Abstract
Porcine circovirus type 4 (PCV4) is a newly emerging virus, with both PCV4 genomic DNA and specific antibodies detected in swine herds in several provinces in China and South Korea. Although the virus was first identified in 2019 in Hunan, China, retrospective research suggests that serum samples collected as early as 2008 were positive for PCV4 antibody. Infections with only PCV4 or co-infections with other pathogens have been associated with several clinical manifestations, but its pathogenesis remains to be determined. The purpose of this review was the following: (1) to characterize PCV4 epidemiology by assessing evolutionary dynamics and genetic diversity of PCV4 strains circulating in swine herds; (2) to reconstruct a computerized 3D model to analyze PCV4 Cap properties; (3) and to summarize the current evidence of PCV4-associated clinical-pathological manifestations. The origin of PCV4 is apparently distinct from other PCV, based on analysis of phylogenetic trees. Of note, PCV4 shares an ancient common ancestor with mink circoviruses. Furthermore, the amino acid residue at position 27 of the PCV4 Cap is a key benchmark to distinguish PCV4a (27S) from PCV4b (27 N), based on PCV4 strains currently available, and variation of this residue may alter Cap antigenicity. In addition, the capsid surface of PCV4 has characteristics of increased polar residues, compared to PCV2, which raises the possibility that PCV4 may target negatively charged host receptors to promote virus infection. Further studies are required, including virus isolation and culture, and more detailed characterization of molecular epidemiology and genetic diversity of PCV4 in swine herds.
Similar content being viewed by others
1 Introduction
Porcine circoviruses (PCV) are members of the Circovirus genus in the Circoviridae family, characterized as non-enveloped viruses composed of a circular, single-stranded genomic DNA within an icosahedral capsid, ~17 nm in diameter [1,2,3]. To date, there are four identified types: PCV1, PCV2, PCV3, and a novel PCV4.
PCV4, a newly emerging circovirus, was first identified in 2019 in Hunan, China, in pigs with several clinical disease syndromes, including respiratory and enteric signs as well as porcine dermatitis nephropathy syndrome (PDNS) [3]. However, in retrospective studies, PCV4 genomic DNA was detected in swine tissue samples collected in 2012 from Henan, China [4], with some serum samples collected as early as 2008 from Chinese swine positive for PCV4 antibody [5]. Therefore, there is evidence that PCV4 has been present and circulating in swine herds for more than a decade.
The genome of PCV4 contains 1770 bases and a palindrome stem-loop structure, with the conserved nonanucleotide (CAGTATTAC) located within the intergenic region between two major open reading frames (ORF) (Figure 1). PCV4 has high nucleotide identity (66.9%) to mink circovirus, but low identities (43.2–51.5%) to other PCV [3]. ORF1 encodes the replicase protein (Rep) and the length of PCV4 ORF1 sequences differs from PCV1 and PCV2. In that regard, whereas ORF2 encodes the capsid protein (Cap), the length of PCV4 ORF2 sequences differs among PCV1, PCV2 and PCV3. Alignment of Cap sequences revealed that PCV4 has low identities with PCV1, PCV2 and PCV3 (~43.1, 45 and 24.5%, respectively) [3]. Cap is the sole structural protein of PCV, with a vital role in clathrin-mediated endocytosis, and actin- and small GTPase-dependent pathways for virus cell entry into host cells, as determined in a study with PCV2 [6, 7]. Additionally, it is noteworthy that Cap mutations cause antigenic drifts and potentially enable PCV2 and PCV3 to evade immunity [8, 9]. Evolutionary pressures driving mutations may enable the virus to generate resistance to antiviral treatment, evade host immune responses, and facilitate its adaptation to the environment and hosts. Thus, elucidating the evolutionary dynamics of Cap is key to understanding this emerging PCV4.
Genomic characterization of PCV4. The PCV4 genome, a single-stranded circular DNA genome with 1770 nt, contains two major ORF that differ from those in PCV1, PCV2 and PCV3. However, the stem-loop of PCV4 has a conserved 9-nt nonanucleotide sequence (CAGTATTAC) located within the intergenic region between ORF1 and ORF2.
