Abstract
This study aimed to determine the porcine circovirus type 2 (PCV2) serological and virological dynamics in piglets vaccinated at different ages in a PCV2 subclinical infection (PCV2-SI) scenario. Six hundred and forty-four 2 week-old healthy piglets were selected and distributed into four treatment groups: vaccination at 3, 6 or 10 weeks of age (3W-VAC, 6W-VAC and 10W-VAC groups, respectively) and unvaccinated pigs (NON-VAC group). Blood (n = 112 pigs) and oral fluid (OF) (n = 40 pens) samples were taken throughout the study to assess PCV2 load, humoral immunity and viral genotyping. Percentage of PCV2-DNA positive sera mainly raised by 10 weeks of age, being maximum at 14 weeks of age, and then started to decrease at 18 and 25 weeks of age. Specifically, PCV2 vaccination at 3 or 6 weeks of age yielded similar results, since they produced an earlier seroconversion and reduced, at different sampling points, the proportion of viremic animals in comparison to the unvaccinated group. In contrast, PCV2 vaccination at 10 weeks of age only achieved such reduction at 25 weeks of age; in this case, vaccination coincided with the increase of the percentage of viremic pigs in the population. Both serological techniques used in sera and OF offered similar results with a high and statistically significant correlation. In contrast, a higher percentage of PCV2 DNA positivity was detected in OF in comparison with sera. In conclusion, under the present study conditions, the optimal time for PCV2 piglet vaccination was at either 3 or 6 weeks of age.
Similar content being viewed by others
Introduction
Porcine circovirus type 2 (PCV2) is one of the most prevalent viruses that cause great economic losses to the worldwide pig industry [1]. This virus has an ubiquitous nature in the pig population and is the causative agent of a number of clinical and subclinical conditions named Porcine circovirus diseases (PCVDs) [2].
Efficacy of PCV2 commercial vaccines has been widely demonstrated under field conditions. Major effects have been seen on the reduction of the impact of PCV2 systemic disease (PCV2-SD), improvement of production parameters, decrease of co-infections, and reduction of PCV2 viremia and shedding [3]. Indeed, the wide and constant use of such vaccines in PCV2-SD scenarios has led, in most farms, to a PCV2 subclinical infection (PCV2-SI) scenario [4]. Moreover, most of the pigs from PCV2-SD affected farms but not displaying this condition also suffer from a PCV2-SI. Furthermore, vaccination against PCV2 has been shown to be economically worthy even in PCV2-SI scenarios [5–8].
The most common age of piglet vaccination against PCV2 is at 3–4 weeks of age (around weaning). However, when a sow and piglet vaccination strategy is planned, a delayed piglet vaccination should be considered in order to achieve higher vaccine efficacy [9]. Under no vaccinated sow scenario, little information is available whether the 3–4 week vaccination-age offers the best profit. Although PCV2 vaccines are routinely used in most of the worldwide porcine production systems, peer-reviewed studies comparing the efficacy of PCV2 vaccination at different ages are scarce in experimental [10, 11] and, particularly, under field conditions [12].
Serum is the most commonly used sample to assess PCV2 antibody and genome detection [2]. However, blood sampling is an individual and invasive method. Oral fluid (OF) is an economic and easy-to-take sample for detecting antibodies and pathogens in a pig population [13–15]. This fact allows a more frequent herd monitoring and a greater representativeness of the animal group. During last years, PCV2 dynamics after natural [16, 17] or experimental [18] infections has been efficiently monitored by OF samples, using both enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) techniques. However, information regarding PCV2 assessment in terms of viral load and antibody levels in OF after immunization is limited [19].
The objectives of the present study were: (a) to determine the optimal time for PCV2 vaccination, in terms of serological and virological parameters, in pigs vaccinated at 3, 6 or 10 weeks of age in a PCV2-SI scenario under common PCV2 circulation timings, and (b) to expand the knowledge on the use of OF samples to detect PCV2 DNA and antibodies.
Materials and methods
Farm selection
The study was conducted in a conventional pig farm, located in Catalonia (Spain). In order to assess PCV2 infection status before the start of the study, a cross-sectional seroprofiling was performed including 10 pigs per batch of 6 age groups (3, 7, 11, 15, 19 and 23 weeks of age). Blood samples were processed by standard PCR [20] and ELISA (Ingezim Circo IgG 11.PCV.K1®, Ingenasa, Madrid, Spain) to detect viral nucleic acid and antibodies (IgGs), respectively. PCV2 genome was detected in 50, 30, 20 and 10% of the sampled pigs at 11, 15, 19 and 23 weeks of age, respectively. All the 3 and 7 weeks tested samples were negative by PCR. Seroconversion was detected from the 11th week of age onwards. Therefore, as no PCVDs clinical signs were evident in the farm, PCV2-SI was confirmed.
