Abstract
NorV has been known to be an anaerobic nitric oxide reductase associated with nitric oxide (NO) detoxification. Recently, we showed that the norV gene of Aeromonas hydrophila was highly upregulated after co-culturing with Tetrahymena thermophila. Here, we demonstrated that the transcription and expression levels of norV were upregulated in a dose-dependent manner after exposure to NO under aerobic and anaerobic conditions. To investigate the roles of norV in resisting predatory protists and virulence of A. hydrophila, we constructed the norV gene-deletion mutant (ΔnorV). Compared to the wild type, the ΔnorV mutant showed no significant difference in growth at various NO concentrations under aerobic conditions but significantly stronger NO-mediated growth inhibition under anaerobic conditions. The deletion of norV exhibited markedly decreased cytotoxicity, hemolytic and protease activities under aerobic and anaerobic conditions. Also, the hemolysin co-regulated protein (Hcp) in the ΔnorV mutant showed increased secretion under aerobic conditions but decreased secretion under anaerobic conditions as compared to the wild-type. Moreover, the inactivation of norV led to reduced resistance to predation by T. thermophila, decreased survival within macrophages and highly attenuated virulence in zebrafish. Our data indicate a diverse role for norV in the expression of A. hydrophila virulence-associated traits that is not completely dependent on its function as a nitric oxide reductase. This study provides insights into an unexplored area of NorV, which will contribute to our understanding of bacterial pathogenesis and the development of new control strategies for A. hydrophila infection.
Similar content being viewed by others
Introduction
Aeromonas hydrophila is a Gram-negative bacterium, commonly found in a variety of natural aquatic environments worldwide including seawater, freshwater, sediments and even drinking water [1]. This bacterium is responsible for a variety of diseases in amphibians, fish and reptiles. As a major pathogen causing hemorrhagic septicemia in fish, A. hydrophila causes severe economic losses to aquaculture worldwide [2, 3]. The pathogenesis of A. hydrophila is complex and multifactorial, probably resulting from the expression of virulence factors such as adhesins, enterotoxins, hemolysin, aerolysin, and proteases, and as well as the secretion systems such as type III (T3SS) and type VI (T6SS) secretion systems [2,3,4,5].
Although many virulence factors have already been identified in A. hydrophila, several remain to be discovered. It is noteworthy that environmental factors, such as protistan predation, have an important impact on the virulence evolution of pathogens [6]. Protists may provide a protective reservoir for pathogens and act as a “training ground” for bacterial virulence [7, 8]. It has been demonstrated that pathogens including Legionella pneumophila [9], Salmonella enteritidis [10, 11], and Mycobacterium avium [12] are able to survive protistan predation, subsequently resulting in resistance to adverse situations such as antibiotics, oxidants and bioacids and increasing virulence. Grazing resistance to protists is an evolutionary precursor of bacterial pathogenicity and promotes bacteria to develop some defensive mechanisms for survival, such as new gene expression patterns or new proteins which may emerge as virulence determinants in animal and human infections [13, 14].
In our previous study, the norV gene of A. hydrophila was screened and identified to be about 15-fold upregulated under predation pressure of Tetrahymena thermophila [15]. The norV encodes a flavorubredoxin, a nitric oxide reductase, which is supposed to be inactivated by oxygen and provides physiological protection against nitric oxide (NO) only at low or zero oxygen concentrations [16]. The transcription of norV gene has been reported to be stimulated via a nitric oxide sensor NorR and sigma factor 54 (σ54)-dependent mechanism [17]. The binding of mononuclear iron site to NO activates the ATPase activity of NorR and enables NorR to interact with σ54-containing RNA polymerase to regulate the transcription of norV [18]. NorV, functioning as a major defense factor, contributes to bacterial resistance against oxidative and nitrosative killing [19,20,21]. Bacteria engulfed within the phagosomes of protists share similar defensive mechanisms responsible for survival within macrophages [22, 23]. During infection, bacterial cells are typically internalized into macrophages, enclosed in the phagolysosome and exposed to nitrogen radicals that are derived from inducible nitric oxide synthase (iNOS) [24]. NO is toxic to bacteria and usually deployed by macrophages as a potent antimicrobial for inhibition of pathogen proliferation, metabolic blockade, inactivation of virulence factors and dispersion of bacterial biofilm [25]. It has been reported that the norV mRNA expression was upregulated in Salmonella enterica sv. Typhimurium after macrophage internalization [26]. In addition, norV has been identified to limit NO level and contribute to the production of shiga toxin 2 (Stx2) of enterohaemorrhagic Escherichia coli (EHEC) within macrophages [27]. Whether and how norV gene is involved in stress response and pathogenicity of A. hydrophila are still unclear.
In this study, we evaluated the biological functions of the norV gene both in the aerobic and anaerobic environments. We also proposed a possible role of norV in A. hydrophila virulence and its resistance to predation by T. thermophila.
Materials and methods
Strains, cell lines and media
Aeromonas hydrophila strain NJ-35 (accession number CP006870) and E. coli SM10 were maintained in Luria–Bertani (LB) media at 28 °C and 37 °C, respectively. When required, media were supplemented with ampicillin (100 μg/mL), chloramphenicol (34 μg/mL) or gentamicin (100 μg/mL).
Tetrahymena thermophila SB210 (accession number GCA_000261185.1) was obtained from Dr. Miao Wei, Institute of Hydrobiology, China Academy of Sciences, and cultured in SPP medium (2% protease peptone, 0.1% yeast extract, 0.2% glucose, 0.003% EDTA-Fe) at 28 °C.