The purpose of this review was the following: (1) to characterize PCV4 epidemiology by assessing evolutionary dynamics and genetic diversity of PCV4 strains circulating in swine herds; (2) to reconstruct a computerized 3D model to analyze PCV4 Cap properties; and (3) to summarize clinical diseases associated with PCV4 infection.
2 Evolution and genetic diversity of PCV4
2.1 Evolutionary changes
Although PCV4 was discovered in 2019 in the Hunan province of China, retrospective studies demonstrated that PCV4 DNA was present in swine samples collected in 2012 [4], implying PCV4 has been circulating in pigs for at least a decade. Since 2019, PCV4 has been reported in several provinces of China, including Hunan, Henan, Jiangsu, Anhui, Shanxi, Guangxi, and Inner Mongolia [3, 4, 10,11,12,13,14]. Furthermore, PCV4 was also detected in domestic swine in Korea [15, 16]. Notwithstanding, based on failure to detect PCV4 DNA in swine samples (sera and tissues) from Europe (Italy and Spain) [17], it seems PCV4 appears to have a limited geographic distribution. Thus, distribution of PCV4 in other geographic regions requires further study.
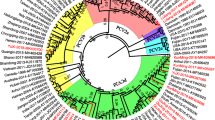
For a better understanding of the evolutionary origin of PCV4, we analyzed a dataset that included sequences from 35 PCV4 and other circoviruses from a variety of host species. RDP4 recombination analysis software was used to detect gene recombination, but there was no evidence of recombination. Sequences were aligned with the Clustal W method conducted in MEGA 7. To trace the origin of PCV, a phylogenetic tree was reconstructed, based on Rep amino acid sequences, using the neighbor joining (NJ) and maximum likelihood (ML) methods. Based on phylogenetic trees, we inferred that PCV1 and PCV2 are closely related to bat-clade 2 circoviruses, whereas PCV3 is closely related to bat-clade 1 circoviruses, indicating a potential bat circovirus origin (Figure 2). However, PCV4 has a distinct origin from other PCV (Figure 2). All PCV4 strains were closely related to mink circoviruses (NJ tree bootstrap = 1.0, ML tree bootstrap = 0.99). The NJ and ML trees had similar topology, emphasizing that PCV4 shares a common ancient ancestor with mink circoviruses, providing evidence that it is reasonable to trace the origin of PCV based on the conserved Rep protein.
Phylogenetic trees of circoviruses, using conserved Rep amino acid sequences. The analysis contained 93 amino acid sequences. Neighbor joining (A) and maximum likelihood (B) trees were reconstructed using the p-distance and Jones-Taylor-Thornton (JTT) model, respectively, with 1000 bootstrap replicates and bootstrap >50%.
2.2 Genetic analysis
Based on complete genomes, rep or cap genes of all the 35 PCV4 sequences deposited in GenBank, phylogenetic trees were constructed, and PCV4 was divided into 2 temporary genotypes: PCV4a and PCV4b [18]. PCV4a contained 29 sequences collected from 2012 to 2021, whereas PCV4b only included 6 PCV4 sequences from 2017 to 2020. To better understand differences between the two genotypes, sequence alignments revealed that residues at positions 4, 155 and 228 in ORF1 and residues at positions 15, 27 and 138 in ORF2 were mutation hot-spots (Figure 3).
Phylogenetic trees of PCV4, based on the complete genome. A neighbor joining tree was reconstructed using p-distance model with 1000 bootstrap replicates and bootstrap >50%. Amino acid sequences of putative ORF1 and ORF2 were aligned with the ESPript 3.0 online tool, and the hot-spots of mutations are represented by the columns with their corresponding positions.