This farm was a two-site herd with 800 sows with all-in/all-out management and 4-week batch farrowing system. PCV2 vaccination in sows and piglets had never been applied in the studied herd. Sows were routinely vaccinated against porcine reproductive and respiratory syndrome virus (PRRSV), Aujeszky’s disease virus, Swine influenza virus, porcine parvovirus, Erysipelothrix rhusiopathiae, Escherichia coli and Clostridium perfringens. Piglets were vaccinated against Mycoplasma hyopneumoniae 3 days pre-weaning. Weaning was performed at 3 weeks of age and pigs were moved to fattening units at 10 weeks of age. Moreover, no signs of any major pig diseases were present and herd immunity status against PRRSV was determined as “positive-stable” (II-A) according to the previously described classification [21].
Study design
Six-hundred and forty-four 2 week-old healthy crossbred piglets were selected in one single farrowing batch. These piglets came from 59 PCV2 non-vaccinated sows with low number of weak and cross-fostered piglets in their litters. Piglets were individually identified (ear-tagged), bled and their gender was recorded. Blood samples were tested by ELISA (Ingezim Circo IgG 11.PCV.K1®). Cross-fostered piglets were not included in the trial. At 3 weeks of age, animals were randomly allocated in four treatment groups (Table 1). Groups were randomized according to PCV2 ELISA S/P values, sex and litter. Animals from different treatment groups were housed in different pens (32 pens in nursery and 56 pens in fattening units) following a chessboard pattern. Pigs were vaccinated by intramuscular injection with 0.5 mL (single dose) of a commercial inactivated PCV2 vaccine (CIRCOVAC®, Merial SAS, Lyon, France) at either 3, 6 or 10 weeks of age (3W-VAC, 6W-VAC and 10W-VAC groups, respectively), and another group of pigs was kept unvaccinated (NON-VAC group).
Among all animals included in the study, 28 animals per group (14 males and 14 females) with a medium antibody titre (ranging from 0.07 to 1.24 ELISA S/P values at 2 weeks of age) and equally distributed in all pens (2 or 4 piglets per pen in nursery and 2 piglets per pen in fattening units) were randomly selected to be bled. From these animals, a blood sample was taken at 6, 10, 14, 18 and 25 weeks of age. Whole blood samples were allowed clotting, and centrifuged at 3200 rpm during 20 min at 4 °C. All sera were aliquoted and stored at −20 °C until testing.
Oral fluid samples were collected from a representative number of pens (24 nursery and 40 fattening pens) located at the entrance, middle and final area of the nursery and fattening units. OF were taken simultaneously to blood collection by suspending a non-treated, 3-strand, 100% cotton rope in each pen for 30 min [22]. Each rope was manually squeezed inside a single-use plastic bag for OF extraction; then, the corner of the bag was cut and the sample was poured into a sterile tube. To avoid cross-contaminations, all materials (bag, globes, tube) were changed or disinfected (scissors) between pens. Once in the laboratory, samples were centrifuged at 1000×g during 10 min at 4 °C for clearing the sample [14]; then, the supernatant was aliquoted and frozen at −80 °C until use.
From 2 to 25 weeks of age (at each vaccination or bleeding point), all pigs included in the study were monitored for clinical signs and mortality. Animals with major pathologies (hernia, lameness, injuries, etc.) were excluded from the study. Housing conditions, feeding system, feed characteristics and health management remained consistent along the course of the trial, and were the same among all experimental groups. The present study was approved by the Ethics Committee for Animal Experimentation from the Universitat Autònoma de Barcelona and the Animal Experimentation Commission from the local government (Dpt. de Medi Ambient i Habitatge from the Generalitat de Catalunya; Reference 5796).
DNA extraction and real-time quantitative PCR in serum and OF samples
DNA was extracted from 200 µL of serum or 300 µL of OF samples, by using the MagMAX™ Pathogen RNA/DNA Kit (Applied Biosystems) following the manufacturer’s instructions. The DNA obtained was suspended in 90 µL of elution solution.
To quantify the PCV2 DNA in serum and OF samples, a real-time qPCR assay (LSI VetMAX™ Porcine Circovirus Type 2 Quantification, Life Technologies) was performed. Each extraction and qPCR plate included negative controls (diethylpyrocarbonate (DEPC)-treated water) and each sample reaction had an internal positive control (IPC) to monitor DNA extraction and amplification procedures. Viral concentrations were expressed as the mean log10 PCV2 genome copies/mL. Area under the curve (AUC) of viral load in serum samples from 2 to 25 weeks of age was calculated according to the trapezoidal method as previously described [23].
Serology
Indirect ELISA for detecting anti-PCV2 IgG antibodies in serum samples
All serum samples were tested by the Ingezim Circo IgG 11.PCV.K1® assay. The optical density (OD) was measured at 450 nm by the PowerWave XS reader (BioTek). Mean positive cut-off was established at 0.3 OD (±SD) following the kit’s instructions (positive cut-off = OD of negative control +0.25). ELISA results were expressed as mean S/P ratio (OD of sample/OD of positive control for each ELISA plate).
