro et al. 2017; Lekha and Lonsane 1997; Li et al. 2018; Martins et al. 2016). In the past decades, both the high-yield production of tannase and the high activity conservation were considered as the research priorities. Since the discovery of tannase, its esterase and depsidase activities have been in debate for a long time (Haslam and Stangroom 1966). However, only few reports described the catalytic mechanism and substrate specificity of tannase. In the previous works, our group reported the crystal structure of LP-tan, which was the first tannase 3D structure (Ren et al. 2013). Based on the apo and complexes structures of LP-tan, the hydrolysis mechanism of tannase was explained. Mutagenesis studies demonstrated that the esterase and depsidase activities of LP-tan shared the same catalytic site. When LP-tan binding the substrates, two hydrogen-bond binding networks were observed, the first network was formed between the amino acids G77, A164, S163, H451 and the carboxyl group of galloyl unit of the substrates; the other hydrogen-bonding network was formed between amino acids E357, K343, P421 and the hydroxyl groups of the galloyl unit of the substrates (Ren et al. 2013). The hydrogen-bonding networks could help to bind the substrates and were necessary for the catalytic.
According to the amino acid alignment, the substrate binding and catalytic triad were conserved in SS-tan (Fig. 1). Site-directed mutagenesis of each residue in the catalytic center (Ser210A) and hydrogen-bonding networks (K371A, E385A, D453A, D455A, H485A) resulted in an almost complete abolished in esterase and depsidase activities of SS-tan (Fig. 2b). Therefore, the esterase and depsidase activities of SS-tan also shared the same catalytic site and mechanism. However, these two enzymes possess different substrate specificity; LP-tan had a higher esterase activity while the SS-tan had a higher depsidase activity (Table 2). Based on the complex structures of LP-tan, the galloyl unit of the substrate was almost buried by the amino acids Gly77 and Pro356, which then combined with the amino acid Ile206 to form the sandwich-like structure to stabilize the bind of galloyl unit. However, the sandwich structure was not found in the SS-tan, because the Pro356 used to form the sandwich structure in LP-tan was replaced with Gly384 at the same position in SS-tan.
To investigate the function of the sandwich structure in the substrate specificity, we mutated Pro356 to Gly356 (LP-tan P356G) in LP-tan and mutated Gly384 to Pro384 in SS-tan (SS-tan G384P). Compared with the wild type, LP-tan P356G showed significantly higher substrate affinity and catalytic efficiency for depside bonds, while SS-tan G384P showed a higher affinity and catalytic efficiency for esterase bonds (Tables 2, 3). The galloyl unit was the only clear part that could be observed from the electron density map in the complex structure of LP-tan, which means that the substrates had high complexity and flexibility. Therefore, the sandwich structure of tannase might have dual functions. When tannase digested small substrates, like methyl gallate (ester bond), hydrogen-bonding network was formed to bind the substrates, and the amino acid proline in sandwich structure could help to stabilize the binding the galloyl unit of substrates. When tannase digested large substrates, like tannic acid (depside bond), hydrogen-bonding network also formed in the activity center with the galloyl unit. However, due to the complexity and flexibility of the substrates, the proline in the sandwich structure might form hydrophobic reactions with the other galloyl units to prevent the substrates from binding to the activity center.
LP-tan displays α/β structure, featured by a large cap domain inserted into the classical serine hydrolase fold, which was familiar with the feruloyl esterase and lipases (Hermoso et al. 2004). The lid/flap in lipases showed a simple rigid-body shift to expose the activity centre, while no obvious conformational changes were found in feruloyl esterases (Ren et al. 2013; Suzuki et al. 2014). The flap in LP-tan worked to guide the substrate entering the catalytic site and strengthen the binding of the substrates, but no major conformational changes were found (Ren et al. 2013). Further studies found that the amino acid residues (His227, Tyr229, Pro231, Pro241, Phe243 and Pro245) of the flap can form hydrophobic interactions with the phenolic rings of substrates. Meanwhile, the hydrophilic and charged residues (Arg228, Lys237, Lys240, and Lys242) of the flap may form hydrogen bonds with the hydroxyl groups of substrates (Ren et al. 2013; Suzuki et al. 2014).