It is noteworthy that two-point mutations (R15W and S27N) occur in the putative nuclear localization signal (NLS) region of the PCV4 Cap, an arginine-rich region within the circovirus genus, which may be implicated in the package of the viral genome [15, 19]. Recently, it was reported that residue 1–20 of NLS is responsible for nucleolar localization of PCV4 Cap. Furthermore, the Cap is capable of directly interacting with nucleolar phosphoprotein nucleophosmin-1 (NPM1) [20]. The positively charged NLS remains buried in the internal surface of the PCV2 capsid, which may externalize in the metastable capsid during viral “breathing” [19]. Nevertheless, our previous study revealed that the peptide containing residues 1–17 of PCV2 NLS (defined NLS-A) was rapidly internalized via direct translocation by increased membrane permeability during cellular uptake [21]. Additionally, the positively charged residue (R) of PCV2 NLS-A changed to an uncharged residue (A), which significantly decreased membrane permeability and inhibited viral entry into cells (unpublished data). Thus, it was predicted that the mutation of R15W may affect PCV4 NLS functionality [18], although effects on membrane permeability remain to be determined. At position 27, a polar residue (27S) was changed to a similar polar one (N). Of note, the residue at position 27 was a critical site to distinguish PCV4a (27S) from PCV4b (27 N), based on all PCV4 sequences deposited in GenBank (Figure 3). Furthermore, epitope prediction suggests that the NLS contained a potential linear B cell epitope (5–35 aa). Furthermore, a previous study revealed that an epitope (26–36 aa) within the NLS of the PCV2 Cap, is a critical B cell epitope capable of eliciting neutralization antibody against PCV2 infection [22]. Therefore, mutations occurring at the two sites (15 and 27) of the NLS may alter antigenicity of the PCV4 Cap. However, potential immunogenic changes due to variations of the Cap NLS between PCV4a and PCV4b remain to be determined.
3 Analysis of PCV4 Cap properties
The Cap is the sole structural protein, capable of eliciting robust immune responses and regarded as the major target antigen for PCV4 serological diagnosis and vaccine design [23]. Since the three-dimensional (3D) structure of PCV4 Cap has not been resolved, we modeled the 3D structure of the PCV4 Cap via homology modeling based on PCV2 Cap structure (PDB ID: 3R0R) in the SWISS-MODEL, as described [23]. There was a typical jelly-roll structure in the PCV4 Cap, although surface-exposed loops were distinctly different between PCV2 and PCV4, similar to the PCV2 Cap [23]. As a non-enveloped virus, the Cap has a vital role in entry of the virus into cells. PCV2 enters host cells via attachment of the PCV2 icosahedral capsid to the moiety of heparan sulfate (HS) or chondroitin sulfate B (CSB) on the cell surface [24]. Residues responsible for binding to HS or CSB have been resolved by Reza Khayat’s group and, interestingly, are discontinuously distributed on the capsid surface [25]. Based on our analysis of electrostatic properties, PCV4 has 14 polar residues on the capsid surface, including seven positively charged residues (R and K) and eight polar amino acid residues (N and Q) (Figure 4A), with more polar residues than PCV2 (Figure 4B). In general, residues on the outer surface of the Cap are locations where the Cap interacts with the environment (e.g., cell receptors). Therefore, a better environment is created for receptor binding by hydrogen bonds, increasing its polarity by increasing polar residues on PCV4 capsid surface. Although the receptor and cell entry mechanism of PCV4 are unknown, we reported that PCV4 virus-like particles (VLP) can efficiently enter porcine cell lines (PK15 and 3D4/21) [23]. Thus, we hypothesized that PCV4 may have evolved with increasing polar residues via nonspecific interactions of the virus with negatively charged HS, CSB or perhaps other cell membrane receptors during PCV4 infection.
3D structures of PCV2 and PCV4 Cap and capsid. The 3D structure of PCV4 Cap was generated via homology modeling, using the PCV2 Cap as a template (PDB ID: 3R0R). 3D structures of Cap and capsid were displayed with Pymol version 1.8.0.3. Electrostatic surface analysis of Cap monomer (far left) was computed in Chimera. The positively charged and polar amino acid residues of the Cap monomer (center) and capsid (far right) were indicated by light blue and red, respectively.