Semi-quantitative ELISA for detecting anti-PCV2 antibodies in OF samples
All OF samples were processed by the SERELISA® PCV2 Ab Mono Blocking kit (Synbiotics, Lyon, France) with some modifications (protocol used at Labocea, Ploufragan—personal communication). The analysis of the samples by this technique led to a semi-quantitative result expressed as 1 (+), 2 (++), 3 (+++) or 4 (++++).
Viral neutralization test (VNT)
The ability to neutralize PCV2 was assessed by VNT in 14 randomly selected serum samples per group (half of collected serum samples). This assay was performed as previously described [24], with the following modifications: (1) serum was tested in fourfold dilutions (from 1:4 to 1:4096) using supplemented DMEM (Dulbecco’s Modified Eagle Medium) in 96-well plates (plates were read using a microscope at 10× magnifications), and (2) number of PCV2 infected cells (nuclear and/or cytoplasmic staining) per well in each sample replica was counted. Percentage of virus neutralization (%VN) at each serum dilution was calculated as follows: % VN = [1 − (mean number of infected cells of the two replicas of each serum dilution/mean number of infected cells in negative control wells)] ×100. Then, VNT50 (i.e. reciprocal of the last dilution of the serum sample in which the number of PCV2 infected cells was reduced to a 50%) was calculated and designated as the neutralizing antibody (NA) titre. Results were expressed as log2 NA titre.
PCV2 amplification and sequencing
With the aim of determining the main PCV2 genotype circulating in the farm, the capsid protein gene (ORF2) was sequenced from two PCV2 qPCR positive samples per treatment group. Amplification was done from nucleotide 1050 to 1735 (PCV2 genome; GenBank Accession Number: AY181948) using primers PCV2all_F (5′ GGGTCTTTAAGATTAAATYC 3′) and PCV2all_R (5′ ATGACGTATCCAAGGAG 3′). PCR was developed in a 25 μL reaction containing 1.25 μL of each mentioned primer at 10 pmol/μL, 5 μL of 5 × PCR buffer, 2.5 μL of MgCl2 at 25 mM, 0.75 U of Taq DNA polymerase, 1 μL of dNTP stock solution at 5 mM, 11.35 μL of DEPC-treated water and 2.5 μL of extracted DNA. The PCR was started with an initial denaturation step of 5 min at 94 °C. The temperature profile of the following 40 cycles consisted of 30 s at 95 °C for denaturation, 30 s at 53 °C for primer annealing and 40 s at 72 °C for elongation. The reaction was terminated by a final elongation step of 7 min at 72 °C. Amplified PCR product was run in an electrophoresis gel with 1.8% agarose. The band was purified using NucleoSpin Gel and PCR Clean-up kit (Macherey–Nagel, GmbH & Co. KG, Germany) according to the manufacturer’s instruction. Sequencing reactions were performed with BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and analysed using a 3130 × l Genetic Analyser (Applied Biosystems).
PCV2 capsid protein (ORF2) phylogenetic and sequence analysis
Nucleotide sequences of the PCV2 capsid protein were analysed using Bioedit v7.0.9.0 [25]. Sequences were aligned using the Clustal W multiple alignment method included in the Bioedit package [26]. Fifteen strains of different PCV2 genotypes retrieved from the GenBank database were included in the comparison. The phylogenetic tree was constructed according to the Neighbor-Joining method with 1000 bootstrap replicates using MEGA version 4 [27].
Statistical analyses
Animal mortality and exclusion rates between groups were compared using the likelihood ratio test. Generalized linear mixed models for longitudinal binary data were performed to analyse the evolution between groups for PCV2 qPCR (positive/negative) values in pigs (serum samples) and pens (OF samples). Treatment group, sampling point and their interaction were considered as fixed effects, and piglet and pen as random effects. Whenever differences between groups were detected, they were further evaluated by pairwise comparisons. p-values were corrected using Tukey’s method. Generalized linear mixed models were applied for longitudinal continuous data such as mean log10 PCV2 copies/mL in qPCR positive serum and OF samples, mean ELISA S/P IgG values in sera, mean ELISA semi-quantitative values in OF and log2 NA titre in sera. The comparison of PCV2 AUC load in serum samples between groups was analysed by a non-parametric test (Kruskall-Wallis statistic). Pearson’s correlation coefficient was used to assess the relationship between serum and OF results (ELISA and qPCR), as well as between ELISA values from both serum and OF samples in comparison to NA titres in serum samples. Statistical analyses were carried out using SAS v9.4, SAS Institute Inc., Cary, NC, USA. The significance level was set at p < 0.05.
Results
Clinical signs and mortality
No clinical signs related to PCV2-SD were observed during the course of the study. No statistically significant differences in terms of mortality rate and animal exclusion (ranging from 2.5 to 7.5% in all groups) were observed among treatment groups during the whole experimental period (data not shown).
