Abstract
Emergence of bovine mastitis caused by Prototheca algae is the impetus to better understand these infections. Both P. bovis and P. ciferrii belong to Prototheca algae, but they differ in their pathogenicity to induce inflammatory responses. The objective was to characterize and compare pathogenesis of inflammatory responses in bMECs induced by P. bovis versus P. ciferrii. Mitochondrial ultrastructure, activity and mtROS in bMECs were assessed with transmission electron microscopy and laser scanning confocal microscopy. Cytokines, including TNF-α, IL-1β and IL-18, were measured by ELISA and real-time PCR, whereas expressions of various proteins in the NF-κB and NLRP3 inflammasome pathways were detected with immunofluorescence or Western blot. Infection with P. bovis or P. ciferrii damaged mitochondria, including dissolution and vacuolation of cristae, and decreased mitochondrial activity, with P. bovis being more pathogenic and causing greater destruction. There were increases in NADPH production and mtROS accumulation in infected bMECs, with P. bovis causing greater increases and also inducing higher cytokine concentrations. Expressions of NF-κB-p65, p-NF-κB-p65, IκBα and p-IκBα proteins in the NF-κB pathway, as well as NLRP3, Pro Caspase1, Caspase1 p20, ASC, Pro IL-1β, and IL-1β proteins in the NLRP3 inflammasome pathway, were significantly higher in P. bovis-infected bMECs. However, mito-TEMPO significantly inhibited production of cytokines and decreased expression of proteins in NF-κB and NLRP3 inflammasome pathways in bMECs infected with either P. bovis or P. ciferrii. In conclusion, P. bovis or P. ciferrii infections induced inflammatory responses in bMECs, with increased mtROS in damaged mitochondria and activated NF-κB and NLRP3 inflammasome pathways, with P. bovis causing a more severe reaction.
Similar content being viewed by others
Introduction
Mastitis is common in dairy cattle worldwide, causing serious reductions in milk yield and quality and large financial losses [1, 2]. Infections with pathogens are an important cause of mastitis. Prototheca spp. are unicellular achlorophyllous algae, 3–30 µm in diameter, that lack a specific glucosamine cell wall or chloroplasts; specific species include P. bovis, P. ciferrii, P. cerasi, P. pringsheimii, P. blaschkeae, P. wickerhamii, P. xanthoriae, P. cookie, P. xanthoriae, P. cutis, P. miyajii, P. tumulicola, P. moriformis, P. stagnora, and P. ulmea [3,4,5]. Bovine mastitis caused by Prototheca spp. is characterized by an abrupt decrease in both milk production and quality, an increased somatic cell count, and frequently culling, with substantial economic losses [6]. Among Prototheca spp., P. bovis was the causative pathogen of bovine mastitis, whereas P. ciferrii occasionally causes granulomatous lesions in experimentally infected bovine udders and protothecosis in humans [7, 8]. Prototheca spp. mastitis has been reported in many countries, including Canada, Poland, Italy, Brazil, and Japan [9,10,11].
An inflammatory response, a typical feature of bovine mastitis, is characterized by release of inflammatory cytokines such as IL-1β, TNF-α and IL-18. Numerous signal molecules or pathways are involved in regulation of an inflammatory response, including reactive oxygen species, inflammasome and NF-κB pathway [12, 13]. The inflammasome is an upstream regulatory mechanism that triggers an inflammatory response when stimulated by pathogens. The best characterized inflammasome is the NLRP3 inflammasone, comprised of NLRP3, apoptosis-associated speck-like protein containing adaptor (ASC), and Caspase1 [12,13,14]. Furthermore, NLRP3 is a cytosolic pattern recognition receptor (PRR) activated by pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [15]. Once activated, the inflammasome recruits NLRP3, ASC, and Caspase1, and cleaves Pro Caspase1 to an active form (cleavage Caspase1) that triggers proteolytic cleavage of Pro IL-1β and Pro IL-18 to mature and secreted forms [12, 15]. Although NLRP3 signaling usually confers protection, excessive activation can damage cells and cause inflammatory diseases [16,17,18]. The NLRP3 inflammasome can be generated and activated by Escherichia coli and Staphylococcus aureus, causing an aggravated inflammatory response and damage in bMECs [19, 20]. Activation of the NLRP3 inflammasome is regulated by various genes. In that regard, NF-κB participates, and has an important regulatory role, in activation of the NLRP3 inflammasome, which triggers an inflammatory response [21]. Furthermore, an activated NF-κB pathway could function as an upstream activator of NLRP3 and contribute to regulating inflammatory cytokines [22, 23].
Mitochondrial reactive oxygen species (mtROS) also activate the NLRP3 inflammasome, promoting inflammation and enhancing immune responses [24, 25]. Accumulation of damaged mitochondria may be essential for NLRP3 activation. In addition to increased mtROS, exposure of mitochondria-derived DAMPs (mtDAMPs) [e.g., mitochondrial DNA (mtDNA)] and cardiolipin to the cytosol, can also promote NLRP3 activation [26, 27]. Activation of the NLRP3 inflammasome has a crucial role in inflammatory responses in many diseases. Clinical bovine mastitis is usually characterized by pain, edema, cytokine production, and cellular infiltration. In Prototheca spp. mastitis, there are interstitial infiltrates of macrophages, plasma cells and lymphocytes into the mammary gland, and an antiserum against bovine keratin had weak positive expression in damaged mammary tissue [28]. We reported that infections with P. bovis or P. ciferrii increased expression of cytokine mRNA in bMECs [29]; however, inflammatory responses in bovine mammary epithelial cells (bMECs) infected with P. bovis or P. ciferrii are not well characterized. Therefore, mitochondrial damage, inflammatory cytokines including TNF-α, IL-1β and IL-18, and protein expression in the NF-κB/NLRP3 pathway that regulate inflammation were measured to characterize and compare the pathogenesis of inflammatory responses in bMECs induced by infection with P. bovis versus P. ciferrii.