4 Epidemiology of PCV4 and clinical diseases associated with this virus
Since the first discovery of PCV4 in the Hunan province of China, there have been several serological surveys and molecular epidemiology reports. PCV4 was detected in pigs of various age groups and in several tissues, as co-infections with other pathogens, and with a variety of clinical signs, as summarized in Table 1. To date, PCV4 genomic DNA has been detected in pigs in several provinces in China, as well as in South Korea, with positive rates ranging from 1.6 to 45.39% [4, 10]. PCV4 can infect almost all ages of pigs, including sucking, weanling, and fattening pigs, as well as sows and fetuses [4, 10, 11, 15]. Additionally, PCV4 genome has been detected in sera and other tissues, including heart, liver, spleen, lung, kidney, lymph node, tonsil, intestine, and brain. Thus, we inferred that PCV4 has a wide tissue tropism, facilitating both horizontal and vertical transmission. Interestingly, PCV4 remains geographically confined to Asia (China and Korea), but it has not been detected in Europe. Several animals (mice, dogs and cattle) may serve as reservoirs for PCV, with potential for cross-species transmission of PCV to swine [26]. However, currently, it is not clear whether intermediate hosts are involved in transmission of PCV4 to swine herds. Therefore, further monitoring and a better understanding of the molecular epidemiology of PCV4 in potential intermediate hosts should provide new insights into the limited PCV4 geographic distribution and assist in controlling PCV4 transmission.
In a serological survey of 1790 serum samples collected from pigs in 17 provinces of China between 2008 and 2020, overall seroprevalence of PCV4 was 43.97% [5]. PCV4 Rep-specific antibodies were detected in sera from pigs of various ages, with the highest prevalence (67.8%) in sows, and the earliest evidence from a sample collected in 2008 [5]. In another serological survey of 1048 pig serum samples collected from Jiangsu province of China between 2018 and 2021, 3.44% of samples had PCV4 Cap-specific antibodies [27]. Although these serological studies provided insights into the prevalence of PCV4 in pigs, dynamics of viremia and antibody responses to PCV4 infections in swine herds require further investigation.
Pathogenesis of PCV4 is not yet well established. So far, PCV4-associated clinical manifestations have been described in various age groups of pigs, including PDNS and respiratory symptoms [3, 4, 12], postweaning multisystemic wasting syndrome (PMWS), neurological symptoms and diarrhea [4, 11], enteritis and encephalitis [4], and skin disease [13]. In addition, PCV4 may also cause reproductive dysfunction via vertical transmission, boosting its prevalence in aborted fetuses and sows, and implying an association with reproductive failure [4, 15]. Of note, co-infections of PCV4 with other PCV are common in swine herds. PCV2 causes PMWS and PDNS [1], and PCV3 may be associated with PDNS and reproductive failure [2, 28]. Co-infections of PCV4 with PCV2, PCV3, or both, have been described in pigs with clinical signs of PMWS, PDNS and reproductive failure [4, 11, 12]. Moreover, the correlation between PCV4 virus titers and clinical manifestations in pigs may be helpful to elucidate PCV4 pathogenesis. Two groups demonstrated that PCV4 produces a moderate viral load [15, 18]. Furthermore, the positive rate and viral DNA loads of PCV2 and PCV4 are higher in spleen and lymph nodes than other tissues [18], indicating PCV2 targets lymphoid tissues and causes lymphocyte depletion, consistent with previous studies [1]. Based on the similar immune tissue tropism between PCV4 and PCV2, PCV4 may act as a co-factor, or independently cause subclinical infections in pigs with moderate viral loads. In addition, there are reports of co-infection of PCV4 with pseudorabies virus (PRV), porcine epidemic diarrhea virus (PEDV) and porcine reproductive and respiratory syndrome virus (PRRSV) in pigs with neurological symptoms, encephalitis, diarrhea, and enteritis [4, 11]. Based on current evidence, PCV4 is often detected in co-infections with other viral pathogens that make it difficult to elucidate the role of PCV4 in disease pathogenesis. Thus, isolating this virus or rescuing PCV4 using infectious clones will provide new insight. Very recently, PCV4 was successfully rescued from an infectious clone of infected piglets, leading to visible pathological changes in several organs in PCV4-inoculated piglets [29]. In addition, PCV4 viremia, PCV4-specific antibody and up-regulated cytokines were detected in PCV4-inoculated piglets. These results implied that PCV4 is pathogenic in piglets, which may pose a great threat to the swine industry.
