Abstract
Background
Post-transplant lymphoproliferative disorders (PTLDs) are a spectrum of hematological malignancies occurring after solid organ and hematopoietic stem cell transplantation. [18F]FDG PET/CT is routinely performed at PTLD diagnosis, allowing for both staging of the disease and quantification of volumetric parameters, such as whole-body metabolic tumor volume (MTV) and total lesion glycolysis (TLG). In this retrospective study, we aimed to determine the prognostic value of MTV and TLG in PTLD patients, together with other variables of interest, such as the International Prognostic Index (IPI), organ transplant type, EBV tumor status, time after transplant, albumin levels and PTLD morphology.
Results
A total of 88 patients were included. The 1-, 3-, 5- year overall survival rates were 67%, 58% and 43% respectively. Multivariable analysis indicated that a high IPI (HR: 1.56, 95% CI: 1.13–2.16) and an EBV-negative tumor (HR: 2.71, 95% CI: 1.38–5.32) were associated with poor overall survival. Patients with a kidney transplant had a longer overall survival than any other organ recipients (HR: 0.38 95% CI: 0.16–0.89). IPI was found to be the best predicting parameter of overall survival in our cohort. Whole-body MTV, TLG, time after transplant, hypoalbuminemia and PTLD morphology were not associated with overall survival.
Conclusion
[18F]FDG PET/CT whole-body volumetric quantitative parameters were not predictive of overall survival in PTLD. In our cohort, high IPI and an EBV-negative tumor were found to predictors of worse overall survival while kidney transplant patients had a longer overall survival compared to other organ transplant recipients
Similar content being viewed by others
Background
Post-transplant lymphoproliferative disorders (PTLDs) are a spectrum of hematological malignancies occurring after solid organ and hematopoietic stem cell transplantation in the setting of pharmacological immunosuppression. In this already vulnerable population, PTLD constitutes a serious health burden, associated with high morbidity and mortality [1]. Although risk-stratified sequential treatment and the introduction of Rituximab have improved outcome, reported 3-year overall survival remains low, ranging from 40 to 70% [2,3,4,5,6]. In an attempt to stratify high-risk patients, various prognostic makers and different prognostic scores have been suggested.
Several classical lymphoma-specific markers have been identified as consistent predictors of overall survival in PTLD cohorts. Indeed, multiple studies have identified age, performance status, elevated lactate dehydrogenase and extra-nodal disease as independent predictors of overall survival [2, 3, 7,8,9,10,11]. Additionally, several other markers have been reported to be predictive of survival including: number/location of involved sites, morphological subtype, time from transplantation, presence of B-symptoms, albumin levels, serum creatine, gender and organ transplanted [2, 3, 7,8,9,10, 12]. Different prognostic scores have also been shown to be significant predictors of overall survival [2, 3, 5, 7,8,9, 12]. The International Prognostic Index (IPI) is a clinically validated tool in the prognostication of aggressive non-Hodgkin lymphoma and its value in PTLD has been established in the PTLD-1 trial [13, 14]. Taking into consideration the particularities of post-transplantation immunocompromised patients, Caillard and colleagues have proposed an PTLD specific prognostic score after kidney transplantation, which nevertheless does not seem to surpass the performance of the IPI [12, 15]. Although current prognostic models allow for some degree of stratification, they fail to perform consistently across all cohorts and are seldomly employed clinically. Therefore, there is a need for new clinically applicable markers.
Quantification of whole-body tumor metabolism may provide additional information, not perceptible with current clinical and biological markers. 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography ([18F]FDG PET/CT) not only allows for anatomical lesion localization, but also for quantification of volumetric parameters, such as whole-body metabolic tumor volume (MTV) and total lesion glycolysis (TLG). As [18F]FDG PET/CT is considered standard-of-care in many institutions and current commercial software packages allow for semi-automatic metabolic quantification, MTV and TLG may become clinically feasible prognostic tools [16]. In immunocompetent lymphoma patients and in particular diffuse large B-cell lymphoma (DLBCL), high baseline MTV and TLG have been reported to be associated with worse survival [17]. However, these volumetric parameters have yet to be evaluated in PTLD.
We performed a retrospective study to determine the prognostic value of baseline whole-body MTV and TLG measurements in patients with newly diagnosed, biopsy-proven PTLD as a primary research goal. Prognostic value of IPI and other markers of interest were analyzed as secondary outcome parameters.