RAW264.7 cells were maintained in Dulbecco’s modified Eagle medium (DMEM; Gibco, New York, USA) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Gibco). All reagents used in this study were supplied by Sigma (St. Louis, MO, USA) unless otherwise indicated.
Inactivation and complementation of norV gene in A. hydrophila
The norV gene was knocked out on the basis of the suicide plasmid pYAK1 via homologous recombination as previously described [28]. Firstly, the two flanking regions of norV were fused and cloned into pYAK1. The resulting plasmid was conjugated into A. hydrophila NJ-35 (resistance to ampicillin, Ampr) from E. coli SM10 and transconjugants were selected on LB agar plates with ampicillin and chloramphenicol. The positive colonies were cultured in LB medium without sodium chloride for 12 h and then counter-selected by growing on LB agar plates containing 20% sucrose. The gene-deletion mutant ΔnorV was verified by PCR.
Genetic complementation was carried out by inserting the norV gene with a synonymous point mutation to the genome of ΔnorV mutant. The norV gene and its two flanking regions were amplified from NJ-35 genomic DNA allowing a point mutation (G1236A) in norV. The fused PCR product was cloned into pYAK1 and conjugated into the ΔnorV (Ampr). Chromosomal integration was achieved via allelic homologous recombination. The complemented strain CΔnorV was screened by antibiotics (ampicillin and chloramphenicol) and 20% sucrose in sequence and further verified by PCR and sequencing. The primers used are listed in Additional file 1.
NO growth inhibition assay
NO growth inhibition assay was performed as previously described [27]. A. hydrophila wild-type, ΔnorV and CΔnorV strains grown overnight were adjusted to OD600 of 1.0 with fresh LB media. The bacterial suspensions were diluted 1:100 with LB containing various concentrations of NO donor (sodium nitroprusside, SNP) and grown statically at 28 °C under aerobic or anaerobic conditions. Cultures without any treatment served as control. Then the OD600 values were measured every 1 h by a spectrophotometer (Bio-Rad, USA). Each sample was repeated in triplicate.
Measurement of LDH release, hemolytic and protease activity
Aeromonas hydrophila wild-type, ΔnorV and CΔnorV strains were cultured with or without NO donor to OD600 of 0.8 both under aerobic and anaerobic conditions. The culture supernatants were collected through centrifugation and filtration.
Cytotoxicity of RAW 264.7 macrophages induced by bacterial extracellular products (ECPs) was evaluated by measuring the release of lactate dehydrogenase (LDH) with a CytoTox 96 nonradioactive cytotoxicity assay (Promega, USA). The assay was performed according to the manufacturer’s instructions. Macrophages grown in 96-well plates were washed and added with 100 μL/well MEM containing 10 μL of the above mentioned culture supernatants. The plate was then incubated for 4 h at 37 °C with 5% CO2. LDH released by lysis of cells with 1% (vol/vol) Triton X-100 was defined as cell maximum release. LDH released by uninfected cells was designated as cell spontaneous release. The release of LDH was determined by measuring OD492 using a micro-plate reader (Tecan, Switzerland). Cytotoxicity was calculated as follows: % cytotoxicity (test LDH release − cell spontaneous release)/(cell maximal release − cell spontaneous release).
The hemolytic activity was measured as previously described [29]. One hundred microliters (100 μL) sterilized saline was added to each well of a 96-well cell plate. A 0.1-mL aliquot of the supernatant from a given strain was added to the first well, followed by a serial twofold dilution, with addition of 100 μL of 2% sheep red blood cells (RBCs). The 2% RBCs added with an equal volume of sterilized saline and water served as negative and positive controls, respectively. The plate was incubated at 37 °C for 1 h and placed at 4 °C overnight. The unlysed cells were precipitated by centrifugation. One hundred microliters of the supernatants were separated and transferred to a new 96-well plate, and the hemoglobin released was measured at OD540. The hemolytic activity was expressed as the reciprocal of the highest dilution of the culture filtrates that lead to the lysis of exceeding 50% RBCs.
The protease activity was measured as previously described [30]. Briefly, 250 μL of the supernatants were mixed with an equal volume of 0.5% (wt/vol) casein in 50 mM Tris–HCl (pH 8.0). After incubation at 37 °C for 2 h, the mixture was added to 500 μL pre-cooled 10% (wt/vol) trichloroacetic acid (TCA) and placed on ice for 30 min to precipitate the proteins. Then 500 μL of the supernatant was collected after centrifugation and mixed with an equal volume of 1 M NaOH. The absorbance was measured at OD440.
Western blot for hemolysin co-regulated protein (Hcp) and NorV protein
Western blot was performed to determine the levels of Hcp protein expression and secretion in A. hydrophila wild-type, ΔnorV and CΔnorV strains as described previously [31]. Briefly, both the cell pellets and culture supernatants from a given strain were collected and treated with 5× SDS-PAGE buffer. Aliquots of the samples were subjected to SDS-PAGE and electrophoretically transferred to NC membranes. The membranes were incubated with anti-Hcp, or anti-NorV, or anti-GroEL polyclonal antiserum followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG. Antibody-antigen complexes were detected using an ECL Pico-detect kit (CMCTAG, USA) and ChemiDoc™ Touch imaging system (Bio-Rad, USA).
qRT-PCR analysis for hcp and norV genes
The mRNA levels of hcp and norV genes in A. hydrophila were measured using qRT-PCR. Bacterial RNA was isolated with an E.Z.N.A. bacterial RNA isolation kit (Omega, Beijing, China). Then cDNA was synthesized in triplicate using HiScript qRT SuperMix (Vazyme Biotech). The cDNA amplification was manipulated using AceQ qPCR SYBR Green kit (Vazyme Biotech) in the Applied Biosystems StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific, USA). All procedures above were performed according to the manufacturer’s instructions. The internal housekeeping gene recA was used as the reference, and the acquired cycle threshold (CT) of each gene was normalized. The fold-change of mRNA levels was calculated using the 2−ΔΔCT method [32]. The primers used are listed in Additional file 1.