PCV4 can be detected with several diagnostic methods, including polymerase chain reaction (PCR), real-time PCR, and indirect enzyme-linked immunosorbent assay (ELISA). Nevertheless, immunochemistry or in situ hybridization should be used to detect virus-specific antigen or viral RNA expression in various tissues and its associated histopathology. Applying these diagnostic techniques to PCV4 would promote the understanding of the pathogenesis and molecular epidemiology of this emerging porcine circovirus.
5 Future perspectives
As four distinct PCV are circulating in swine herds worldwide, exploring serologic cross-reactivity of PCV4 with other PCV is important to establish reliable serological diagnostic methods. Two studies demonstrated that the established PCV4 ELISA, based on Rep or Cap, had no serological cross-reactions with positive sera of other PCV [5, 27]. Moreover, our previous study also demonstrated an absence of cross-reactions between PCV4 and either PCV2 or PCV3 [23]. VLP, morphologically and immunogenically similar to their native viruses, are widely used for novel vaccine design and serological diagnosis. VLP, assembled by a number of subunits (e.g., PCV2 VLP are assembled from 60 Cap subunits), contain multivalent epitopes (i.e., conformational epitopes), with higher avidity to antibodies compared to the subunit protein. Therefore, VLP are advantageous as an antigen for serological diagnostic tests. PCV4 VLP, prepared in E. coli, were highly immunogenic in vivo, and can be used as candidate vaccine and research tools for PCV4, as we reported [23]. Thus, PCV4 VLP have much potential for the development of serological diagnostics for PCV4. In addition, Misinzo et al. used PCV2 VLP to investigate the effects of HS or CSB on binding of PCV2 VLP to host cells [24]. Due to the lack of an efficient cell cultivation method for PCV4, the use of PCV4 VLP has great potential to further characterize this virus. That PCV4 VLP can be produced with high purity and yields make them a preferred tool instead of virus to study cellular receptors during early stages of virus attachment.
As co-infections of PCV4 with other PCV are common in swine herds, there is rationale for developing multivalent PCV combinations to protect against these co-infections of PCV. Based on sequence alignment, PCV4 had low identities (<50%) with three other PCV Reps and Caps. Nevertheless, PCV4 had identities of ~43–48% with PCV1 or PCV2 Reps and Caps, and it is important to analyze the conserved epitopes among the 4 distinct PCV for vaccine design or antiviral strategies against PCV co-infections; However, whether these PCV share conserved epitopes requires further investigation.