Methods
Study design and patient selection
This retrospective study was performed at the University Medical Center Groningen (UMCG) and the University Hospitals Leuven (UZ Leuven) including biopsy-proven de novo PTLD patients between 2009 and 2019. Patients included in this study underwent an [18F]FDG PET/CT at baseline with reconstruction parameters according to The European Association of Nuclear Medicine Research Ltd (EARL) recommendations [18, 19]. Patients excluded were those in whom accurate segmentation either semi-automatically or visually was not possible (i.e., areas of high background physiological uptake), previously treated PTLD or those with more than 30 days between histopathological confirmation and the [18F]FDG PET/CT. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the approval of the respective ethical committees.
[18F]FDG PET/CT acquisition and semi-quantification.
[18F]FDG PET/CT scans were performed using a Siemens Biograph mCT 40- or 64-slice (Siemens Healthcare, Knoxville, Tennessee, United States) at the UMCG and a Siemens Biograph 16 HiRez, Siemens Truepoint 40 (Siemens Healthcare, Erlangen, Germany) or GE Healthcare Discovery MI4 (GE Healthcare, Chicago, IL, USA) at the UZ Leuven. Patients fasted for a minimum of 6 h and glucose levels were targeted at < 11 mmol/L (range: 3.3 to 14.5 mmol/L) before intravenous [18F]FDG administration (range: 3 to 4.25 MBq [18F]FDG/kg body weight). Sixty minutes after [18F]FDG administration a low-dose CT scan was performed, immediately followed by a whole-body (vertex to mid-thigh) PET scan using a multi-bed position, with 70 to 180 s per bed position. Low-dose CT data were used for attenuation correction of the PET images.
Semi-quantification of volumetric parameters was performed on the Hermes Hybrid 3D software (Hermes Medical Solutions AB, Stockholm, Sweden) by F.M.J. (nuclear medicine research fellow) blinded for all other results with the support of two experienced nuclear physicians (A.W.J.M.G. & W.N.). Extracted volumetric parameters included: whole-body MTV defined as, the total metabolically active volume of the segmented tumors, and whole-body TLG, defined as whole-body MTV × mean standardized uptake value (SUVmean) contained within the volume of interest. TLG was corrected for fasting glucose using the formula: (TLG × fasting glucose in mmol/L)/5. MTV and TLG were interpreted as continuous variables. Lesion segmentation was performed with the “Tumor Finder” application in Hermes Hybrid 3D, in line with PERCIST recommendations [20]. Based on a 14.1-ml spherical volume placed in the right lobe of the liver, lesions above a threshold of 1.5 × liver SUVmean + 2 standard deviations were selected. If the use of the right lobe of the liver as a reference region was not possible (ongoing liver pathology which would impact physiological liver metabolism i.e. diffuse metastatic disease), a 1.6-ml spherical volume was placed in the mediastinal blood pool and lesions selected based on a threshold above 2 × mediastinal blood pool SUVmean + 2 standard deviations [20]. Lesions not automatically segmented but suspected of malignancy were manually added, while any metabolically active focus interpreted as physiological was removed. During manual segmentation, particular attention was paid to extra-nodal lesions and splenic involvement. By diffuse splenic involvement the whole spleen was segmented while by focal involvement, lesions with [18F]FDG uptake higher than background were selected.
International Prognostic Index and prognostic parameters.
The IPI score of each patient was calculated retrospectively, interpreted as a continuous variable [13]. Other potential prognostic markers evaluated were: organ transplant type, Epstein-Barr virus (EBV) tumor status by in situ hybridization (EBV-positive versus EBV-negative), time after transplant (early-PTLD ≤ 1 year versus late-PTLD > 1 year), hypoalbuminemia (defined as albumin < 35 g/L) and PTLD morphology (non-destructive PTLD, polymorphic PTLD, monomorphic PTLD or classic Hodgkin lymphoma-type PTLD).