Bacterial resistance to predation by T. thermophila
The anti-predation ability of A. hydrophila was expressed as the relative survival of bacteria after co-cultured with T. thermophila [15]. Briefly, A. hydrophila (1 × 109 CFU/mL) and T. thermophila SB210 (2 × 105 cells/mL) in TBSS (2 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2, and 1 mM Tris [pH 6.8–7.2]) were well mixed in equal volume. One hundred microliters of the mixture was transferred to each well of a 96-well plate. Meanwhile, A. hydrophila and SB210 mixed with same volume of TBSS respectively acted as the controls. TBSS alone served as the blank control. The plate was placed at 28 °C without shaking for 12 h, and the bacterial population was measured at OD450. The relative survival of bacteria was expressed as the OD450 value of the bacteria co-cultured with T. thermophila divided by that of bacteria grown alone at 12 h. The absorbance of T. thermophila cells was negligible [33]. The assay was duplicated in quadruplicate in three independent experiments.
Determination of intracellular growth efficiency
The intracellular growth efficiency was determined using RAW264.7 macrophages as previously described [34]. A. hydrophila wild-type, ΔnorV and CΔnorV strains grown to logarithmic phase were washed and resuspended in fresh serum-free DMEM. The RAW264.7 macrophages cultured in 24-well plate were infected with A. hydrophila at a multiplicity of infection (MOI) of 1:1 for 1 h at 37 °C. Extracellular bacteria were removed by washing and addition of DMEM containing gentamicin. To measure the intracellular bacterial survival rate, cells were sampled 1 h after the interaction with antibiotics (time point 0) and subsequently at a regular intervals of 20 min. Infected macrophages were washed three times with PBS and then treated with 0.1% (vol/vol) Triton X-100 for 10 min to fully lyse the cells and release intracellular bacteria. The CFU of intracellular bacteria was quantified using LB agar plates. The relative survival rate over time was calculated as follows: (average CFU at a specific time point/average CFU at time point 0) × 100.
Determination of the bacterial median lethal dose (LD50)
The LD50 assay was performed using a zebrafish model as described by Pang et al. [33]. Zebrafish were provided by the Pearl River Fishery Research Institute, Chinese Academic of Fishery Science. A. hydrophila grown to logarithmic phase were washed three times and adjusted to 5 × 108 CFU/mL, followed by a serial tenfold dilution with sterilized PBS. The zebrafish were divided into seven groups for each strain and each group consisted of ten zebrafish. The zebrafish were intraperitoneally injected with 20 μL of each dilution (101 to 107 CFU). Meanwhile, another ten zebrafish were injected with 20 μL of sterile PBS as the negative control. LD50 studies were carried out in triplicate for all of the strains. The numbers of dead fish showing clinical symptoms were recorded within 7 days. The LD50 values were calculated by the Reed and Muench method [35].
Statistical analyses
The statistical analyses in this study were performed using the GraphPad Prism version 5 software. Student’s t test was used to analyze the difference between the ΔnorV mutant and the wild type strain. Tukey’s multiple comparisons were preformed to analyze the norV mRNA levels of A. hydrophila NJ-35 grown under various conditions using one-way analysis of variance (ANOVA) with 95% confidence intervals. P-value < 0.05 was considered as a significant difference.
Results
NO activates the expression of norV gene
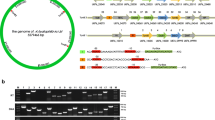
To determine how the norV gene in A. hydrophila responds to NO exposure, qRT-RCR analysis and Western blot were preformed. As shown in Figure 1, the transcription and expression levels of norV were increased when cells were exposed to SNP in a dose-dependent manner under both aerobic and anaerobic conditions, showing that norV could be activated by NO exposure regardless of the presence or absence of oxygen. Notably, the mRNA level of norV was significantly enhanced under anaerobic conditions compared to that under aerobic conditions (P < 0.001), suggesting that norV expression to some extent may be inhibited by oxygen.
The expression of norV in response to NO donor (sodium nitroprusside, SNP) under aerobic and anaerobic conditions. The A. hydrophila strains were cultured to OD600 of 0.8 with various concentrations of NO donor under aerobic and anaerobic conditions. A The mRNA level of norV in the wild type strain determined by qRT-PCR. The expression value of norV without SNP treatment in the aerobic condition was normalized to 1.0. Values are means ± SD from three biological replicates. Different uppercase letters (a–g) indicate significant differences (P < 0.05) among different treatments. B The expression levels of NorV protein detected by Western blot. Lines 1, 4, 7, 10 represent the wild type strain NJ-35; Lines 2, 5, 8, 11 represent the ΔnorV mutant; and Lines 3, 6, 9, 12 represent the complemented CΔnorV strain.
NorV affords protection against NO-mediated growth inhibition
To examine how NorV in A. hydrophila responds to NO, we determined the growth difference between the wild-type and ΔnorV mutant strains exposed to NO donor. As shown in Figures 2A and B, the growth of the wild-type strain and ΔnorV mutant showed no difference both under the aerobic and anaerobic conditions in the absence of NO. In the aerobic cultures containing SNP, the growth rates of ΔnorV were comparable to the wild type strain (Figures 2C and E). However, the ΔnorV mutant displayed a reduction of anaerobic growth in response to SNP in a dose-dependent manner (Figures 2D and F). The growth capacity was restored in the complemented strain CΔnorV.