Materials and methods
Reagents and antibody
Cell Counting Kit-8 (CCK-8), NADP + /NADPH assay kit, Bicinchoninic acid (BCA) protein assay kit, radioimmunoprecipitation assay (RIPA) lysis buffer, Mito-Tracker Green staining solution and Hoechst 33342 live cell staining solution were purchased from Beyotime (Shanghai, China). ELISA assay kit was purchased from mlbio (Shanghai, China). 4’, 6-Diamidine-2’-phenylindole dihydrochloride (DAPI), coverslips and Triton X-100, penicillin, streptomycin and bovine serum albumin (BSA) was purchased from Solarbio (Beijing, China). Enhanced chemiluminescence (ECL) kits were obtained from Thermo Fisher Scientific Pierce (Rockford, IL, USA). Fetal Bovine Serum (FBS) and Dulbecco’s Modified Eagle’s medium (DMEM) were purchased from Hyclone (Logan, UT, USA). Rotenone was purchased from MCE (Shanghai, China). Mito-SOX red mitochondrial superoxide indicator was purchased from Yeasen (Shanghai, China). Trizol Reagent, cDNA synthesis superMix and Two-step RT-PCR superMix were purchased from TransGen Biotech (Beijing, China). Primary antibodies, including NLRP3, ASC, Caspase-1, IL-1β and α-Tubulin, were purchased from Proteintech (Wuhan, China), and NF-κB p65, Phospho-NF-κB p65, IκBα and Phospho-IκBα were purchased from Cell Signaling Technology (Danvers, MA, USA). Peroxidase-conjugated goat anti-mouse IgG and goat anti-rabbit IgG were purchased from Proteintech (Wuhan, China).
P. bovis and P. ciferrii isolates
Prototheca bovis was isolated in 2016 from 105 clinical mastitis milk samples collected on 6 large (> 500 cows) Chinese dairy farms, whereas the 58 P. ciferrii isolates were recovered in the same year from environmental samples from 3 large dairy farms, located in suburbs of Beijing, Tianjin and Shandong [3]. The isolates were stored at 4 °C at the College of Veterinary Medicine, China Agricultural University, Beijing, China [3]. These Prototheca spp. were characterized as P. bovis and P. ciferrii by several methods. Firstly, based on cellular fatty acid pattern, P. bovis had more eicosadienoic acid (C20:2) compared to P. ciferrii [7]. Secondly, we determined 18S rDNA sequences using genotype-specific PCR. For this, a PCR mix (20 µL) containing Prototheca (450 bp) fragment internal amplification control Proto18-4f (GACATGGCGAGGATTGACAGA) and Proto18-4r (AGCACACCCAATCGGTAGGA) primers (2.5 µL each primer), DNA template (1 µL), ddH2O (4 µL), and 2 × EasyTaq PCR supermix (10 µL) was amplified under specific conditions (2 min at 95 °C, followed by 34 cycles of 30 s at 95 °C, 30 s at 50 °C, and 30 s at 72 °C, with a final extension of 5 min at 72 °C). Amplified fragments were sent for sequencing (Sangon Biotech, Shanghai, China). Then, P. bovis and P. ciferrii were characterized by genotype-specific primers [3, 7]. Additionally, the P. bovis and P. ciferrii genotypes were further confirmed by restriction fragment length polymorphism analysis targeting the cytb gene fragment [7, 30]. Taken together, we confirmed P. bovis and P. ciferrii genotypes in the isolates recovered from clinical mastitis milk and environmental samples. These strains in within a species (P. bovis and P. ciferrii), strains had the same genotype, colony morphology and similar biochemical characteristics. We randomly selected 3 strains of each species for the following experiments, which were performed independently in triplicate. The 3 strains of P. bovis and 3 strains of P. ciferrii were isolated from clinical mastitis milk and environmental samples of 3 large farms located near suburbs of Beijing, Tianjin and Shandong, respectively [3]. Furthermore, within each species, genotype and colony morphology were the same and the biochemical characteristics were similar. Additionally, P. ciferrii grew more slowly than P. bovis on SDA and their colonies had differences in morphological characteristics; P. ciferrii produced small colonies with smooth surface and folded edges compared to P. bovis, whereas the latter had more eicosadienoic acid (C20:2) compared to the former [7]. P. bovis and P. ciferrii isolates were multiplied by streaking on sabouraud dextrose agar (SDA) and incubated at 37 °C for 48 h. Then, a single colony was placed in sabouraud dextrose broth (SDB) and incubated for 72 h. Thereafter, organisms were diluted in DMEM to achieve required concentrations.
Cell culture and treatment
The MAC-T line of bMECs (Shanghai Jingma Biological Technology Co., Ltd. China) was used for cell culture. bMECs were placed in DMEM medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL) and streptomycin (100 U/mL) and grown in cell culture plates. Cells were incubated in 5% CO2 at 37 °C, and cells from passages 2–8 were used for experiments. Before infection, cells were put in 6-well plates (1 × 106 cells per well) and cultured overnight. Next, cells were infected with P. bovis or P. ciferrii at a 5:1 multiplicity of infection (MOI; ratio of P. bovis or P. ciferrii to bMECs) and incubated in 5% CO2 at 37 °C for 12 h. Then, culture supernatants were collected and frozen (−80 °C) to subsequently determine cytokine concentrations, whereas cells were collected to extract and characterize proteins. Each experiment was conducted in triplicate.
Transmission electron microscopy
The bMECs were fixed as described [29] and transmission electron microscopy (TEM) used to assess ultrastructure. Briefly, cells were washed 3 times with phosphate buffered solution (PBS) and then fixed with 2.5% glutaraldehyde solution (pH 7.4) for 2–4 h at room temperature. After fixation, samples were routinely processed and examined with a transmission electron microscope (H7650, Hitachi, Tokyo, Japan) at an accelerating voltage of 80 kV.
Cell viability assay
Cell viability was measured with a Cell Counting Kit-8 (CCK-8). The bMECs were seeded into 96-well plates at a density of 5 × 103 cells/well, allowed to adhere overnight, and then treated for 12 h with various concentrations of rotenone (mitochondrial electron transport chain complex I inhibitor) used to enhance mitochondrial reactive oxygen species production (i.e., a positive control). Then, bMECs were washed 3 times with PBS and 10 µL CCK-8 solution added to each well. After incubation for 1.5 h at 37 °C with 5% CO2, OD values were read at 570 nm.
Mito-tracker green staining
The bMECs were cultured in 6-well plates overnight and then infected with P. bovis or P. ciferrii at a 5:1 MOI. After 12 h, bMECs were washed 3 times with PBS and 2 mL of warm (37 °C) Mito-Tracker Green staining solution was added. After incubation for 30 min at 37 °C, Mito-Tracker Green staining solution was removed and 2 mL fresh cell culture solution (37 °C) was added. Then, 10 μL Hoechst 33342 live cell staining solution was added to each well. After incubating for 10 min at 37 °C, the dye-containing culture medium was aspirated, cells were washed 3 times with culture medium and observed with laser scanning confocal microscopy (Olympus-FV3000, Olympus, Tokyo, Japan).