Statistical analysis
Categorical variables were presented as counts and percentages, while continuous variables as median with interquartile range (IQR). Variables were graphically checked for normality. Cox proportional hazards model was used for survival analysis with overall survival as endpoint, defined as time from diagnosis until death (from any cause). Surviving patients were censored at the last date of follow-up as mentioned in the patient record files. A combination of backward and forward likelihood-ratio model was used, with probability for stepwise removal set at p ≤ 0.1 and probability for stepwise entry set at p ≤ 0.05. Variables remaining in the backward likelihood-ratio model were further analyzed with a forward likelihood-ratio model and dummy variables created for categorical variables. The stability of the model selection procedure was tested by bootstrap resampling with 1000 replications and statistical significance set at p ≤ 0.05. Results were reported as hazard ratio (HR), with a 95% confidence interval (95% CI). Log base 10 transformation was used for highly skewed variables. Correlations between the variables included in the model were assessed using Spearman’s rank correlation coefficient (ρ). Correlations were categorized as very weak (ρ = 0–0.19), weak (ρ = 0.20–0.39), moderate (ρ = 0.40–0.5), strong (ρ = 0.60–0.79) and very strong (ρ = 0.80–1.00). The following list of variables were considered in the model: MTV, TLG, IPI, organ transplant type, EBV tumor status, time after transplant, albumin levels and PTLD morphology. Statistical and graphical analysis were performed using SPSS, version 23.0 (IBM Corporation, Armonk, NY, USA).
Results
Demographic characteristics
A total of 116 PTLD patients with baseline, EARL reconstructed [18F]FDG PET/CT were identified from the patient record files. From these patients, 13 were excluded because accurate segmentation was not possible (mostly due to central nervous system-PTLD). Seven patients were excluded due to previously treated PTLD and in 5 patients, histopathological confirmation was not available within 30 days of the [18F]FDG PET/CT. Finally, 2 patients were excluded because fasting glucose prior to the [18F]FDG PET/CT scan was not reported and in 1 patient multiple variables could not be retrieved, preventing inclusion in the survival model. In total, 88 patients were included in this study, 47 patients from UZ Leuven and 41 from the UMCG. There were 53 (60%) males and 35 (40%) females with a median age at diagnosis of 51 years (IQR: 33.3–62.8 years). Kidney was the most often transplanted organ in 35% of patients, followed by lung (23%) and liver (17%). Morphology was predominantly monomorphic (77%), with 57% of all tumors being EBV-positive. The majority of cases (76%) occurred more than 1-year post transplantation, defined as late-PTLD. Median baseline IPI was 2 (IQR: 1–3). Baseline therapy was most often given as single-agent Rituximab (66%) or chemotherapy (21%). Forty-one percent of patients were deceased mostly due to PTLD (53%) or therapy-related complications (17%). Median whole-body MTV and TLG values were 272 (IQR:42–566) and 1825 (IQR: 232–5610), respectively (Table 1).
Survival analysis
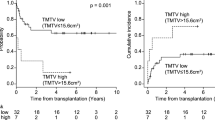
The 1-, 3-, 5-year overall survival rates were 67%, 58% and 43% respectively. Median survival for all patients was 35 months (IQR: 5–67), with a median follow-up for the 51 living patients of 58 months (IQR: 35–101). MTV and TLG underwent log-transformation due to the right-sided skewed distribution. In backwards stepwise elimination, TLG and MTV were eliminated in step 3 and step 4, respectively and were not included in further analysis. Glucose uncorrected TLG values were likewise not prognostic of overall survival (data not shown). IPI (p = 0.01), EBV status of the tumor (p = 0.01) and transplanted organ (p = 0.04) were retained in the model (Table 2). These variables were selected for forward selection analysis and the categorical ‘transplanted organ’ variable coded into a dummy variable for each organ transplant type (kidney, lung, liver, HSCT, heart or multiorgan). A high IPI (HR: 1.56, 95% CI: 1.13–2.16) and an EBV-negative tumor (HR: 2.71, 95% CI: 1.38–5.32) were associated with lower overall survival (Figs. 1, 2). Patients with a kidney transplant had longer overall survival than transplant recipients of any other organ (HR: 0.38 95% CI: 0.16–0.89). IPI was the first variable to be included in the forward selection model, suggesting it as the best fitting variable in our model. All variables retained statistical significance after bootstrapping (Table 3). Variables included in the final model (IPI, EBV tumor status and transplanted organ-kidney) were not correlated to each other.
Discussion
In this 88-patient PTLD cohort, multivariable overall survival analysis indicated that a high IPI and an EBV-negative tumor were associated with lower overall survival. Kidney transplant patients seemed to have a longer overall survival compared to other transplant organ recipients. Whole-body MTV, TLG, time after transplant, hypoalbuminemia and PTLD morphology were not associated with overall survival. Based on these findings, clinical use of IPI may be applicable in PTLD patients while [18F]FDG PET/CT derived volumetric parameters do not to add any prognostic value.