Growth ability of the wild-type, ΔnorV mutant, and complemented CΔnorV strains exposed to NO donor. Growth curves of A. hydrophila strains were determined under aerobic (A) and anaerobic (B) conditions without SNP treatment. After the addition of 0.5 mM SNP, the growth yields of A. hydrophila strains under aerobic (C) or anaerobic (D) conditions were detected by measuring the OD600 values. NO growth inhibition assay was performed in bacteria grown for 8 h at 28 °C in LB broth containing various concentrations of SNP under aerobic (E) or anaerobic (F) conditions. Values are the means ± SD from three biological replicates. ***P < 0.001.
The norV is involved in cytotoxicity, hemolytic and protease activities
To determine whether NorV is associated with the production of extracellular products, the cytotoxicity, hemolytic and protease activities were determined. As shown in Figures 3A and B, the culture filtrates collected from the ΔnorV, grown both under aerobic and anaerobic conditions, produced significantly lower injury to macrophages than those of the wild-type strain (P < 0.001). In addition, the ΔnorV mutant displayed poorer hemolytic activity than the wild-type strain when cultured both under aerobic and anaerobic conditions (P < 0.001) (Figures 3C and D). Similarly, the protease activity of ΔnorV was also markedly decreased (P < 0.001) (Figures 3E and F). Notably, the cytotoxicity, hemolytic and protease activities of ΔnorV mutant when exposed to NO under anaerobic conditions were decreased in a dose-dependent manner, whereas their activities in the wild-type strain showed no obvious difference. Moreover, the hemolytic activity of the ΔnorV mutant under anaerobic conditions was lower than that under aerobic conditions. High concentrations of NO could exert reduced effect on hemolytic activity of the wild-type. All the activities of extracellular products were restored in the CΔnorV.
Cytotoxicity, hemolytic and protease activities of the wild-type, ΔnorV mutant, and complemented CΔnorV strains grown under various conditions. Cytotoxicity was expressed as LDH release of RAW264.7 macrophages induced by culture supernatants collected from bacteria grown under aerobic (A) and anaerobic (B) conditions after exposure to various concentrations of SNP. The hemolytic activities of each strain grown under aerobic (C) and anaerobic (D) conditions after treating with various concentrations of SNP were expressed as the dilution-fold of the A. hydrophila culture filtrates that led to the lysis of 50% erythrocytes. The protease activities of A. hydrophila strains cultured in LB media containing various concentrations of SNP under aerobic (E) and anaerobic (F) conditions were measured at OD440 with azocasein as the substrate. Values are the means ± SD from three biological replicates. ***P < 0.001.
The norV is diversely involved in production and secretion of Hcp protein
To determine whether the norV gene is involved in regulating the function of T6SS in response to NO, the expression and secretion of Hcp in A. hydrophila wild-type, ΔnorV mutant and CΔnorV strains were examined. When exposed to NO, Hcp expression showed no obvious difference between ΔnorV mutant and wild-type strain under aerobic conditions (Figures 4A and B). However, under anaerobic conditions, Hcp expression displayed a decreased tendency after exposure to NO and that of ΔnorV mutant was significantly lower than the wild-type strain (P < 0.05) with SNP at concentrations of 0.5 mM and 2.5 mM (Figures 4A and C). The mRNA levels of hcp were consistent with protein expression results (Figures 4D and E). However, as shown in Figure 5A, NO exposure led to an increase in Hcp secretion of the wild-type strain but a decrease in ΔnorV mutant both under aerobic and anaerobic conditions. Hcp secretion of ΔnorV mutant was higher in the aerobic environment but poorer under anaerobic conditions than the wild-type strain (Figures 5B and C). The cytoplasmic chaperone protein GroEL was not detectable in the supernatants, demonstrating that the Hcp detected was the result of bacterial secretion but not cell lysis.
Effects of norV on Hcp protein expression. A The Hcp proteins in the whole cells of bacteria grown under aerobic and anaerobic conditions exposing to various concentrations of SNP were determined by Western blot. Polyclonal anti-Hcp antibodies were used to measure the production of Hcp and anti-GroEL antibodies served as internal reference. Lines 1, 4, 7, 10 represent the wild type strain NJ-35; Lines 2, 5, 8, 11 represent the ΔnorV mutant; and Lines 3, 6, 9, 12 represent the complemented CΔnorV strain. The relative changes of Hcp production of bacteria cultured under aerobic (B) and anaerobic (C) conditions are given between the blots. The intensities of the Hcp and GroEL bands were measured and Hcp was equalized to that of GroEL. The relative mRNA levels of hcp gene in A. hydrophila strains grown under aerobic (D) and anaerobic (E) conditions after treated with various concentrations of SNP were determined by qRT-PCR. Values are the means ± SD from three biological replicates. *P < 0.05, **P < 0.01.
The norV is diversely involved in secretion of Hcp protein. A Hcp proteins in the culture supernatants of bacteria grown under aerobic and anaerobic conditions after exposure with various concentrations of SNP were determined by Western blot. Polyclonal anti-Hcp antibodies were used to measure the secretion of Hcp and anti-GroEL antibodies served as internal reference. Lines 1, 4, 7, 10 represent the wild type strain NJ-35; Lines 2, 5, 8, 11 represent the ΔnorV mutant; and Lines 3, 6, 9, 12 represent the complemented CΔnorV strain. The relative changes of Hcp secretion of bacteria grown under aerobic (B) and anaerobic (C) conditions are given between the blots. Values are the means ± SD from three biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001.