Mitochondrial ROS measurement
To detect intracellular mtROS production, bMECs were seeded into 6-well plates with cell climbing films and infected with P. bovis or P. ciferrii at a 5:1 MOI. After 12 h, Mito-SOX red mitochondrial superoxide indicator was used to label mitochondrial reactive oxygen species. To induce accumulation of mtROS (positive control), bMECs were treated with 2.5 μM rotenone for 12 h. Next, cells were incubated with Mito-SOX (5 μM) in the dark for 10 min at 37 °C and then washed 3 times with PBS. Nuclei were stained with 300 nM 4. 6-diamimo-2-phenyl indole (DAPI) for 5 min at 37 °C and washed with PBS. Slides were covered with glass cover slips and intracellular mtROS assessed with laser scanning confocal microscopy (Olympus-FV3000).
NADPH analysis
The bMECs were cultured into 6-well plates overnight and then infected with P. bovis or P. ciferrii at a 5:1 MOI for 12 h. The NADPH content in cells was determined with a commercial NADP + /NADPH Assay Kit, according to the manufacturer’s protocol. Briefly, 200 μL NADP + /NADPH extract was added into each hole of the 6-well plate, gently blown to promote cell lysis, and supernatant collected for subsequent experiments. Then, 50 μL supernatant and 200 μL of G6PDH working solution were added into each 96-well plate. After incubation for 10 min at 37 °C, 10 μL chromogenic solution was added into each well and after incubation for 20 min at 37 °C, absorbance was measured at 450 nm.
ELISA
The bMECs were infected with the 3 P. bovis or 3 P. ciferrii isolates at a 5:1 MOI for 12 h, and 10 µM of 2-(2,2,6,6-Tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenyl-phosphonium chloride, monohydrate (mito-TEMPO), a mitochondria-targeted superoxide dismutase mimetic with superoxide and alkyl radical scavenging properties, was used to scavenge superoxide. Cytokines in supernatants of bMECs culture medium were quantified by ELISA kits, according to the manufacturer’s instructions. Cell culture supernatants were collected and concentrations of TNF-α, IL-1β and IL-18 in supernatants were measured, based on OD values at 450 nm.
RNA extraction and real time PCR
The bMECs were treated as described above for ELISA, washed 3 times with PBS, and cells collected for total RNA extraction. Trizol Reagent was pre-chilled on ice and 1 mL added to cell samples for 5 min to lyse cells. Mixed liquid was centrifuged at 12 000 × g for 15 min at 4 °C and supernatant collected. Total mRNA of bMECs was extracted with mRNA extraction kit according to manufacturer’s instructions. Relative expression levels of TNF-α, IL-1β and IL-18 mRNA were determined using the StepOnePlus Real-Time PCR systems. Data were analyzed according to the 2−∆∆Ct method and results were expressed as relative mRNA levels [7]. Primer sequences for GAPDH (housekeeping gene), TNF-α, IL-1β, and IL-18 are presented in Table 1.
Immunofluorescence
The bMECs were treated as described above for ELISA, washed 3 times with PBS and then fixed in 4% paraformaldehyde for 30 min and subsequently permeabilized in 0.25% Triton X-100. Cells were incubated with 3% bovine serum for 30 min at room temperature and then incubated overnight at 4 °C with the following primary antibodies: NLRP3, NF-κB p65, Phospho-NF-κB p65, ASC, and IL-1β. Next, samples were washed with PBS and incubated with Alexa Fluor 488-labeled goat anti-rabbit IgG (H + L) for 1 h at room temperature. Then, samples were washed with PBS and stained with DAPI for 20 min. After washing with PBS, slides were covered with glass cover slips and observed under a laser scanning confocal microscope (Olympus-FV3000).
Western blot
The bMECs were treated as described above for ELISA and then lysed on ice and the cell lysate suspension collected and centrifuged (12 000 × g, 4 °C) for 15 min. Total protein concentration in the supernatant was determined with a BCA protein assay kit. Protein samples were denatured in boiling water for 10 min and then separated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. These membranes were blocked with 5% nonfat dry milk for 2 h at room temperature, then incubated overnight at 4 °C with the following primary antibodies: α-Tubulin, NLRP3, NF-κB p65, Phospho-NF-κB p65, IκBα, Phospho-IκBα, ASC, Caspase-1, and IL-1β. For α-Tubulin, membranes were incubated with mouse anti-α-Tubulin antibody, whereas for all other proteins, they were incubated with secondary antibody against rabbit IgG for 1 h at room temperature. After washing with Tris-buffered saline, the membrane was developed using ECL reagents and visualized with a chemiluminescence system. Results were normalized to α-Tubulin, and band density was analyzed with Image J (National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
After visually confirming that the data were normally distributed, independent Student’s t-tests or one-way ANOVA were used to analyze effects of Prototheca spp. on cell viability, NADPH content, inflammatory cytokines including TNF-α, IL-1β and IL-18, and protein expression in NF-κB/NLRP3 pathway, with a Bonferroni method used to correct multiple comparisons, P < 0.05 was divided by the number of tests to be considered statistically significant. Data are reported as means ± standard deviation (SD) of 3 independent experiments (3 technical replicates were carried out in each experiment).
Results
Prototheca spp. infection caused mitochondrial damage in bMECs
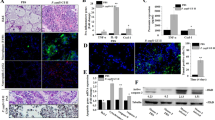
In uninfected bMECs (controls), mitochondria had reticulated morphologies and mitochondrial cristae were clearly visible with TEM (Figure 1, panels A1 and A2), whereas in bMECs infected with P. ciferrii, mitochondria had relatively minor damage, including slight vacuolization (Figure 1, panels B1 and B2). In contrast, in bMECs infected with P. bovis, mitochondria had dissolution of their cristae and large areas of vacuolation (Figure 1, panels C1 and C2). Intensity of green fluorescence was profoundly decreased in bMECs infected with P. bovis, with less suppression in the P. ciferrii infection group, although both were lower than the control (Figure 2), indicating decreased mitochondrial activity in infected bMECs. Both P. bovis and P. ciferrii induced mitochondrial damage, with more severe damage caused by P. bovis.
Mitochondrial ultrastructure in bMECs. A1 and A2: Control group, with normal mitochondria and mitochondrial cristae in the bMECs. B1 and B2: P. ciferrii infection group; note the slight vacuolization in mitochondria in P. ciferrii-infected bMECs. C1 and C2: P. bovis infection group; note the mitochondrial cristae dissolution and large areas of vacuolation in bMECs.