Compared with the wild type, LP-tan variants showed a higher catalytic efficiency to depside bond and a lower catalytic efficiency to ester bond, while the SS-tan variants showed a lower affinity and catalytic efficiency to tannic acid, but has no obvious changes to methyl gallate (Table 3). The activity assay also showed the similar results (Table 2). Based on these results, we can suggest that the flap in LP-tan can help to bind the small substrates, like methyl gallate; but the substrates with two or more than two aromatic rings, the extra aromatic rings out the activity center may form hydrophobic interactions to prevent the binding.
For SS-tan, the flap-like domain only worked on the binding of tannic acid. So the flap-like domain in SS-tan may has a flexible structure like the esterases, when SS-tan binds large substrates like tannic acid, the flap-like domain might tend to shift away to expose the catalytic site to accommodate the large substrates and the amino acids in the flap-like domain might facilitate the hydrophobic and hydrogen bond interactions with the substrates, which combined with the two hydrogen bond networks to stabilize the binding. While the enzyme binds small substrates like methyl gallate, the flap-like domain might tend to hang above the catalytic center and forge to strengthen the small substrates binding. Such a finding maybe help to guide the application of tannases and provide the theoretical basis for the modification for tannases.
Change history
19 April 2019
The Editor-in-Chief has retracted this article (Wang et al. 2018) because the authors do not have ownership of the data they report. An investigation by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) has concluded that the data reported in this article are the sole property of the CSIRO. Mingbo Wu agrees with this retraction. Dan Wang, Yao Liu, Die Lv, Xueli Hu, Qiumei Zhong and Ye Zhao have not responded to correspondence about this retraction.
References
Aguilar CN, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan LA, Ramírez-Coronel A, Contreras-Esquivel JC (2007) Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol 76:47–59
Chamorro S, Viveros A, Rebolé A, Arija I, Romero C, Alvarez I, Rey A, Brenes A (2017) Addition of exogenous enzymes to diets containing grape pomace: effects on intestinal utilization of catechins and antioxidant status of chickens. Food Res Int 96:226–234
Curiel JA, Rodríguez H, Acebrón I, Mancheño JM, de las Rivas B, Muñoz R (2009) Production and physicochemical properties of recombinant Lactobacillus plantarum tannase. J Agric Food Chem 57:6224–6230
Haslam E, Stangroom JE (1966) The esterase and depsidase activities of tannase. Biochem J 99:28–31
Hermoso JA, Sanzaparicio J, Molina R, Juge N, González R, Faulds CB (2004) The crystal structure of feruloyl esterase a from Aspergillus niger suggests evolutive functional convergence in feruloyl esterase family. J Mol Biol 338(3):495–506
Iwamoto K, Tsuruta H, Nishitaini Y, Osawa R (2008) Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from Lactobacillus plantarum ATCC 14917(T). Syst Appl Microbiol 31:269–277
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase: state of the art. Adv Appl Microbiol 44:215–260
Li R, Fu FG, Liu C, Wan Y, Wang S, Liu T (2018) Tannase immobilisation by amino-functionalised magnetic Fe3O4-chitosan nanoparticles and its application in tea infusion. Int J Biol Macromol 114:1134–1143
Lopes LMM, Costa Batista LH, Gouveia MJ, Leite TCC, de Mello MRF, de Assis SA, de Sena AR (2018) Kinetic and thermodynamic parameters, and partial characterization of the crude extract of tannase produced by Saccharomyces cerevisiae CCMB 520. Nat Prod Res 32(9):1068–1075
Martins IM, Roberto BS, Blumberg JB, Chen CO, Macedo GA (2016) Enzymatic biotransformation of polyphenolics increases antioxidant activity of red and white grape pomace. Food Res Int 89(Pt 1):533–539