6 Conclusion
PCV4 is a potential pathogen associated with several clinical signs or syndromes, including PDNS, respiratory or enteric diseases and reproductive failures. This virus has been detected in almost all porcine tissues, particularly in spleen and lymph nodes. Moreover, co-infection of PCV4 with other PCV or pathogens is common in pigs. Therefore, we should focus on monitoring the prevalence and co-infection of PCV4 with other pathogens, as well as continue to closely monitor dynamic changes in genetic diversity and molecular epidemiology of dominant PCV4 strains. Meanwhile, as the exact pathogenesis of PCV4 remains to be elucidated, virus isolation of PCV4 in clinical samples or PCV4 rescued using infectious clones should provide more insight to better elucidate PCV4 pathogenesis and associated diseases.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
References
Meng XJ (2013) Porcine circovirus type 2 (PCV2): pathogenesis and interaction with the immune system. Annu Rev Anim Biosci 1:43–64
Palinski R, Piñeyro P, Shang P, Yuan F, Guo R, Fang Y, Byers E, Hause BM (2017) A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. J Virol 91:e01879-16
Zhang HH, Hu WQ, Li JY, Liu TN, Zhou JY, Opriessnig T, Xiao CT (2020) Novel circovirus species identified in farmed pigs designated as porcine circovirus 4, Hunan province, China. Transbound Emerg Dis 67:1057–1061
Hou CY, Zhang LH, Zhang YH, Cui JT, Zhao L, Zheng LL, Chen HY (2021) Phylogenetic analysis of porcine circovirus 4 in Henan Province of China: a retrospective study from 2011 to 2021. Transbound Emerg Dis, in press. https://doi.org/10.1111/tbed.14172
Ge M, Hu WQ, Ning KM, Li SY, Xiao CT (2021) The seroprevalence of the newly identified porcine circovirus type 4 in China investigated by an enzymed-linked immunosorbent assay. Transbound Emerg Dis 68:2910–2914
Misinzo G, Meerts P, Bublot M, Mast J, Weingartl HM, Nauwynck HJ (2005) Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J Gen Virol 86:2057–2068
Nauwynck HJ, Sanchez R, Meerts P, Lefebvre DJ, Saha D, Huang L, Misinzo G (2012) Cell tropism and entry of porcine circovirus 2. Virus Res 164:43–45
Wei R, Xie J, Theuns S, Nauwynck HJ (2019) Changes on the viral capsid surface during the evolution of porcine circovirus type 2 (PCV2) from 2009 till 2018 may lead to a better receptor binding. Virus Evol 5:vez26
Ha Z, Li J, Xie CZ, Yu CD, Hao PF, Zhang Y, Xu W, Nan F, Xie YB, Li YW, Rong FL, Wang GY, Guo YC, Lu HJ, Jin NY (2020) Prevalence, pathogenesis, and evolution of porcine circovirus type 3 in China from 2016 to 2019. Vet Microbiol 247:108756
Ha Z, Yu CD, Xie CZ, Wang GY, Zhang Y, Hao PF, Li JF, Li ZX, Li YW, Rong FL, Nan FL, Zhang H, Zhuang XY, Xie YB, Shi N, Lu HJ, Jin NY (2021) Retrospective surveillance of porcine circovirus 4 in pigs in Inner Mongolia, China, from 2016 to 2018. Arch Virol 166:1951–1959
Tian RB, Zhao Y, Cui JT, Zheng HH, Xu T, Hou CY, Wang ZY, Li XS, Zheng LL, Chen HY (2021) Molecular detection and phylogenetic analysis of porcine circovirus 4 in Henan and Shanxi Provinces of China. Transbound Emerg Dis 68:276–282
Sun WC, Du Q, Han ZX, Bi JS, Lan T, Wang W, Zheng M (2021) Detection and genetic characterization of porcine circovirus 4 (PCV4) in Guangxi, China. Gene 773:145384
Zhang D, Bai CX, Ge K, Li YD, Gao WH, Jiang SD, Wang Y (2020) Establishment of an SYBR Green-based real-time PCR assay for porcine circovirus type 4 detection. J Virol Methods 285:113963
Chen NH, Xiao YZ, Li XS, Li SB, Xie NJ, Yan XL, Li XD, Zhu JZ (2021) Development and application of a quadruplex real-time PCR assay for differential detection of porcine circoviruses (PCV1 to PCV4) in Jiangsu province of China from 2016 to 2020. Transbound Emerg Dis 68:1615–1624
Nguyen VG, Do HQ, Huynh TM, Park YH, Park BK, Chung HC (2022) Molecular-based detection, genetic characterization and phylogenetic analysis of porcine circovirus 4 from Korean domestic swine farms. Transbound Emerg Dis 9:538–48