Mitochondrial activity in bMECs. Mito-Tracker Green is a mitochondrial green fluorescent probe, with intensity of green fluorescence reflecting mitochondrial activity. A1, A2, and A3: In the Control group, mitochondria in normal bMECs had strong green fluorescence, indicating good mitochondrial activity. B1, B2, and B3: In the P. ciferrii infection group, there was weak green fluorescence, indicating decreased mitochondrial activity in bMECs. C1, C2, and C3: In the P. bovis infection group, there was weak green fluorescence in bMECs, indicating mitochondrial activities were decreased.
Prototheca spp. infection enhanced mtROS accumulation in bMECs
In bMECs infected with P. ciferrii, the mtROS assay had weak red fluorescence (Figure 3A). As a positive control, bMECs were treated with various concentrations of rotenone, with 2.5 µM rotenone used to treat bMECs (Figure 3B). However, strong red fluorescence was observed in bMECs infected with P. bovis or treated with rotenone (Figure 3A); therefore, P. bovis induced greater mtROS accumulation. Furthermore, NADPH content was higher in bMECs infected with P. bovis or P. ciferrii compared to the control (P < 0.05), with the highest NADPH in the P. bovis infection group (Figure 3C). P. bovis and P. ciferrii decreased bMECs viability at 12 h post infection, although P. bovis caused a more profound decrease than P. ciferrii in the viability of bMECs (Figure 3D).
Mitochondrial accumulation of reactive oxygen species in bMECs. A Mit-SOX red mitochondrial superoxide indicator (5.0 µM) was used to label reactive oxygen species in mitochondria (mtROS) of bMECs infected with P. bovis or P. ciferrii; both induced mtROS accumulation, with P. bovis being more severe. Rotenone (2.5 µM), a mitochondrial electron transport chain complex I inhibitor, was used to induce production of mtROS. B Effects of various concentrations of rotenone on bMECs viability (note the gradual decrease with increasing rotenone concentrations). As a positive control, bMECs were treated with 2.5 µM rotenone. C NADPH in bMECs infected with either P. bovis or P. ciferrii. D Cell viability in infected bMECs. Data represent means ± SD of 3 independent experiments. *P < 0.05 or **P < 0.01, difference compared to the control.
Prototheca spp. infection increased production of TNF-α, IL-1β and IL-18 in bMECs
Infection with P. ciferrii increased production of IL-1β, and IL-18 proteins and mRNAs in bMECs (P < 0.05), with more profound increases in bMECs infected with P. bovis (P < 0.05) (Figures 4B and C). P. bovis infection increased production of TNF-α proteins and mRNAs in bMECs (P < 0.05) (Figure 4A). In contrast, treatment with mito-TEMPO inhibited production of TNF-α, IL-1β and IL-18 proteins and mRNAs in bMECs infected with P. bovis or P. ciferrii (P < 0.05) (Figures 4A, B and C). Expression of TNF-α, IL-1β and IL-18 was not significantly different among isolates within P. bovis nor among P. ciferrii species (Additional file 1).
Production of TNF-α, IL-1β, and IL-18 in bMECs. A, B and C Changes of TNF-α, IL-1β and IL-18 in bMECs measured by ELISA and real time PCR, respectively. Treatment with MT (10 µM) for 12 h inhibited cytokine production in bMECs infected with P. bovis or P. ciferrii. The mRNA expression was analyzed according to the 2−∆∆Ct method and results were expressed as relative mRNA levels. Data represent means ± SD of 3 independent experiments. *P < 0.05 or **P < 0.01, difference compared to the control; #P < 0.05 or ##P < 0.01, difference compared to the P. ciferrii infection group; &P < 0.05 or &&P < 0.01, difference compared to the P. bovis infection group.
Prototheca spp. infection promoted protein expression of NF-κB pathway in bMECs
Infection with P. bovis increased the green fluorescence intensity of NF-κB p65 and p-NF-κB p65 in bMECs (Figures 5A and B). Furthermore, in Western blots, protein expression levels of NF-κB p65 and p-NF-κB p65 were also upregulated in P. bovis-infected bMECs (P < 0.05) compared to the control (Figures 5C, D and E). Although P. ciferrii infection in bMECs also increased expression levels of NF-κB p65 and p-NF-κB p65 proteins, changes were less profound than in the P. bovis infection group, with the immunofluorescence consistent with the Western blot (Figures 5A, B, C, D and E). In addition, expression level of p-IκBα proteins was upregulated in P. bovis-infected cells (P < 0.05); there were fewer profound increases induced by P. ciferrii, but p-IκBα (P > 0.05) was higher than the control (Figures 5C and G). Mito-TEMPO inhibited expression levels of NF-κB p65 and p-NF-κB p65 proteins in bMECS infected with P. bovis or P. ciferrii (P < 0.05) (Figures 5C, D and E). A similar trend in expression levels of IκBα and p-IκBα protein in bMECs infected with P. bovis or P. ciferrii was also observed after treatment with mito-TEMPO, but IκBα and p-IκBα were decreased compared to the P. bovis group (P < 0.05) (Figures 5C, F and G). Protein expression in the NF-κB pathway was not significantly different among isolates within P. bovis or P. ciferrii species (Additional file 2).
Expression of NF-κB pathway proteins in bMECs. A and B Green fluorescence is expression of NF-κB p65 and p-NF-κB p65 proteins; treatment with MT (10 µM) for 12 h inhibited expression of these proteins in bMECs infected with P. bovis or P. ciferrii. C–G Treatment with MT (10 µM) for 12 h reduced expression levels of NF-κB p65, p-NF-κB p65, IκBα, and p-IκBα proteins in bMECs infected with P. bovis or P. ciferrii. “-” and “ + ” after MT indicated that MT was not or was added, respectively; “-” and “ + ” after P. bovis indicated that P. bovis was not or were added, respectively; “-” and “ + ” after P. ciferrii indicated that P. ciferrii was not or were added, respectively. Data represent means ± SD of 3 independent experiments. *P < 0.05 or **P < 0.01, difference compared to the control; #P < 0.05 or ##P < 0.01, difference compared to the P. ciferrii infection group; &P < 0.05 or &&P < 0.01, difference compared to the P. bovis infection group.