Quantification of PCV2 DNA
Serum samples
While very few pigs were qPCR positive at 3 and 6 weeks of age, the percentage of PCV2-DNA positive pig sera raised at 10 weeks of age, was maximum at 14 weeks of age and then started to decrease by 18 and 25 weeks of age. Particularly, animals from 3W-VAC to 6W-VAC groups had a significantly lower (p < 0.05) percentage of viremic animals (Figure 1A) compared to the NON-VAC group at 14, 18 and 25 weeks of age (in 3W-VAC group) and at 14 and 18 weeks of age (in 6W-VAC group). In contrast, the 10W-VAC group showed a higher percentage of viremic pigs than 3W-VAC and 6W-VAC groups at 10, 14 and 18 weeks of age (only significantly different at 18 weeks of age), but lower than that of control group at 14, 18 and 25 weeks of age (only significant at 25 weeks of age). At the peak of infection (14 weeks of age), the 3W-VAC group showed a significantly lower (p < 0.05) PCV2 load (Figure 1B) than the NON-VAC group. The 6W-VAC and 10W-VAC groups also showed the same trend with lower viral loads than the NON-VAC group but these differences were not statistically significant. The 3W-VAC and 6W-VAC groups experienced a significantly lower AUC of viral load than the NON-VAC group (p < 0.05) (Table 2). However, PCV2 AUC of 10W-VAC group was only numerically lower than that of the NON-VAC group.
OF samples
At 3 weeks of age, OF collection was not possible since piglets did not chew the ropes. Percentage of PCV2 qPCR positive pens (Figure 2A) was high at all sampling points and no statistically significant differences among treatment groups were observed. At the peak of infection (14 weeks of age), 100% of positivity was observed in all groups. At this time point, viral load (Figure 2B) was numerically lower in all the vaccinated groups compared to the control group.
Virological results obtained from serum and OF samples showed positive but non-significant correlations: percentage of PCV2 qPCR positive samples (r = 0.86; p = 0.06) and viral load (r = 0.76; p = 0.13).
Serology
Anti-PCV2 IgG antibody levels in serum samples
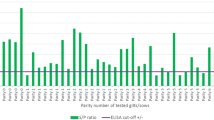
The course of antibodies against PCV2 in the four treatment groups is shown in Figure 3. From 2 to 6 weeks of age, all groups presented a decrease of ELISA S/P values and no differences between groups were observed. At 10 weeks of age, 3W-VAC and 6W-VAC groups showed significantly higher (p < 0.05) S/P values than 10W-VAC and NON-VAC groups. The 10W-VAC group seroconverted by 14 weeks of age, reaching significantly higher antibody levels at 18 weeks of age compared to the other groups. The NON-VAC group seroconverted by 14 to 18 weeks of age. From this time point onwards, S/P values of the vaccinated groups began to decrease whereas the ones of the control group remained stable.
Anti-PCV2 antibody levels in OF samples
Mean semi-quantitative antibody values in OF are summarized in Figure 4. At 10 weeks of age, the 3W-VAC group displayed a statistically significant increase of antibody response compared to all other groups. At the same time point, the other groups experienced a decrease of antibody levels, being the ones of the 6W-VAC group significantly higher than the ones of the NON-VAC group. Four weeks later, i.e. at the peak of infection, all vaccinated groups showed significantly higher antibody values than the NON-VAC group. From 18 weeks of age onwards, antibody levels from all groups remained high and no significant differences were observed between them.
A high and statistically significant correlation (r = 0.95, p = 0.015) between serum and OF ELISA results was observed.
Neutralizing antibody titres in serum samples
Mean NA titres (± SD) dynamics for each treatment group is depicted in Figure 5. From 2 to 6 weeks of age, all groups showed a decrease of NA titres and no differences between groups were observed. Subsequently, pigs from groups 3W-VAC and 6W-VAC had significantly higher NA levels compared to the 10W-VAC (at 10 weeks of age) and NON-VAC (at 10 and 14 weeks of age) pigs. In the 10W-VAC group, the increase of NA titres was observed 4 weeks after vaccination, i.e. 14 weeks of age, being significantly higher than the ones in NON-VAC pigs. The NA response for animals of the NON-VAC group was detected at 14 weeks of age, reaching maximum levels at 18 weeks of age. After this sampling point, NA levels from all groups began to decrease.
High and statistically significant correlations were found between NA titres tested in serum samples in comparison to ELISA values detected in serum (r = 0.97, p = 0.001) and OF (r = 0.90, p = 0.038) samples.
PCV2 genotyping
A Neighbor-Joining phylogenetic tree including the relationships among the PCV2 isolates sequenced in this study (two per experimental group) and reference strains is represented in Figure 6. All serum samples sequenced (GenBank accession numbers: KX670778–KX670785) were genetically closely related and clustered within PCV2a genotype.