The norV contributes to resisting predation by T. thermophila
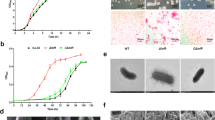
In order to study whether norV inactivation exerts influence on the interaction between A. hydrophila and T. thermophila, the relative survivals of the wild type strain NJ-35 and its norV derivatives were detected after co-culture with T. thermophila for 12 h. The relative survival of ΔnorV mutant (51.83 ± 4.02%) exhibited a significant reduction (P = 0.0054) compared to that of the wild-type strain (68.10 ± 3.20%). The anti-protistan predation level was restored in the complemented strain (63.72 ± 4.73%).
The norV enhances the intracellular survival of A. hydrophila in macrophages
The bacteria within RAW264.7 macrophages were quantified by CFU counts. Compared with the wild-type, more intracellular viable bacteria of the ΔnorV mutant were observed, suggesting that the ΔnorV is more susceptible to phagocytosis by macrophages (Figure 6A). Moreover, the ΔnorV mutant displayed impaired survival rate in macrophages in a time-dependent manner (Figure 6B), indicating that the ΔnorV engulfed was more likely to be cleared by macrophages.
Survival efficiency of the wild-type, ΔnorV mutant, and complemented CΔnorV strains in RAW264.7 macrophages. A CFU of intracellular bacteria at 1 h post-infection (time point 0). B Intracellular survival rate of A. hydrophila in macrophages. Phagocytosis was allowed to proceed for 1 h at a MOI of 1.0. The CFU of intracellular bacteria was estimated every 20 min. The relative survival was calculated by dividing the mean CFU at a specific time point by the CFU at time point 0. The survival rate at time point 0 was arbitrarily set to 100%. Data are presented as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
The norV is required for the virulence of A. hydrophila in zebrafish
To investigate the effect of norV on the virulence of A. hydrophila, zebrafish were injected intraperitoneally with the wild-type or ΔnorV mutant strain. The mortality of zebrafish was recorded within 7 days after infection. The LD50 value of the ΔnorV mutant (104.09 ± 103.03 CFU) was 87-fold higher than that of the wild-type strain (102.16 ± 101.37 CFU), indicating a significant reduction in the virulence of the ΔnorV mutant (P < 0.0001). Virulence was almost restored when the CΔnorV (102.75 ± 101.89 CFU) was used to infect zebrafish.
Discussion
Nitric oxide is an important component of host defence against invading pathogenic bacteria [19, 25, 27, 36, 37]. It is likely to be encountered by bacteria in diverse environments. To overcome the deleterious effects of NO, bacteria have evolved multiple mechanisms to protect against NO stress, for example, detoxifying NO via the NorV [38]. In this study, we used SNP as NO donor and investigated the biological functions of NorV in A. hydrophila. Before entering the study, we evaluated the kinetics of NO release by SNP using Griess assay and found NO could be released continuously for more than 10 h (Additional file 2). Based on the releasing dynamic, SNP of 2.5 mM could produce a concentration of NO2− of about 10 μM in LB medium. Shimizu et al. [27] revealed that the NO2− concentration in the supernatants of infected RAW264.7 cells by E. coli could be up to nearly 20 μM after 10 h post-infection. Thus, it is possible that bacteria will have an opportunity to encounter relatively high amount of NO during infection. The SNP concentrations used in this study are, therefore, biologically relevant.
NorV has been known for the detoxification of NO under anaerobic, but not aerobic conditions [19, 20]. However, there is evidence that the norV promoter can also be activated by NO exposure during aerobic growth [39]. To determine whether the activation of NorV correlated with the presence or absence of oxygen, we examined its mRNA and protein expression under aerobic and anaerobic conditions. Our data demonstrated that the transcription of norV in A. hydrophila was regulated in a NO-dependent manner in both conditions, and NorV expression would be activated by NO exposure with or without oxygen. Surprisingly, however, NorV could only exert a protective growth effect by eliminating NO under anoxic conditions. Hence, these findings prompt us to explore whether and which physiological role NorV plays in the presence of oxygen in A. hydrophila.
Both environmental oxygen and NO signals are able to reprogram gene expression and to stimulate bacterial secretion systems, effector molecules, or toxins to promote survival and resist injury [36, 37, 40]. Extracellular proteins such as hemolysins, proteases and cytotoxins are belived to exhibit potential pathogenicity in Aeromonas spp. [41, 42]. In this study, we demonstrated for the first time that the norV gene plays important roles in the secretion of bacterial extracellular products. The activities of ECPs from the ΔnorV strain remained aerobically unaffected by NO but anaerobically decreased in a NO dose-dependent manner. This phenomenon leads us to speculate that NO can anaerobically inhibit the production of ECPs and NorV serves as a NO scavenger to minimize the inhibition effect of NO. Notably, however, the activities of ECPs in ΔnorV mutant were significantly lower than those in the wild-type strain even without exposure to NO, indicating that NorV could regulate the production of ECPs beyond depending on its nitric oxide reductase activity. To exclude the potential polar effect of norV disruption on the production of ECPs, we studied the transcriptional levels of both upstream (norR) and downstream (norW) genes of the norV deletion region. The data showed that the inactivation of norV did not affect the transcription of either norR or norW (Additional file 3), indicating no polar mutation had occurred. In A. hydrophila, type II secretion system (T2SS) has been known to be essential for the translocation of ECPs from the periplasm into the extracellular milieu [43, 44]. Thus, it is speculated that NorV might affect the secretion of extracellular proteins through T2SS. In this regard, it may be of interest to further investigate the relationship of norV and T2SS.