Prototheca spp. infection contributed to NLRP3 inflammasome activation in bMECs
The green fluorescence intensity of NLRP3 and ASC was higher after infection with P. bovis or P. ciferrii compared to the control (Figures 6A and B). In contrast, treatment with mito-TEMPO decreased the green fluorescence intensity of NLRP3 and ASC compared to infection with either P. bovis or P. ciferrii (Figures 6A and B). Furthermore, expression levels of NLRP3, Pro Caspase1, Caspase1 p20, and ASC proteins were upregulated in P. bovis-infected cells (P < 0.05) compared to the control (Figures 6C, D, E, F and G). Infection of bMECs with P. ciferrii also increased expression of these proteins, but there was no significant change compared to the control (lowest P = 0.14) (Figures 6C, D, E, F and G). Expression of these proteins were all upregulated after P. bovis or P. ciferrii infections, with more pronounced increases for P. bovis. However, in bMECs pretreated with mito-TEMPO, expression of NLRP3, Pro Caspase1, Caspase1 p20, and ASC proteins were inhibited in bMECs infected with P. ciferrii and P. bovis (except ASC, P < 0.05) (Figures 6C, D, E, F and G). Protein expression in NLRP3 inflammasome pathway was not significantly different among isolates within P. bovis or P. ciferrii species (Additional file 2).
Expression of NLRP3 inflammasome pathway proteins in bMECs. A and B in P. bovis- or P. ciferrii-infected bMECs. Treatment with MT (10 µM) for 12 h inhibited protein expression of NLRP3 and ASC in P. bovis- or P. ciferrii-infected bMECs. Green fluorescence is expression of NLRP3 and ASC proteins. C–G Treatment with MT (10 µM) for 12 h inhibited expressions of NLRP3, Pro Caspase1, Caspase1 p20, and ASC proteins in P. bovis- or P. ciferrii-infected bMECs. “-” and “ + ” after MT indicated that MT was not or were added, respectively; “-” and “ + ” after P. bovis indicated that P. bovis was not or were added, respectively; “-” and “ + ” after P. ciferrii indicated that P. ciferrii was not or were added, respectively. Data represent means ± SD of 3 independent experiments. *P < 0.05 or **P < 0.01, difference compared to the control; #P < 0.05 or ##P < 0.01, difference compared to the P. ciferrii infection group; &P < 0.05 or &&P < 0.01, difference compared to the P. bovis infection group.
Prototheca spp. infection enhanced protein expression of IL-1β in bMECs
Infection with P. bovis or P. ciferrii increased the red fluorescence intensity of IL-1β in bMECs compared to the control (Figure 7A). However, treatment with mito-TEMPO decreased the red fluorescence intensity of IL-1β in bMECs infected with P. bovis or P. ciferrii (Figure 7A). Furthermore, expression of Pro IL-1β protein was upregulated in bMECs infected with P. bovis (P < 0.05) (Figures 7B and C). In addition, IL-1β protein was also upregulated in bMECs infected with P. bovis or P. ciferrii (P < 0.05) (Figures 7B and D). Expression of Pro IL-1β and IL-1β proteins were higher after P. bovis compared to P. ciferrii infection. Treatment with mito-TEMPO inhibited expression of Pro IL-1β after P. bovis infection (P < 0.05) and IL-1β protein in P. bovis and P. ciferrii infected bMECs were downregulated (P < 0.05) (Figures 7B, C and D). Protein expression of IL-1β was not significantly different among isolates within P. bovis or P. ciferrii species (Additional file 2).
Expression IL-1β protein in bMECs. A Treatment with MT (10 µM) for 12 h inhibited IL-1β expression in P. bovis- or P. ciferrii-infected bMECs. Red fluorescence is expression of IL-1β protein. B–D Treatment with MT (10 µM) for 12 h inhibited expression of Pro IL-1β and IL-1β proteins in P. bovis- or P. ciferrii-infected bMECs. “-” and “ + ” after MT indicated that MT was not or were added, respectively; “-” and “ + ” after P. bovis indicated that P. bovis was not or were added, respectively; “-” and “ + ” after P. ciferrii indicated that P. ciferrii was not or were added, respectively. Data represent means ± SD of 3 independent experiments. *P < 0.05 or **P < 0.01, difference compared to the control; #P < 0.05 or ##P < 0.01, difference compared to the P. ciferrii infection group; &P < 0.05 or &&P < 0.01, difference compared to the P. bovis infection group.
Discussion
In this study, Prototheca spp. infection in bMECs induced an inflammatory response through the NF-κB and NLRP3 inflammasome pathways. Infection of bMECs with Prototheca spp., especially P. bovis, damaged mitochondria and promoted mtROS accumulation, which activated an inflammatory response through the NF-κB and NLRP3 inflammasome pathways and enhanced IL-1β production. However, scavenging mtROS decreased expressions of proteins in NF-κB/NLRP3 inflammasome pathways and IL-1β production in bMECs infected with P. bovis or P. ciferrii. Accumulation of mtROS may be important in inflammatory responses to P. bovis or P. ciferrii infections. Furthermore, mtROS activated NF-κB/NLRP3 inflammasome pathways were involved in inflammation in bMECs infected with P. bovis and P. ciferrii.
Pathogenic infections can cause mitochondrial damage, including swelling and vacuolation, increase ROS, decrease membrane potential, and increase oxidative stress, both in vitro and in vivo [31,32,33]. Mitochondrial damage is closely related to development of inflammatory diseases [34]. In the current study, Prototheca spp. infections in bMECs, especially P. bovis, caused dissolution and large area vacuolation of mitochondrial cristae, and decreased mitochondrial activity. Mitochondria are the main site for mtROS production [35] and mitochondrial damage may contribute to mtROS production. Based on Mito-SOX, mtROS in bMECs increased after infection with P. bovis, with a lesser increase in P. ciferrii-infected bMECs. NADPH is closely linked to ROS production and there is increasing evidence that increases in both ROS production and expression of NADPH oxidase were upregulated both in vitro and in vivo [36, 37]. In the present study, P. bovis or P. ciferrii infections in bMECs increased NADPH, which also provided evidence for production of mtROS. In this study, although strains for each species (P. bovis and P. ciferrii) were isolated from different samples, the pathogenicity of strains in each species to bMECs was similar, as both P. bovis and P. ciferrii induced mitochondrial damage and mtROS accumulation, with the former causing more profound damage.
Increased mitochondrial ROS promoted inflammatory responses in peritoneal mesothelial cells, macrophages and T cells [15, 38]. Infection of bMECs with P. bovis or P. ciferrii stimulated inflammatory responses, characterized by release of inflammatory cytokines which activate immune effector cells to eliminate invading pathogens. There were significant increases in production of TNF-α, IL-18, and IL-1β at 12 h after bMECs were infected with P. bovis or P. ciferrii, indicating a marked inflammatory response. However, responses to P. bovis were more severe than P. ciferrii, consistent with its greater pathogenicity. However, scavenging mtROS with mito-TEMPO significantly decreased cytokine production. Therefore, we inferred that infection with P. bovis or P. ciferrii induced inflammatory responses in bMECs that were mitigated by suppression of mtROS.