Neighbor-Joining phylogenetic tree with 1000 bootstrap replicates showing the relationships among the nucleotide sequences of the PCV2 capsid protein. PCV2 strains sequenced in this study from 3W-VAC (*), 6W-VAC (**), 10W-VAC (***) and NON-VAC (****) groups are compared to PCV2 types a, b, c and d strains. Horizontal branches indicate the sequence distance (number of base differences per site).
Discussion
Several studies have shown that PCV2 piglet vaccination at weaning age (3–4 weeks of age) is effective regardless of the PCVD farm status (PCV2-SD or PCV2-SI) and the brand of commercial vaccine used [3]. Vaccination schedules at earlier ages with one single dose are rarely applied, since high levels of maternally derived antibodies (MDA) at the vaccination time may cause a lower humoral immune response (interference with the seroconversion) elicited by the vaccine [6, 11, 28, 29] and may eventually jeopardize the efficacy of vaccination [8, 30].
In the peer-reviewed literature, little information does exist on PCV2 vaccine efficacy obtained by comparing vaccination of piglets (coming from non-vaccinated sows) at different postweaning ages [11, 12]. In the present study, PCV2 vaccination in piglets at 3 or 6 weeks of age yielded similar virological and serological results, producing a relatively early humoral immune response and reducing the proportion of viremic animals in comparison to the unvaccinated group. These results are in accordance with two previously published trials, where no statistically significant differences in terms of PCV2 viremia and/or humoral and cellular immunity were found between pigs vaccinated at 3 and 6 weeks of age [12], and at 3–7 weeks of age [11]. In addition, it has also been demonstrated that vaccination of older animals (8.5 weeks of age) with a subunit vaccine and under a PCV2-SD scenario resulted in a significantly lower mortality in vaccinates than in controls [31]. Although in this latter study, serological and virological parameters from pigs vaccinated at this time point were not tested, the vaccination took place, most probably, when a proportion of pigs were already infected. As far as the authors knowledge, no more information is available on the efficacy of piglet vaccination at older ages. Thus, the present study represents the first time comparing the use of vaccination at 10 weeks of age (entering to the fattening facilities) with earlier ages. Under the conditions of the present farm trial, PCV2 vaccination at 10 weeks of age was probably done too late for an optimal performance as it coincided with the increase of the percentage of viremic pigs in all the treatment groups. Under field conditions, PCV2 viremia usually starts at the end of the nursery or at the beginning of fattening periods, although it is variable depending on the farm and even the production batch [32]. In the present farm scenario, vaccination at 10 weeks of age was able to numerically reduce the percentage of viremic animals at 14 (peak of infection), 18 and 25 weeks of age in comparison with the control group, being statistically significant at the latter time point. This evidence is in agreement with a previous experimental trial [33], showing that vaccination of PCV2 viremic and seropositive piglets leads to a humoral and cellular immune response able to reduce PCV2 viremia. Therefore, although not optimal, vaccination of viremic pigs seems to exert a positive effect compared to viremic, non-vaccinated ones.
The current work further demonstrated the ability of an inactivated vaccine to produce a NA response after piglet immunization at different ages. This response led to a significantly greater protection (in terms of PCV2 viremia) of groups vaccinated before natural infection compared to the group vaccinated after the onset of infection, i.e. at 10 weeks of age, and the control group. The inverse dynamics between NA titres and PCV2 load in serum found in the present study had previously been described [24, 34, 35]. In addition, an immune response analysis of the four major vaccines available on the market was performed in a recent study [36], confirming the ability of the inactivated vaccine used in this study to induce a NA response after vaccination, producing higher NA levels than the ones from subunit vaccines. Moreover, in the current work, high and statistically significant correlations were found between ELISA values from both serum and OF samples in comparison to NA titres in sera. This finding suggests that antibody levels tested by the used ELISA kits might be used as potential predictor of NA titres.
Both serological techniques used in serum and OF samples offered similar results with a high and statistically significant correlation among them. These results would suggest that OF samples can be an alternative to serum for studying PCV2 antibody dynamics. This outcome is in agreement with a reported trial [16] in which positive correlation between OF and pooled sera in terms of antibody detection was found. In contrast, a higher PCV2 qPCR positivity was detected in the present study from OF in comparison with sera at all sampling points, and no significant differences between treatment groups were observed by using OF. Moreover, PCV2 circulation was detected earlier in OF (from 6 weeks of age) compared to sera (from 10 weeks of age) in all groups of pigs. These findings are in accordance with a previous study [16] and support the fact that the starting time of PCV2 circulation and viremia might be different. However, in terms of PCV2 load, whereas similar levels of PCV2 DNA in OF and serum samples with a significant correlation were described [16], higher mean viral loads in OF (over one logarithm) with no significant correlation to PCV2 loads in sera were detected in the current study at infection peak (14 weeks of age). The higher qPCR positivity percentages and PCV2 load in OF compared to sera may be explained by a number of reasons. First, serum samples were obtained from only two or four pigs per pen, but OF sample was a collective sample representing around 23 or 11 pigs per nursery or fattening pen, respectively. Therefore, there is a reasonable probability that some viremic/shedder animals were not bled or alternatively that the bled subjects were not the ones with the highest viral loads. Second, since PCV2 replicates firstly in the tonsil [37, 38], it might be probably detected at an earlier stage and with a greater concentration in OF with regards to sera as has been previously suggested [16]. Finally, PCV2 is an endemic and very stable virus [39] that might be ever-present in pens [40]. In fact, it has been demonstrated that PCV2 subclinically infected pigs may excrete medium to high viral loads in faeces [41, 42]. Therefore, it should be taken into account that ropes might be spoiled by the traces of faeces present in the mouth/skin of the pigs.