Kim DY, Kim HR, Park JH, Kwon NY, Kim JM, Kim JK, Park JH, Lee KK, Kim SH, Kim WI, Lyoo YS, Park CK (2021) Detection of a novel porcine circovirus 4 in Korean pig herds using a loop-mediated isothermal amplification assay. J Virol Methods. 299:114350
Franzo G, Ruiz A, Grassi L, Sibila M, Drigo M, Segalés J (2020) Lack of porcine circovirus 4 genome detection in pig samples from Italy and Spain. Pathogens 9:433
Xu T, Hou CY, Zhang YH, Li HX, Chen XM, Pan JJ, Chen HY (2021) Simultaneous detection and genetic characterization of porcine circovirus 2 and 4 in Henan province of China. Gene 808:145991
Khayat R, Brunn N, Speir JA, Hardham JM, Ankenbauer RG, Schneemann A, Johnson JE (2011) The 2.3-angstrom structure of porcine circovirus 2. J Virol 85:7856–7862
Zhou JW, Qiu YH, Zhu N, Zhou LY, Dai BN, Feng XF, Hou L, Liu J (2021) The nucleolar localization signal of porcine circovirus type 4 capsid protein is essential for interaction with serine-48 residue of nucleolar phosphoprotein nucleophosmin-1. Front Microbiol 12:751382
Yu WT, Zhan Y, Xue BX, Dong YP, Wang YF, Jiang P, Wang AB, Sun YJ, Yang Y (2018) Highly efficient cellular uptake of a cell-penetrating peptide (CPP) derived from the capsid protein of porcine circovirus type 2. J Biol Chem 293:15221–15232
Guo L, Lu Y, Huang L, Wei Y, Liu C (2011) Identification of a new antigen epitope in the nuclear localization signal region of porcine circovirus type 2 capsid protein. Intervirology 54:156–163
Wang DL, Mai JH, Lei B, Zhang YJ, Yang Y, Wang ND (2021) Structure, antigenic properties, and highly efficient assembly of PCV4 capsid protein. Front Vet Sci 8:695466
Misinzo G, Delputte PL, Meerts P, Lefebvre DJ, Nauwynck HJ (2006) Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J Virol 80:3487–3494
Dhindwal S, Avila B, Feng S, Khayat R (2019) Porcine circovirus 2 uses a multitude of weak binding sites to interact with heparan sulfate, and the interactions do not follow the symmetry of the capsid. J Virol 93:e02222-18
Turlewicz-Podbielska H, Augustyniak A, Pomorska-Mól M (2022) Novel porcine circoviruses in view of lessons learned from porcine circovirus type 2-epidemiology and threat to pigs and other species. Viruses 14:261
Lian ZM, Liu JB, Liu PR, Zhu ZB, Yao XH, Yuan L, Hu D, Jiang Y, Chen CH, Chen NH, Li XD (2021) Development and application of an indirect ELISA for the detection of antibody to porcine circovirus 4 in pigs. Transbound Emerg Dis 68:2975–2979
Assao VS, Santos MR, Pereira CER, Vannucci F, Silva-Júnior A (2021) Porcine circovirus 3 in North and South America: epidemiology and genetic diversity. Transbound Emerg Dis 68:2949–2956
Niu GY, Zhang XW, Ji WL, Chen S, Li X, Yang L, Zhang LY, Ouyang HS, Li C, Ren LZ (2022) Porcine circovirus 4 rescued from an infectious clone is replicable and pathogenic in vivo. Transbound Emerg Dis, in press https://doi.org/10.1111/tbed.14498
Acknowledgements
We thank all the members of Research Center of Reverse Vaccinology for their constructive comments concerning our manuscript.
Funding
This study was supported by Hunan Provincial Natural Science Foundation of China (No. 2018JJ2177) and Double first-class construction project of Hunan Agricultural University (No. SYL2019048).
Author information
Authors and Affiliations
Contributions
DW and NW contributed to development and writing of the manuscript, reviewing relevant literature, and preparation of tables for the manuscript. YY and CX contributed to writing the manuscript and provided suggestions on the revision. DW and JM contributed to the drawing of the figure. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, D., Mai, J., Yang, Y. et al. Current knowledge on epidemiology and evolution of novel porcine circovirus 4. Vet Res 53, 38 (2022). https://doi.org/10.1186/s13567-022-01053-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-022-01053-w