In some Gram-negative bacteria, T6SS is often involved in virulence-related mechanisms and environmental competitive fitness [45, 46]. Intriguingly, similar to the impact on ECPs, we found that NorV anaerobically contributed to Hcp production in a NO-dependent fashion. Compared to the wild-type, Hcp secretion in the ΔnorV mutant was higher under oxic conditions but lower under anoxic conditions, indicating that NorV is involved in Hcp secretion and the involvement effect might be strongly related to the presence of oxygen. Oxygen is a constantly changing environmental parameter in bacterial infections [47]. To adapt to the variable oxygen conditions, bacteria have to convert metabolic strategies [40]. A study from Babujee et al. [40] has demonstrated that oxygen limitation was associated to varying degrees with the Hcp production in Dickeya dadantii and Pectobacterium atrosepticum. Also, it should be noted that Hcp secretion in the wild type strain was increased in a NO dose-dependent manner but decreased in the ΔnorV mutant. This indicates that the promotion on Hcp secretion by NO may positively correlate with NorV, and the NO-dependent decrease of Hcp secretion in the ΔnorV mutant probably imply an indirect effect. Some bacteria have been reported to develop other NO defense mechanisms, including flavohemoglobin (Hmp) [48] and cytochrome c nitrite reductase (NrfA) [49] in addition to the NorV. However, we demonstrated that the inactivation of norV gene did not affect the transcription levels of hmp or nrfA (Additional file 4). We can, however, not exclude the possibility that the inactivation of norV may affect other unknown NO defense systems. Also, various classes of regulators sensitive to environmental cues have been demonstrated to specifically modulate the T6SS activity, for example, iron, σ54-dependent activator proteins and surface association [50]. We could not determine whether these regulators may be involved in the Hcp secretion in A. hydrophila. Maybe future transcriptomic study will be helpful to explain the molecular basis of the altered Hcp secretion due to NorV.
In the present study, we also determined that norV gene had ability to confer a protective advantage for A. hydrophila in resisting predation by Tetrahymena and improving the bacterial survival within macrophages, and made an important contribution to bacterial virulence in zebrafish. NorV was confirmed to play an important role for the survival of EHEC within macrophages by eliminating NO produced by iNOS [27]. However, we believe the mechanism by which NorV increased A. hydrophila survival in macrophages cannot be due to its NO consumption activity. Some previous studies have indicated that NO could only be produced by macrophages after 8 h post-infection [27, 51], but in our study, A. hydrophila was almost cleared by cells within 3 h. Additional regulatory mechanisms may account for the contribution of norV in bacterial survival within macrophages.
In summary, our results reveal that norV gene has various effects on the virulence-associated traits of A. hydrophila. Regulation of these virulence traits in response to external conditions reflects that this bacterium has the ability to regulate its cellular activity in order to adapt to the environment in which it is growing.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files.
Abbreviations
- NorV:
-
nitric oxide reductase
- NO:
-
nitric oxide
- iNOS:
-
inducible nitric oxide synthase
- SNP:
-
sodium nitroprusside
- OD:
-
optical density
- LB:
-
Luria–Bertani
- Ampr :
-
ampicillin resistance
- Cmr :
-
chloramphenicol resistance
- PCR:
-
polymerase chain reaction
- CFU:
-
colony forming unit
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- FBS:
-
fetal bovine serum
- LDH:
-
lactate dehydrogenase
- MOI:
-
multiplicity of infection
- PBS:
-
phosphate buffered saline
- ECPs:
-
extracellular products
- RBCs:
-
red blood cells
- Hcp:
-
hemolysin co-regulated protein
- TCA:
-
trichloroacetic acid
- GroEL:
-
heat shock protein Hsp60
- LD50 :
-
median lethal dose
- T2SS:
-
type II secretion system
- T6SS:
-
type VI secretion system
References
Janda JM, Abbott SL (2010) The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 23:35–73
Ji Y, Li J, Qin Z, Li A, Gu Z, Liu X, Lin L, Zhou Y (2015) Contribution of nuclease to the pathogenesis of Aeromonas hydrophila. Virulence 6:515–522
Tomás JM (2012) The main Aeromonas pathogenic factors. ISRN Microbiol 2012:256261
Citterio B, Francesca B (2015) Aeromonas hydrophila virulence. Virulence 6:417–418
Pemberton JM, Kidd SP, Schmidt R (1997) Secreted enzymes of Aeromonas. FEMS Microbiol Lett 152:1–10
Erken M, Lutz C, McDougald D (2013) The rise of pathogens: predation as a factor driving the evolution of human pathogens in the environment. Microb Ecol 65:860–868
Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y (2005) Amoebae as training grounds for intracellular bacterial pathogens. Appl Environ Microbiol 71:20–28
Barker J, Brown MR (1994) Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253–1259
Berk SG, Faulkner G, Garduno E, Joy MC, Ortiz-Jimenez MA, Garduno RA (2008) Packaging of live Legionella pneumophila into pellets expelled by Tetrahymena spp. does not require bacterial replication and depends on a Dot/Icm-mediated survival mechanism. Appl Environ Microbiol 74:2187–2199
Brandl MT, Rosenthal BM, Haxo AF, Berk SG (2005) Enhanced survival of Salmonella enterica in vesicles released by a soilborne Tetrahymena species. Appl Environ Microbiol 71:1562–1569