In all sequenced samples (n = 8), PCV2a genotype was identified. Although this genotype has a worldwide distribution [43], the most current prevalent genotype in the pig population is PCV2b [1, 44]. Indeed, it has been proposed that PCV2b is more prevalent than PCV2a in PCV2-SD cases and in vaccinated farms [45]. The PCV2-SI scenario in the studied farm and the fact that no PCVD compatible clinical signs had ever been observed before the start of the study (and in consequence, vaccination had never been applied before this trial) might be related with the detection of PCV2a genotype in the farm. The apparent sole presence of PCV2a genotype was not enough to produce overt disease in this farm. In fact, the speculation that PCV2a might not be as efficient as PCV2b to trigger clinically evident disease came from the demonstration of a worldwide genotype shift from PCV2a to PCV2b coinciding with major outbreaks of PCV2-SD around the globe [1].
In conclusion, under the conditions of this study, the optimal age for piglet vaccination was at 3 or 6 weeks of age, since it was applied when the percentage of viremic pigs was minimal, triggering an effective humoral immune response before the peak of infection. These strategies were able to reduce, at different sampling points, the proportion of viremic animals in comparison to the unvaccinated group. In contrast, PCV2 vaccination at 10 weeks of age (coinciding with the increase of the percentage of viremic pigs in the population) only achieved such reduction at 25 weeks of age. Therefore, age at PCV2 vaccination should be adapted according to the viral infection dynamics present in the studied farm. Moreover, both serological techniques used in sera and OF were useful to study PCV2 antibody dynamics. In contrast, viral detection in OF might be useful to have an idea of the infection dynamics at population level but should remain only as a raw indicative method.
Abbreviations
- AUC:
-
area under the curve
- DEPC:
-
diethylpyrocarbonate
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- ELISA:
-
enzyme-linked immunosorbent assay
- Ig:
-
immunoglobulin
- IPC:
-
internal positive control
- MDA:
-
maternally derived antibodies
- NA:
-
neutralizing antibodies
- OD:
-
optical density
- OF:
-
oral fluid
- PCR:
-
polymerase chain reaction
- PCV2:
-
porcine circovirus type 2
- PCV2-SD:
-
PCV2 systemic disease
- PCV2-SI:
-
PCV2 subclinical infection
- PCVD:
-
porcine circovirus disease
- PRRSV:
-
porcine reproductive and respiratory syndrome virus
- qPCR:
-
quantitative PCR
- S/P:
-
sample to positive
- SD:
-
standard deviation
- VNT:
-
viral neutralization test
- %VN:
-
percentage of virus neutralization
References
Segalés J, Kekarainen T, Cortey M (2013) The natural history of porcine circovirus type 2: from an inoffensive virus to a devastating swine disease? Vet Microbiol 165:13–20
Segalés J (2012) Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res 164:10–19
Segalés J (2015) Best practice and future challenges for vaccination against porcine circovirus type 2. Expert Rev Vaccines 14:473–487
Dvorak CM, Yang Y, Haley C, Sharma N, Murtaugh MP (2016) National reduction in porcine circovirus type 2 prevalence following introduction of vaccination. Vet Microbiol 189:86–90
Young MG, Cunningham GL, Sanford SE (2011) Circovirus vaccination in pigs with subclinical porcine circovirus type 2 infection complicated by ileitis. J Swine Health Prod 19:175–180
Fraile L, Grau-Roma L, Sarasola P, Sinovas N, Nofrarías M, López-Jimenez R, López-Soria S, Sibila M, Segalés J (2012) Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: improvement of production parameters and interaction with maternally derived immunity. Vaccine 30:1986–1992
Alarcón P, Rushton J, Wieland B (2013) Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England - an economic disease model. Prev Vet Med 110:88–102
Feng H, Segalés J, Fraile L, López-Soria S, Sibila M (2016) Effect of high and low levels of maternally derived antibodies on porcine circovirus type 2 (PCV2) infection dynamics and production parameters in PCV2 vaccinated pigs under field conditions. Vaccine 34:3044–3050
Martelli P, Saleri R, Ferrarini G, De Angelis E, Cavalli V, Benetti M, Ferrari L, Canelli E, Bonilauri P, Arioli E, Caleffi A, Nathues H, Borghetti P (2016) Impact of maternally derived immunity on piglets’ immune response and protection against porcine circovirus type 2 (PCV2) after vaccination against PCV2 at different age. BMC Vet Res 12:77