Inflammatory responses have many regulatory mechanisms, including the NF-κB and NLRP3 inflammasome pathways [39, 40]. In the NF-κB pathway, both IκBα and NF-κB p65 are inactive in the cytoplasm [41, 42]. However, when an upstream signal activates inhibitor of nuclear factor kappa-B kinase (IKK), it will be ubiquitinated, phosphorylated and degrade IκBα, so that NF-κB p65 will be activated and translocated from the cytoplasm to the nucleus to bind to the corresponding inflammation-related genes, promote transcription of inflammatory cytokines, and induce inflammation [42, 43]. In the current study, infection of bMECs with P. bovis or P. ciferrii activated the NF-κB pathway, upregulating expression of IκBα and NF-κB p65 proteins. Furthermore, expression levels of phosphorylated IκBα and NF-κB p65 proteins were upregulated after P. bovis or P. ciferrii infection, with greater upregulation of protein expression in the NF-κB pathway induced by P. bovis, indicating higher pathogenicity. Activation of the NF-κB pathway promoted inflammation, including a massive increase in cytokine production. In addition, mtROS activation of the IKK complex and subsequent signaling through the NF-κB pathway led to secretion of proinflammatory cytokines by inducing the intermolecular disulfide linkage of nuclear factor IκBα essential modulator [44], whereas quenching mtROS in vivo decreased the NF-κB-guided anti-inflammatory phenotype [45]. In the present study, in bMECs infected with P. bovis or P. ciferrii, mito-TEMPO decreased expression of various proteins in the NF-κB pathway, including IκBα, NF-κB p65, p-IκBα and p-NF-κB p65. Thus, infection of bMECs with Prototheca spp., especially P. bovis, caused overexpression of proteins in the NF-κB pathway and enhanced inflammatory responses through generation of mtROS.
Activation of inflammasomes is critical in inflammatory responses, with key roles in regulating inflammation caused by pathogenic bacteria [46, 47]. The NLRP3 inflammasome is well characterized [48]. Once activated, ASC self assembles and activates Pro Caspase1; the activated Caspase1 induces maturation of IL-1β and IL-18 for subsequent release [48, 49]. Activation of the NLRP3 inflammasome is regulated by many factors, including bacterial infections and mtROS [50, 51]. In the present study, P. bovis or P. ciferrii infection in bMECs promoted activation of NLRP3 inflammasomes to varying degrees, modulating upregulation of expression of NLRP3, ASC, and Caspase1 proteins, and promoting cleavage of Caspase1. We inferred that infection with either P. bovis or P. ciferrii contributed to the assembly of ASC, Pro Caspase1, and NLRP3 during inflammasome formation. Although P. bovis or P. ciferrii infections in bMECs promoted NLRP3 inflammasome activation, P. bovis induced larger increases in proteins of the NLRP3 inflammasome, indicating greater pathogenicity. Furthermore, expression levels of proteins of the downstream genes Pro IL-1β and IL-1β were upregulated in cells infected with P. bovis or P. ciferrii, although P. bovis caused more pronounced increases. Therefore, P. bovis induced a greater inflammatory response than P. ciferrii vai the NLRP3 inflammasom pathway.
Generation of mtROS is one of the first identified triggers of NLRP3 inflammasome activation, although mtROS-independent activation of the NLRP3 inflammasome has been reported [52, 53]. In the present study, P. bovis or P. ciferrii infections induced mtROS in bMECs. However, treatment with mito-TEMPO downregulated expression levels of NLRP3, ASC and Caspase1 proteins in bMECs infected with P. bovis or P. ciferrii, whereas expression of Pro IL-1β and IL-1β proteins was also downregulated. Therefore, we inferred that mtROS has an important role in activation of NLRP3 inflammasomes and enhances production of IL-β, resulting in an inflammatory response in bMECs. Mito-TEMPO suppressed expression of proteins in NF-κB and NLRP3 inflammasomes pathways and reduced inflammatory responses in P. bovis- and P. ciferrii-infected bMECs (Figure 8). In this study, 3 strains in each species were randomly selected to infect bMECs. Consequently, we maximized the probability of choosing strains that represented other strains within each species in terms of pathogenicity. Regardless, mechanisms of Prototheca spp.-infected cells inducing mtROS generation need further study.
The pattern of Prototheca spp. infection induced inflammatory responses through NF-κB and NLRP3 inflammasome pathways in bMECs. Infection of bMECs with P. bovis or P. ciferrii, increased mtROS, which can promote activation of NF-κB and NLRP3 inflammasome pathways and further enhance production of IL-β, resulting in an inflammatory response in bMECs. However, in bMECs treated with mito-TEMPO, which scavenged mtROS, protein expression in NF-κB and NLRP3 inflammasome pathways was inhibited and there was suppression of the inflammatory response in P. bovis- or P. ciferrii-infected bMECs.