Rehfuss MY, Parker CT, Brandl MT (2011) Salmonella transcriptional signature in Tetrahymena phagosomes and role of acid tolerance in passage through the protist. ISME J 5:262–273
Cirillo JD, Falkow S, Tompkins LS, Bermudez LE (1997) Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun 65:3759–3767
Adiba S, Nizak C, van Baalen M, Denamur E, Depaulis F (2010) From grazing resistance to pathogenesis: the coincidental evolution of virulence factors. PLoS One 5:e11882
King CH, Shotts EB Jr, Wooley RE, Porter KG (1988) Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl Environ Microbiol 54:3023–3033
Pang M, Lin X, Liu J, Guo C, Gao S, Du H, Lu C, Liu Y (2016) Identification of Aeromonas hydrophila genes preferentially expressed after phagocytosis by Tetrahymena and involvement of methionine sulfoxide reductases. Front Cell Infect Microbiol 6:199
Gardner AM, Helmick RA, Gardner PR (2002) Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J Biol Chem 277:8172–8177
Gardner AM, Gessner CR, Gardner PR (2003) Regulation of the nitric oxide reduction operon (norRVW) in Escherichia coli. Role of NorR and sigma54 in the nitric oxide stress response. J Biol Chem 278:10081–10086
D’Autreaux B, Tucker NP, Dixon R, Spiro S (2005) A non-haem iron centre in the transcription factor NorR senses nitric oxide. Nature 437:769–772
Muhlig A, Kabisch J, Pichner R, Scherer S, Muller-Herbst S (2014) Contribution of the NO-detoxifying enzymes HmpA, NorV and NrfA to nitrosative stress protection of Salmonella Typhimurium in raw sausages. Food Microbiol 42:26–33
Mills PC, Richardson DJ, Hinton JC, Spiro S (2005) Detoxification of nitric oxide by the flavorubredoxin of Salmonella enterica serovar Typhimurium. Biochem Soc Trans 33:198–199
Kulasekara BR, Jacobs M, Zhou Y, Wu Z, Sims E, Saenphimmachak C, Rohmer L, Ritchie JM, Radey M, McKevitt M, Freeman TL, Hayden H, Haugen E, Gillett W, Fong C, Chang J, Beskhlebnaya V, Waldor MK, Samadpour M, Whittam TS, Kaul R, Brittnacher M, Miller SI (2009) Analysis of the genome of the Escherichia coli O157:H7 2006 spinach-associated outbreak isolate indicates candidate genes that may enhance virulence. Infect Immun 77:3713–3721
Cosson P, Soldati T (2008) Eat, kill or die: when amoeba meets bacteria. Curr Opin Microbiol 11:271–276
Jacobs ME, DeSouza LV, Samaranayake H, Pearlman RE, Siu KW, Klobutcher LA (2006) The Tetrahymena thermophila phagosome proteome. Eukaryot Cell 5:1990–2000
Vazquez-Torres A, Fang FC (2001) Oxygen-dependent anti-Salmonella activity of macrophages. Trends Microbiol 9:29–33
Bogdan C (2015) Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol 36:161–178
Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC (2003) Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol 47:103–118
Shimizu T, Tsutsuki H, Matsumoto A, Nakaya H, Noda M (2012) The nitric oxide reductase of enterohaemorrhagic Escherichia coli plays an important role for the survival within macrophages. Mol Microbiol 85:492–512
Dong Y, Liu J, Pang M, Du H, Wang N, Awan F, Lu C, Liu Y (2016) Catecholamine-stimulated growth of Aeromonas hydrophila requires the TonB2 energy transduction system but is independent of the amonabactin siderophore. Front Cell Infect Microbiol 6:183
Khajanchi BK, Kozlova EV, Sha J, Popov VL, Chopra AK (2012) The two-component QseBC signalling system regulates in vitro and in vivo virulence of Aeromonas hydrophila. Microbiology 158:259–271
Swift S, Lynch MJ, Fish L, Kirke DF, Tomas JM, Stewart GS, Williams P (1999) Quorum sensing-dependent regulation and blockade of exoprotease production in Aeromonas hydrophila. Infect Immun 67:5192–5199
Wang N, Wu Y, Pang M, Liu J, Lu C, Liu Y (2015) Protective efficacy of recombinant hemolysin co-regulated protein (Hcp) of Aeromonas hydrophila in common carp (Cyprinus carpio). Fish Shellfish Immunol 46:297–304
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Pang M, Lin X, Hu M, Li J, Lu C, Liu Y (2012) Tetrahymena: an alternative model host for evaluating virulence of Aeromonas strains. PLoS One 7:e48922
Wang Z, Guo C, Xu Y, Liu G, Lu C, Liu Y (2014) Two novel functions of hyaluronidase from Streptococcus agalactiae are enhanced intracellular survival and inhibition of proinflammatory cytokine expression. Infect Immun 82:2615–2625
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent endpoints12. Am J Epidemiol 27:493–497
Vareille M, de Sablet T, Hindre T, Martin C, Gobert AP (2007) Nitric oxide inhibits Shiga-toxin synthesis by enterohemorrhagic Escherichia coli. Proc Natl Acad Sci U S A 104:10199–10204
Branchu P, Matrat S, Vareille M, Garrivier A, Durand A, Crepin S, Harel J, Jubelin G, Gobert AP (2014) NsrR, GadE, and GadX interplay in repressing expression of the Escherichia coli O157:H7 LEE pathogenicity island in response to nitric oxide. PLoS Pathog 10:e1003874
Tucker NP, D’Autreaux B, Spiro S, Dixon R (2006) Mechanism of transcriptional regulation by the Escherichia coli nitric oxide sensor NorR. Biochem Soc Trans 34:191–194
Hutchings MI, Mandhana N, Spiro S (2002) The NorR protein of Escherichia coli activates expression of the flavorubredoxin gene norV in response to reactive nitrogen species. J Bacteriol 184:4640–4643
Babujee L, Apodaca J, Balakrishnan V, Liss P, Kiley PJ, Charkowski AO, Glasner JD, Perna NT (2012) Evolution of the metabolic and regulatory networks associated with oxygen availability in two phytopathogenic enterobacteria. BMC Genomics 13:110
Parker JL, Shaw JG (2011) Aeromonas spp. clinical microbiology and disease. J Infect 62:109–118