O’Neill KC, Shen HG, Lin K, Hemann M, Beach NM, Meng XJ, Halbur PG, Opriessnig T (2011) Studies on porcine circovirus type 2 vaccination of 5-day-old piglets. Clin Vaccine Immunol 18:1865–1871
Oh Y, Seo HW, Park C, Chae C (2014) Comparison of sow and/or piglet vaccination of 3 commercial porcine circovirus type 2 (PCV2) single-dose vaccines on pigs under experimental PCV2 challenge. Vet Microbiol 172:371–380
Cline G, Wilt V, Diaz E, Edler R (2008) Efficacy of immunising pigs against porcine circovirus type 2 at three or six weeks of age. Vet Rec 163:737–740
Prickett JR, Zimmerman JJ (2010) The development of oral fluid-based diagnostics and applications in veterinary medicine. Anim Health Res Rev 11:207–216
Kittawornrat A, Prickett J, Chittick W, Wang C, Engle M, Johnson J, Patnayak D, Schwartz T, Whitney D, Olsen C, Schwartz K, Zimmerman J (2010) Porcine reproductive and respiratory syndrome virus (PRRSV) in serum and oral fluid samples from individual boars: will oral fluid replace serum for PRRSV surveillance? Virus Res 154:170–176
Prickett J, Simer R, Christopher-Hennings J, Yoon KJ, Evans RB, Zimmerman JJ (2008) Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: a longitudinal study under experimental conditions. J Vet Diagn Invest 20:156–163
Kim WI (2010) Application of oral fluid sample to monitor porcine circovirus-2 infection in pig farms. J Vet Clin 27:704–712
Ramirez A, Wang C, Prickett JR, Pogranichniy R, Yoon KJ, Main R, Johnson JK, Rademacher C, Hoogland M, Hoffmann P, Kurtz A, Kurtz E, Zimmerman J (2012) Efficient surveillance of pig populations using oral fluids. Prev Vet Med 104:292–300
Prickett JR, Johnson J, Murtaugh MP, Puvanendiran S, Wang C, Zimmerman JJ, Opriessnig T (2011) Prolonged detection of PCV2 and anti-PCV2 antibody in oral fluids following experimental inoculation. Transbound Emerg Dis 58:121–127
Zanotti C, Martinelli N, Lelli D, Amadori M (2015) Correlates of protection following vaccination with inactivated porcine circovirus 2 vaccines. Viral Immunol 28:600–608
Quintana J, Balasch M, Segalés J, Calsamiglia M, Rodríguez-Arrioja GM, Plana-Duran J, Domingo M (2002) Experimental inoculation of porcine circoviruses type 1 (PCV1) and type 2 (PCV2) in rabbits and mice. Vet Res 33:229–237
Holtkamp DJ, Polson DD, Torremorell M, Morrison B, Classen DM, Becton L, Henry S, Rodibaugh MT, Rowland RR, Snelson H, Straw B, Yeske P, Zimmerman J (2011) Terminology for classifying the porcine reproductive and respiratory syndrome virus (PRRSV) status of swine herds. Tierarztl Prax Ausg G Grosstiere Nutztiere 39:101–112 (in German)
Seddon YM, Guy JH, Edwards SA (2012) Optimising oral fluid collection from groups of pigs: effect of housing system and provision of ropes. Vet J 193:180–184
López-Soria S, Sibila M, Nofrarías M, Calsamiglia M, Manzanilla EG, Ramirez-Mendoza H, Minguez A, Serrano JM, Marin O, Joisel F, Charreyre C, Segalés J (2014) Effect of porcine circovirus type 2 (PCV2) load in serum on average daily weight gain during the postweaning period. Vet Microbiol 174:296–301
Fort M, Olvera A, Sibila M, Segalés J, Mateu E (2007) Detection of neutralizing antibodies in postweaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs. Vet Microbiol 125:244–255
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Fort M, Sibila M, Pérez-Martin E, Nofrarías M, Mateu E, Segalés J (2009) One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 27:4031–4037
Fraile L, Sibila M, Nofrarías M, López-Jimenez R, Huerta E, Llorens A, López-Soria S, Pérez D, Segalés J (2012) Effect of sow and piglet porcine circovirus type 2 (PCV2) vaccination on piglet mortality, viraemia, antibody titre and production parameters. Vet Microbiol 161:229–234
Haake M, Palzer A, Rist B, Weissenbacher-Lang C, Fachinger V, Eggen A, Ritzmann M, Eddicks M (2014) Influence of age on the effectiveness of PCV2 vaccination in piglets with high levels of maternally derived antibodies. Vet Microbiol 168:272–280
Desrosiers R, Clark E, Tremblay D, Tremblay R, Polson D (2009) Use of a one-dose subunit vaccine to prevent losses associated with porcine circoviruses type 2. J Swine Health Prod 17:148–154
Segalés J, Gordon A, Domingo M (2012) Porcine circoviruses. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW (eds) Diseases of swine, 10th edn. Wiley, Ames