In contrast with other [18F]FDG-avid lymphomas in immunocompetent patients (IC-lymphomas), MTV and TLG measurements were not predictive of overall survival in our PTLD cohort. Despite some conflicting results, several studies have reported high baseline MTV and TLG to be associated with worse overall survival in IC-lymphomas [17, 21,22,23,24]. Nevertheless, characteristics inherent to PTLD prevent direct extrapolation of these previous findings. PTLD occurs in immunocompromised patients after solid organ/hematopoietic stem cell transplantation with distinct pathophysiology and clinical manifestations [25]. PTLD in EBV-positive cases is more reliant on the oncogenic effects of the virus, with greater infiltration of immune cells such as cytotoxic T-cells and M2 macrophages. On the other hand, lymphoma in immunocompetent patients, is characterized by a greater number of genetic mutations (as compared to EBV-positive cases) [26, 27]. Therefore, it can be speculated that while [18F]FDG uptake may be mostly due to underlying inflammation in EBV-positive PTLD, genetic mutations may account for the [18F]FDG uptake observed in IC-lymphomas (and in EBV-negative cases). This is particularly true for the p53 mutations, associated with higher SUV uptake [28]. Another distinct feature of PTLD as compared to IC-lymphomas, is the higher incidence of extra-nodal disease, particularly in the allograft [29]. Similar to IC-lymphomas, extra-nodal disease involvement has been associated with lower overall survival in PTLD patients [2, 5, 7]. Yet, as PTLD is characterized by frequent extra-nodal disease, the metabolic tumor volume may be less significant than the location of the lesions in this patient population. In previous studies, involvement of the central nervous system, bone marrow, graft organ and serous membranes have all been associated with poorer survival in PTLD patients [2, 10, 12]. Therefore, even a small tumor with low MTV and TLG may greatly impact survival depending on the extra-nodal lesion location. Finally, considering the recent studies on the prognostic value of baseline whole-body volumetric parameters in IC-lymphomas, the vast majority uses optimal cutoff values derived from retrospective receiver operating curve analysis [17, 30]. Consequently, the prognostic value of whole-body MTV and TLG may have been frequently overestimated in previous studies.
From the remaining parameters evaluated in the multivariable analysis model, high IPI (HR: 1.56, 95% CI: 1.13–2.16) was the first variable to be included in our forward likelihood-ratio model. Although the IPI is widely used for aggressive lymphomas in immunocompetent patients, some authors have questioned its applicability to PTLD. While some studies have demonstrated the prognostic value of IPI in PTLD, others have argued that their own PTLD specific model was superior at predicting survival or that IPI failed to predict survival altogether [3, 7, 8, 10, 31]. Criticism against the use of the IPI in PTLD has included: the inappropriate cutoff age (taking into consideration the vulnerability of this patient population) and the inability of the IPI to account for the predominance of extra-nodal lesions in PTLD patients (leading to generalized higher IPI scores in PTLD as compared to IC-lymphomas) [12]. Similar to the IPI, the role of EBV tumor status on the survival of PTLD patients has been inconsistent. While some studies have found EBV tumor status to be a predictor of overall survival in either univariable or multivariable models, others have dismissed these findings [3, 5, 9, 32]. Although the role of EBV tumor status is undefined, evidence seems to be mounting on the hypothesis that EBV-positive and EBV-negative PTLD are distinct entities [33,34,35]. EBV-negative PTLD has been shown to have a complex genetic profile with a distinct microenvironment, similar to that found in IC-lymphomas [33, 34]. Furthermore, a recent study by Menter et al. has identified three distinct PTLD subgroups, two of which related to EBV infection status [33]. How this distinction may affect overall survival was not reported but the EBV-negative cluster had a poorer relapse-free survival compared to the other two groups. Considering that EBV tumor status and time of onset after transplant are usually associated, it is perhaps surprising that EBV tumor status was prognostic of survival in our analysis while time of onset after transplant was not. However, in our cohort these two variables were only moderately correlated (ρ = 0.43) which may explain the present results. Finally, kidney transplant patients seemed to have a longer overall survival in our cohort. Although a crucial parameter, specific to PTLD patients and not included in the IPI, few studies have focused on the type of organ transplant. In a study by Dierickx et al., liver transplant patients with PTLD were identified as having a worse overall survival as compared to PTLD patients after kidney transplant [10]. One possible explanation is the higher number of kidney transplants performed per year and the subsequent greater clinical expertise. Another reason may be the ability to better adjust immunosuppression in order to preserve allograft function and to perform dialysis is case of graft failure.