Noguchi N, Ohashi T, Shiratori T, Narui K, Hagiwara T, Ko M, Watanabe K, Miyahara T, Taira S, Moriyasu F, Sasatsu M (2007) Association of tannase-producing Staphylococcus lugdunensis with colon cancer and characterization of a novel tannase gene. J Gastroenterol 42:346–351
Ren B, Wu M, Wang Q, Peng X, Wen H, McKinstry WJ, Chen Q (2013) Crystal structure of tannase from Lactobacillus plantarum. J Mol Biol 425:2737–2751
Rodríguez-Durán LV, Valdivia-Urdiales B, Contreras-Esquivel JC, Rodríguez-Herrera R, Aguilar CN (2011) Novel strategies for upstream and downstream processing of tannin acyl hydrolase. Enzyme Res 1:823619
Sharma KP, John PJ (2011) Purification and characterization of tannase and tannase gene from Enterobacter sp. Process Biochem 46:240–244
Suzuki K, Hori A, Kawamoto K, Thangudu RR, Ishida T, Igarashi K, Samejima M, Yamada C, Arakawa T, Wakagi T, Koseki T, Fushinobu S (2014) Crystal structure of a feruloyl esterase belonging to the tannase family: a disulfide bond near a catalytic triad. Proteins 82(10):2857–2867
Tsai CL, Chiu YM, Ho TY, Hsieh CT, Shieh DC, Lee YJ, Tsay GJ, Wu YY (2018) Gallic acid induces apoptosis in human gastric adenocarcinoma cells. Anticancer Res 38(4):2057–2067
Varadharajan V, Vadivel SS, Ramaswamy A, Sundharamurthy V, Chandrasekar P (2017) Modeling and verification of process parameters for the production of tannase by Aspergillus oryzae under submerged fermentation using agro-wastes. Biotechnol Appl Biochem 64(1):100–109
Wu M, Peng X, Wen H, Wang Q, Chen Q, McKinstry WJ, Ren B (2013) Expression, purification, crystallization and preliminary X-ray analysis of tannase from Lactobacillus plantarum. Acta Crystallogr Sect F Struct Biol Cryst Commun 69:456–459
Wu M, Wang Q, McKinstry WJ, Ren B (2015) Characterization of a tannin acyl hydrolase from Streptomyces sviceus with substrate preference for digalloyl ester bonds. Appl Microbiol Biotechnol 99(6):2663–2672
Wu C, Zhang F, Li L, Jiang Z, Ni H, Xiao A (2018) Novel optimization strategy for tannase production through a modified solid-state fermentation system. Biotechnol Biofuels 11:92
Authors’ contributions
WD, LY and LD performed out the whole study and participated in its design. HX and ZQ participated in the experiments. WM and ZY designed the experiments and wrote the manuscript. WM was responsible for initiation and supervision of the study. All authors read and approved the final manuscript.
Acknowledgements
This study was founded by Scientific Research Project of the Sichuan Province Education Department (18ZB0152), Key Scientific Research Project of the Sichuan Province Education Department (16CZ0028,16ZA0287), Natural Science Foundation of Chengdu Medical College (15Z106), State Undergraduate Innovative Experiment Program (508-2033020, 508-2019023), Application and Basic Project of Sichuan provincial Science and Technology Department (2018JY0208), Scientific and Technological Support Project of Sichuan Provincial Science and Technology Department (2016GZ0364), Scientific Research Project of Sichuan Provincial Health Department (18PJ586, 18PJ006) and all support is gratefully acknowledged.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data supporting the conclusions are presented in the main article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The Editor-in Chief has retracted this article because the authors do not have ownership of the data they report. An investigation by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) has concluded that the data reported in this article are the sole property of the CSIRO. Mingbo Wu agrees with this retraction. Dan Wang, Yao Liu, Die Lv, Xueli Hu, Qiumei Zhong and Ye Zhao have not responded to correspondence about this retraction.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, D., Liu, Y., Lv, D. et al. RETRACTED ARTICLE: Substrates specificity of tannase from Streptomyces sviceus and Lactobacillus plantarum. AMB Expr 8, 147 (2018). https://doi.org/10.1186/s13568-018-0677-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-018-0677-1