Valerio E, Chaves S, Tenreiro R (2010) Diversity and impact of prokaryotic toxins on aquatic environments: a review. Toxins 2:2359–2410
Li G, Howard SP (2010) ExeA binds to peptidoglycan and forms a multimer for assembly of the type II secretion apparatus in Aeromonas hydrophila. Mol Microbiol 76:772–781
Strozen TG, Stanley H, Gu Y, Boyd J, Bagdasarian M, Sandkvist M, Howard SP (2011) Involvement of the GspAB complex in assembly of the type II secretion system secretin of Aeromonas and Vibrio species. J Bacteriol 193:2322–2331
Sha J, Rosenzweig JA, Kozlova EV, Wang S, Erova TE, Kirtley ML, van Lier CJ, Chopra AK (2013) Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis. Microbiology 159:1120–1135
Suarez G, Sierra JC, Sha J, Wang S, Erova TE, Fadl AA, Foltz SM, Horneman AJ, Chopra AK (2008) Molecular characterization of a functional type VI secretion system from a clinical isolate of Aeromonas hydrophila. Microb Pathog 44:344–361
Lopes JP, Stylianou M, Backman E, Holmberg S, Jass J, Claesson R, Urban CF (2018) Evasion of immune surveillance in low oxygen environments enhances Candida albicans virulence. MBio 9:e02120-18
Poole RK, Hughes MN (2000) New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol Microbiol 36:775–783
van Wonderen JH, Burlat B, Richardson DJ, Cheesman MR, Butt JN (2008) The nitric oxide reductase activity of cytochrome c nitrite reductase from Escherichia coli. J Biol Chem 283:9587–9594
Silverman JM, Brunet YR, Cascales E, Mougous JD (2012) Structure and regulation of the type VI secretion system. Ann Rev Microbiol 66:453–472
Shatalin K, Gusarov I, Avetissova E, Shatalina Y, McQuade LE, Lippard SJ, Nudler E (2008) Bacillus anthracis-derived nitric oxide is essential for pathogen virulence and survival in macrophages. Proc Natl Acad Sci U S A 105:1009–1013
Acknowledgements
The authors thank Dr Furqan Awan for editing the manuscript.
Funding
This study was funded by the Three New Aquatic Projects in Jiangsu Province (D2017-3-1), Independent Innovation Fund of Agricultural Science and Technology in Jiangsu Province (CX(17)2027, CX(18)2011), the National Nature Science Foundation of China (31372454) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
JL carried out most of the experiments described in the manuscript and wrote the article; YD, NW, SM and CL participated in the design of the study and performed the statistical analysis. YL provided expertise and conceived the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The animal experiment was performed in accordance with the animal welfare standards, complied with the guidelines of the Animal Welfare Council of China, and was approved by the Ethical Committee for Animal Experiments of Nanjing Agricultural University, China (approval number: SYXK(Su) 2017-0007).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 2. Kinetics of NO release in LB medium by SNP.
(A) Standard curve between NO2− concentration and optical density (OD540) was determined by Greiss assay using sodium nitrite (NaNO2) as a standard. (B) LB media containing various concentrations of SNP were incubated at 28 °C. The concentration of NO2− in LB medium was evaluated by Greiss assay over time. Data are presented as the mean ± SD of three independent experiments.
Additional file 3. The mRNA levels of upstream (
norR) and downstream (norW) genes of the norV-deletion region. A. hydrophila wild-type, ΔnorV mutant, and complemented CΔnorV strains were cultured with or without SNP to OD600 of 0.8 both under aerobic and anaerobic environment. Cells were collected and the RNA was extracted. The expression values of norR or norW gene were determined by qRT-PCR. The relative change in corresponding gene expression was normalized to the expression of a housekeeping gene (recA) and calculated by the 2−ΔΔCT method. The fold changes in mRNA level of genes in ΔnorV mutant and complemented CΔnorV strain were normalized to that in A. hydrophila NJ-35 under the same condition. (A) The mRNA levels of norR under aerobic conditions. (B) The mRNA levels of norR under anaerobic conditions. (C) The mRNA levels of norW under aerobic conditions. (D) The mRNA levels of norW under anaerobic conditions. Values are the means ± SD from three biological replicates.
Additional file 4. The mRNA levels of flavohemoglobin coding gene
hmp and cytochrome c nitrite reductase coding gene nrfA. A. hydrophila wild-type, ΔnorV mutant, and complemented CΔnorV strains were cultured with or without SNP to OD600 of 0.8 both under aerobic and anaerobic environment. Cells were collected and the RNA was extracted. The expression values of hmp and nrfA genes were determined by qRT-PCR. The relative change in corresponding gene expression was normalized to the expression of a housekeeping gene (recA) and calculated by the 2−ΔΔCT method. The fold changes in mRNA level of genes in ΔnorV mutant and complemented CΔnorV strain were normalized to that in A. hydrophila NJ-35 under the same condition. (A) The mRNA levels of hmp under aerobic conditions. (B) The mRNA levels of hmp under anaerobic conditions. (C) The mRNA levels of nrfA under aerobic conditions. (D) The mRNA levels of nrfA under anaerobic conditions. Values are the means ± SD from three biological replicates.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, J., Dong, Y., Wang, N. et al. Diverse effects of nitric oxide reductase NorV on Aeromonas hydrophila virulence-associated traits under aerobic and anaerobic conditions. Vet Res 50, 67 (2019). https://doi.org/10.1186/s13567-019-0683-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-019-0683-6