The retrospective nature of this study and the lack of model validation constitute an inherent limitation. Additionally, group distribution was not balanced, with only 8 non-destructive PTLD and 10 polymorphic PTLD cases regarding morphology and only 6 heart and 6 multi-organ transplant patients. As a result, we may not have had enough patients to reach statistical significance in these subgroups. Our cohort also included 3 patients with plasma glucose levels above the 11 mmol/L recommended by the European Association of Nuclear Medicine [36]. Nevertheless, when excluding these patients from our analysis, the overall results did not change. Finally, in the present study we limited our analysis to overall survival as a sole endpoint. This was however deliberately chosen, as other common endpoints such as progression-free survival or disease-free survival may have introduced incorporation or assessment bias into our results.
The lack of established prognostic parameters in PTLD highlights the challenging and complex nature of this disease. Its rarity, broad pathologic spectrum, heterogenous patient population and multiple treatment modalities have difficulted model validation in large patient cohorts. Whole-body MTV and TLG were not applicable for PTLD prognostication. In our cohort and similar to the PTLD-1 trial, IPI may be applicable, but is far from perfect as illustrated by the conflicting results in the literature. Due to the distinct pathophysiology and epidemiology of PTLD, it remains counter intuitive to use IPI instead of a PTLD specific prognostic score. Therefore, future prospective multicenter trials to determine more appropriate prognostic parameters and scores for PTLD are encouraged. Additionally, end-of-treatment [18F]FDG PET/CT has been reported to identify PTLD patients with low risk of relapse and volumetric parameters may further be explored in this group [37, 38].
Conclusion
[18F]FDG PET/CT whole-body volumetric quantitative parameters were not predictive of overall survival in PTLD. In our cohort, high IPI and an EBV-negative tumor were found to predictors of worse overall survival while kidney transplant patients had a longer overall survival compared to other organ transplant recipients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DLBCL:
-
Diffuse large B-cell lymphoma
- EARL:
-
The European Association of Nuclear Medicine Research
- [18F]FDG:
-
2-[18F]fluoro-2-deoxy-D-glucose
- HSCT:
-
Hematopoietic stem cell transplantation
- IPI:
-
International Prognostic Index
- IQR:
-
Interquartile range
- MTV:
-
Metabolic tumor volume
- PET:
-
Positron emission tomography
- PTLD:
-
Post-transplant lymphoproliferative disorder
- SUV:
-
Standardized uptake value
- TLG:
-
Total lesion glycolysis
- UMCG:
-
University Medical Center Groningen
- UZ Leuven:
-
University Hospital Leuven
References
D Dierickx TM Habermann 2018 Post-transplantation lymphoproliferative disorders in adults N Engl J Med 378 549 562
AM Evens KA David I Helenowski B Nelson D Kaufman SM Kircher 2010 Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: Outcomes and prognostic factors in the modern era J Clin Oncol 28 1038 1046
V Leblond N Dhedin M-FM Bruneel S Choquet O Hermine R Porcher 2001 Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders J Clin Oncol. 19 772 778
DE Tsai CL Hardy JE Tomaszewski RM Kotloff KM Oltoff BG Somer 2001 Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients Transplantation 71 1076 1088
B Maecker T Jack M Zimmermann H Abdul-Khaliq M Burdelski A Fuchs 2007 CNS or bone marrow involvement as risk factors for poor survival in post-transplantation lymphoproliferative disorders in children after solid organ transplantation J Clin Oncol 25 4902 4908
RU Trappe D Dierickx H Zimmermann F Morschhauser P Mollee JM Zaucha 2017 Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful stratification into rituximab or R-CHOP consolidation in an international, prospective, multicenter phase II trial J Clin Oncol 35 536 543
IM Ghobrial TM Habermann MJ Maurer SM Geyer KM Ristow TS Larson 2005 Prognostic analysis for survival in adult solid organ transplant recipients with post-transplantation lymphoproliferative disorders J Clin Oncol 23 7574 7582