Seo HW, Park C, Han K, Chae C (2014) Effect of porcine circovirus type 2 (PCV2) vaccination on PCV2-viremic piglets after experimental PCV2 challenge. Vet Res 45:13
Seo HW, Han K, Oh Y, Park C, Chae C (2012) Efficacy of a reformulated inactivated chimeric PCV1-2 vaccine based on clinical, virological, pathological and immunological examination under field conditions. Vaccine 30:6671–6677
Meerts P, Misinzo G, Lefebvre D, Nielsen J, Botner A, Kristensen CS, Nauwynck HJ (2006) Correlation between the presence of neutralizing antibodies against porcine circovirus 2 (PCV2) and protection against replication of the virus and development of PCV2-associated disease. BMC Vet Res 2:6
Seo HW, Lee J, Han K, Park C, Chae C (2014) Comparative analyses of humoral and cell-mediated immune responses upon vaccination with different commercially available single-dose porcine circovirus type 2 vaccines. Res Vet Sci 97:38–42
Allan GM, McNeilly F, Meehan BM, Ellis JA, Connor TJ, McNair I, Krakowka S, Kennedy S (2000) A sequential study of experimental infection of pigs with porcine circovirus and porcine parvovirus: immunostaining of cryostat sections and virus isolation. J Vet Med 47:81–94
Rosell C, Segalés J, Plana-Duran J, Balasch M, Rodriguez-Arrioja GM, Kennedy S, Allan GM, McNeilly F, Latimer KS, Domingo M (1999) Pathological, immunohistochemical, and in situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol 120:59–78
Opriessnig T, Meng XJ, Halbur PG (2007) Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest 19:591–615
Dvorak CM, Lilla MP, Baker SR, Murtaugh MP (2013) Multiple routes of porcine circovirus type 2 transmission to piglets in the presence of maternal immunity. Vet Microbiol 166:365–374
López-Rodriguez A, Dewulf J, Meyns T, Del-Pozo-Sacristan R, Andreoni C, Goubier A, Chapat L, Charreyre C, Joisel F, Maes D (2016) Effect of sow vaccination against porcine circovirus type 2 (PCV2) on virological profiles in herds with or without PCV2 systemic disease. Can Vet J 57:619–628
McIntosh KA, Tumber A, Harding JC, Krakowka S, Ellis JA, Hill JE (2009) Development and validation of a SYBR green real-time PCR for the quantification of porcine circovirus type 2 in serum, buffy coat, feces, and multiple tissues. Vet Microbiol 133:23–33
Franzo G, Cortey M, Segales J, Hughes J, Drigo M (2016) Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol Phylogenet Evol 100:269–280
Franzo G, Tucciarone CM, Dotto G, Gigli A, Ceglie L, Drigo M (2015) International trades, local spread and viral evolution: the case of porcine circovirus type 2 (PCV2) strains heterogeneity in Italy. Infect Genet Evol 32:409–415
Shen HG, Halbur PG, Opriessnig T (2012) Prevalence and phylogenetic analysis of the current porcine circovirus 2 genotypes after implementation of widespread vaccination programmes in the USA. J Gen Virol 93:1345–1355
Competing interests
Salvador Oliver-Ferrando, Antonio Callén, Olivier Merdy and François Joisel are employees of MERIAL.
Authors’ contributions
SOF participated in the design of the study, was involved in the field trial, performed the laboratory tests and drafted the manuscript. SLS contributed to the design, coordination and execution of the field trial. OM and FJ conceived the study and participated in its design. MS, JS and AC participated in the study design, were involved in the field trial, and coordinated all activities of this project. All authors read and approved the final manuscript.
Acknowledgements
This work was funded by Merial S.A.S. and by Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya (DI2013-0013). The authors would like to thank Eva Huerta, Anna Llorens, Patricia Pleguezuelos, Rosa López and Diego Pérez and personnel from CATALANA DE PINSOS S.A., for their excellent technical support and willingness to participate in the study. The authors are also very grateful to Luc Mieli from LDA22 (France), for providing the protocol of OF ELISA test as well as the positive control to be used in such test, and Tuija Kekarainen and Martí Cortey (CReSA), for their assistance in the sequencing and phylogenetic tree construction.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Oliver-Ferrando, S., Segalés, J., López-Soria, S. et al. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet Res 47, 121 (2016). https://doi.org/10.1186/s13567-016-0405-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-016-0405-2