Infections of bMECs with either P. bovis or P. ciferrii damaged mitochondria and induced inflammatory responses, with P. bovis causing a more severe inflammatory response. Accumulation of mtROS had an important role in activation of NF-κB and NLRP3 inflammasomes and suppression of mtROS reduced inflammatory responses in bMECs infected with either P. bovis or P. ciferrii.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- bMECs:
-
bovine mammary epithelial cells
- SDA:
-
sabouraud dextrose agar
- SDB:
-
sabouraud dextrose broth
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
- mtROS:
-
mitochondrial reactive oxygen species
- mito-TEMPO:
-
mitochondrial specific antioxidant
- CCK-8:
-
Cell Counting Kit-8
- BCA:
-
Bicinchoninic acid
- RIPA:
-
radioimmunoprecipitation assay
- BSA:
-
bovine serum albumin
- DAPI:
-
4’, 6-Diamidine-2’-phenylindole dihydrochloride
- PVDF:
-
polyvinylidene difluoride
- ECL:
-
enhanced chemiluminescence
- FBS:
-
fetal bovine serum
- DMEM:
-
Dulbecco’s Modified Eagle’s medium
- PBS:
-
phosphate buffered solution
- MOI:
-
multiplicity of infection
- TEM:
-
transmission electron microscopy
References
Gao J, Barkema HW, Zhang L, Liu G, Deng Z, Cai L, Shan R, Zhang S, Zou J, Kastelic JP, Han B (2017) Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J Dairy Sci 100:4797–4806
Deng ZJ, Shahid M, Zhang LM, Gao J, Gu XL, Zhang SY, Zou J, Fanning S, Han B (2016) An investigation of the innate immune response in bovine mammary epithelial cells challenged by Prototheca zopfii. Mycopathologia 181:823–832
Shahid M, Ali T, Zhang LM, Hou RG, Zhang SY, Ding LD, Han DD, Deng ZJ, Rahman A, Han B (2016) Characterization of Prototheca zopfii genotypes isolated from cases of bovine mastitis and cow barns in China. Mycopathologia 181:185–195
Jagielski T, Bakułaa Z, Gaworb J, Kr M, Kusberd WH, Dyląge M, Nowakowskaf J, Gromadkab R, Karnkowskac A (2019) The genus Prototheca (Trebouxiophyceae, Chlorophyta) revisited: Implications from molecular taxonomic studies. Algal Res 43:101639
Hirose N, Hua Z, Kato Y, Zhang Q, Li R, Nishimura K, Masuda M (2018) Molecular characterization of Prototheca strains isolated in China revealed the first cases of protothecosis associated with Prototheca zopfii genotype 1. Med Mycol 56:279–287
Gao J, Zhang HQ, He JZ, He YH, Li SM, Hou RG, Wu QX, Gao Y, Han B (2012) Characterization of Prototheca zopfii associated with outbreak of bovine clinical mastitis in herd of Beijing, China. Mycopathologia 173:275–281
Shahid M, Cavalcante PA, Knight CG, Barkema HW, Han B, Gao J, Cobo ER (2020) Murine and human cathelicidins contribute differently to hallmarks of mastitis induced by pathogenic Prototheca bovis algae. Front Cell Infect Microbiol 10:31
Pieper L, Godkin A, Roesler U, Polleichtner A, Slavic D, Leslie KE, Kelton DF (2012) Herd characteristics and cow-level factors associated with Prototheca mastitis on dairy farms in Ontario, Canada. J Dairy Sci 95:5635–5644
Sobukawa H, Yamaguchi S, Kano R, Ito T, Suzuki K, Onozaki M, Hasegawa A, Kamata H (2012) Short communication: molecular typing of Prototheca zopfii from bovine mastitis in Japan. J Dairy Sci 95:4442–4446
Morandi S, Cremonesi P, Capra E, Silvetti T, Decimo M, Bianchini V, Alves AC, Costa GM, Ribeiro MG, Brasca M (2016) Molecular typing and differences in biofilm formation and antibiotic susceptibilities among Prototheca strains isolated in Italy and Brazil. J Dairy Sci 99:6436–6445
Jagielski T, Roeske K, Bakuła Z, Piech T, Wlazło Ł, Bochniarz M, Woch P, Krukowski H (2019) A survey on the incidence of Prototheca mastitis in dairy herds in Lublin province, Poland. J Dairy Sci 102:619–628
Bai BC, Yang YY, Wang Q, Li M, Tian C, Liu Y, Aung LHH, Li PF, Yu T, Chu XM (2020) NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis 11:776
Sun LB, Ma W, Gao WL, Xing YM, Chen LX, Xia ZY, Zhang ZJ, Dai ZL (2019) Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis 10:542
Petr B, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16:407–420
Missiroli S, Patergnani S, Caroccia N, Pedriali G, Perrone M, Previati M, Wieckowski MR, Giorgi C (2018) Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis 9:329
Matthew MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17:588–606
Beckwith KS, Beckwith MS, Ullmann S, Sætra RS, Kim H, Marstad A, Åsberg SE, Strand TA, Haug M, Niederweis M, Stenmark HA, Flo TH (2020) Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nat Commun 11:2270
Petr B (2016) Inflammasomes: Intracellular detection of extracellular bacteria. Cell Res 26:859–860
Ma MR, Pei YF, Wang XX, Feng JX, Zhang Y, Gao MQ (2019) LncRNA XIST mediates bovine mammary epithelial cell inflammatory response via NF-κB/NLRP3 inflammasome pathway. Cell Prolif 52:12525
Wu Q, Liu MC, Yang J, Wang JF, Zhu YH (2015) Lactobacillus rhamnosus GR-1 ameliorates Escherichia coli-induced inflammation and cell damage via attenuation of ASC-independent NLRP3 inflammasome activation. Appl Environ Microbiol 82:1173–1182
Yu S, Liu XSBJ, Yu D, Yong EC, Yang JH (2020) Morin protects LPS-induced mastitis via inhibiting NLRP3 inflammasome and NF-κB signaling pathways. Inflammation 43:1293–1303
Zhong ZY, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, He F, Boassa D, Perkins G, Ali SR, McGeough MD, Ellisman MH, Seki E, Gustafsson AB, Hoffman HM, Diaz-Meco MT, Moscat J, Karin M (2016) NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164:896–910
Afonina IS, Zhong ZY, Karin M, Beyaert R (2017) Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol 18:861–869
Han YC, Xu XX, Tang CY, Gao P, Chen XH, Xiong XF, Yang M, Yang S, Zhu X, Yuan S, Liu F, Xiao L, Kanwar YS, Sun L (2018) Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol 16:32–46
Xu MM, Wang L, Wang MY, Wang HY, Zhang H, Chen YQ, Wang X, Gong J, Zhang JJ, Adcock IM, Chung KF, Li F (2019) Mitochondrial ROS and NLRP3 inflammasome in acute ozone-induced murine model of airway inflammation and bronchial hyperresponsiveness. Free Radic Res 53:780–790
Kim BR, Kim BJ, Kook YH, Kim BJ (2020) Mycobacterium abscessus infection leads to enhanced production of type 1 interferon and NLRP3 inflammasome activation in murine macrophages via mitochondrial oxidative stress. PLoS Pathog 16:1008294
Zhong ZY, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, Wong J, Ding S, Seki E, Schnabl B, Hevener AL, Greenberg HB, Kisseleva T, Karin M (2018) New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560:198–203
Corbellini LG, Driemeier D, Cruz C, Dias MM, Ferreiro L (2001) Bovine mastitis due to Prototheca zopfii: clinical, epidemiological and pathological aspects in a Brazilian dairy herd. Trop Anim Health Prod 33:463–470
Shahid M, Cobo ER, Chen LB, Cavalcante PA, Barkema HW, Gao J, Xu S, Liu Y, Knight CG, Kastelic JP, Han B (2020) Prototheca zopfii genotype II induces mitochondrial apoptosis in models of bovine mastitis. Sci Rep 10:698
Jagielski T, Gawor J, Bakuła Z, Decewicz P, Maciszewski K, Karnkowska A (2018) Cytb as a new genetic marker for differentiation of Prototheca species. J Clin Microbiol 56:e00584-e618
Spier A, Stavru F, Cossart P (2019) Interaction between intracellular bacterial pathogens and host cell mitochondria. Microbiol Spectr 7:7.2.10
Ramond E, Jamet A, Coureuil M, Charbit A (2019) Pivotal role of mitochondria in macrophage response to bacterial pathogens. Front Immunol 10:2461
Zavala-Alvarado C, Sismeiro O, Legendre R, Varet H, Bussotti G, Bayram J, Huete S, Rey G, Coppée JY, Picardeau M, Benaroudj N (2020) The transcriptional response of pathogenic Leptospira to peroxide reveals new defenses against infection-related oxidative stress. PLoS Pathog 16:1008904
Grazioli S, Pugin J (2018) Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front Immunol 9:832
Dan Dunn JD, Alvarez LA, Zhang XZ, Soldati T (2015) Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol 6:472–485
Guerby P, Tasta O, Swiader A, Pont F, Bujold E, Parant O, Vayssiere C, Salvayre R, Negre-Salvayre A (2021) Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol 40:101861
Gaspar RS, Ferreira P, Mitchell JL, Pula G, Gibbins JM (2021) Platelet-derived extracellular vesicles express NADPH oxidase-1 (Nox-1), generate superoxide and modulate platelet function. Free Radic Biol Med 165:395–400
Ohl K, Tenbrock K (2018) Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol 9:2499
Chen XL, Liu GL, Yuan YY, Wu GT, Wang SL, Yuan LW (2019) NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis 10:906
Wang SA, Lin YK, Yuan X, Li F, Guo LX, Wu BJ (2018) REV-ERBα integrates colon clock with experimental colitis through regulation of NF-κB/NLRP3 axis. Nat Commun 9:4246
Mulero MC, Huxford T, Ghosh G (2019) NF-κB, IκB, and IKK: integral components of immune system signaling. Adv Exp Med Biol 1172:207–226
Sun SC (2017) The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol 17:545–558
Zhu MM, Wang L, Yang D, Li C, Pang ST, Li XH, Li R, Yang B, Lian YP, Ma L, Lv QL, Jia XB, Feng L (2019) Wedelolactone alleviates doxorubicin-induced inflammation and oxidative stress damage of podocytes by IκK/IκB/NF-κB pathway. Biomed Pharmacother 117:109088
Herb M, Gluschko A, Wiegmann K, Farid A, Wolf A, Utermöhlen O, Krut O, Krönke M, Schramm M (2019) Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO. Sci Signal 12:5926
Formentini L, Santacatterina F, Núñez de Arenas C, Stamatakis K, López-Martínez D, Logan A, Fresno M, Smits R, Murphy MP, Cuezva JM (2017) Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell Rep 19:1202–1213
Man SM, Karki R, Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277:61–75
Yang Y, Wang HN, Kouadir M, Song HH, Shi FS (2019) Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis 10:128
Wang Z, Zhang SM, Xiao Y, Zhang WA, Wu SA, Qin T, Yue Y, Qian W, Li L (2020) NLRP3 inflammasome and inflammatory diseases. Oxid Med Cell Longev 2020:4063562
Kelley N, Jeltema D, Duan YH, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci 20:3328
Yu X, Lan PX, Hou XB, Han QJ, Lu N, Li T, Jiao C, Zhang J, Zhang C, Tian Z (2017) HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. J Hepatol 66:693–702
Wang XG, Eagen WJ, Lee JC (2020) Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles. Proc Natl Acad Sci U S A 117:3174–3184
Chung C, Chen LC, Tsang NM, Chuang WY, Liao TC, Yuan SN, OuYang CN, Ojcius DM, Wu CC, Chang YS (2020) Mitochondrial oxidative phosphorylation complex regulates NLRP3 inflammasome activation and predicts patient survival in nasopharyngeal carcinoma. Mol Cell Proteomics 19:142–154
Chung C, Yuan SN, Yang CN, Lin HC, Huang KY, Chen YJ, Chung AK, Chu CL, Ojcius DM, Chang YS, Chen LC (2018) Src-family kinase-Cbl axis negatively regulates NLRP3 inflammasome activation. Cell Death Dis 9:1109
Acknowledgements
The authors thank our laboratory members who helped us to improve the research and the manuscript with their skillful technical assistance, invaluable comments and suggestions.
Funding
This study was supported financially by: Ningxia Key R&D Project (No. 2019BBF02027), Hebei Key R&D Project (19226607D), the National Natural Science Foundation of China (No. 31572587), and the High-end Foreign Experts Recruitment Program (No. GDT20171100013).
Author information
Authors and Affiliations
Contributions
BH, JG and WZ conceived and designed the experiment. WZ and FH performed the research and wrote the manuscript, WZ, FH, SX, YS and ZD performed the correlation of the genes to inflammation and associated interpretation, WZ, JG, GL, MS and ZD wrote the first draft of the manuscript. JG assisted in the result analysis and re-edited the manuscript. BH, HWB and JPK revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work has received approval for research ethics from Ethical Committee of the College of Veterinary Medicine, China Agricultural University (CAU), Beijing and the study was conducted according to standard ethical guidelines implemented at CAU (SYXK, 2016-0008).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. The coefficient of variation values (ELISA and Real time PCR) of P. ciferrii and P. bovis infections of bMECs.
The coefficient of variation (cv) is the standard deviation divided by the mean. We calculated the cv values among biological replicates within P. bovis and P. ciferrii, respectively. These cv values reflect the variation among different biological replicates and the cv values were in the range of 0.001–0.098, therefore we consider the variation among biological replicates was acceptable.
Additional file 2. The coefficient of variation values (Western blot) of P. ciferrii and P. bovis infections of bMECs.
The coefficient of variation (cv) is the standard deviation divided by the mean. We calculated the cv values among biological replicates within P. bovis and P. ciferrii, respectively. These cv values reflect the variation among different biological replicates and the cv values were in the range of 0.006–0.298, therefore we consider the variation among biological replicates was acceptable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, W., He, F., Barkema, H.W. et al. Prototheca spp. induce an inflammatory response via mtROS-mediated activation of NF-κB and NLRP3 inflammasome pathways in bovine mammary epithelial cell cultures. Vet Res 52, 144 (2021). https://doi.org/10.1186/s13567-021-01014-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-021-01014-9