S Choquet S Oertel V LeBlond H Riess N Varoqueaux B Dörken 2007 Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution Ann Hematol 86 599 607
MJ Hourigan J Doecke PN Mollee DS Gill D Norris DW Johnson 2008 A new prognosticator for post-transplant lymphoproliferative disorders after renal transplantation Br J Haematol 141 904 907
D Dierickx T Tousseyn X Sagaert S Fieuws I Wlodarska J Morscio 2013 Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors Leuk Lymphoma 54 2433 2440
S Caillard FX Lamy C Quelen J Dantal Y Lebranchu P Lang 2012 Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas Am J Transplant 12 682 693
S Caillard R Porcher F Provot J Dantal S Choquet A Durrbach 2013 Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score J Clin Oncol 31 1302 1309
Dockery 1993 A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma N Engl J Med 329 987 994
RU Trappe S Choquet D Dierickx P Mollee JM Zaucha MH Dreyling 2015 International prognostic index, type of transplant and response to rituximab are key parameters to tailor treatment in adults with cd20-positive b cell ptld: clues from the ptld-1 trial Am J Transplant 15 1091 1100
D Dierickx T Tousseyn J Morscio S Fieuws G Verhoef 2013 Validation of prognostic scores in post-transplantation lymphoproliferative disorders J Clin Oncol 31 3443 3444
FM Montes de Jesus TC Kwee M Nijland XU Kahle G Huls RAJO Dierckx 2018 Performance of advanced imaging modalities at diagnosis and treatment response evaluation of patients with post-transplant lymphoproliferative disorder: a systematic review and meta-analysis Crit Rev Oncol Hematol 132 27 38
B Guo X Tan Q Ke H Cen 2019 Prognostic value of baseline metabolic tumor volume and total lesion glycolysis in patients with lymphoma: a meta-analysis PLoS ONE 14 e0210224
R Boellaard WJG Oyen CJ Hoekstra OS Hoekstra EP Visser AT Willemsen 2008 The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials Eur J Nucl Med Mol Imaging 35 2320 2333
Boellaard R, Willemsen A, Arends B, Visser EP. EARL procedure for assessing PET/CT system specific patient FDG activity preparations for quantitative FDG PET/CT studies. April 2013. 2010; pp 1–3
RL Wahl H Jacene Y Kasamon MA Lodge 2009 From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors J Nucl Med 50 122S 150S
HJA Adams JMH Klerk de R Fijnheer BGF Heggelman SV Dubois RAJ Nievelstein 2015 Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole-body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma Eur J Haematol 94 532 539
A-SS Cottereau A Versari A Loft O Casasnovas M Bellei R Ricci 2018 Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial Blood 131 1456 1463
M-K Song J-S Chung H-J Shin S-M Lee S-E Lee H-S Lee 2012 Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement Ann Hematol 91 697 703
NG Mikhaeel D Smith JT Dunn M Phillips H Møller PA Fields 2016 Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL Eur J Nucl Med Mol Imaging 43 1209 1219
International Agency for Research on Cancer. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition. 2017. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. Lyon: WHO; 2017.
J Morscio D Dierickx T Tousseyn 2013 Molecular pathogenesis of B-cell posttransplant lymphoproliferative disorder: what do we know so far? Clin Dev Immunol 2013 1 13
J Morscio T Tousseyn 2016 Recent insights in the pathogenesis of post-transplantation lymphoproliferative disorders World J Transplant 6 505
GH Chang R Kurzrock L Tran M Schwaederle CK Hoh 2018 TP53 mutations and number of alterations correlate with maximum standardized uptake value (SUVmax) determined by positron emission tomography/computed tomography (PET/CT) [18F] fluorodeoxyglucose (18F-FDG PET) Oncotarget 9 14306 14310
NA Bakker GW Imhoff van EAM Verschuuren WJ Son van JJ Homan van der Heide NJGM Veeger 2005 Early onset post-transplant lymphoproliferative disease is associated with allograft localization Clin Transplant 19 327 334
L Kostakoglu S Chauvie 2018 Metabolic tumor volume metrics in lymphoma Semin Nucl Med 48 50 66
R Trappe S Oertel V Leblond P Mollee M Sender P Reinke 2012 Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial Lancet Oncol 13 196 206
V Leblond F Davi F Charlotte R Dorent MO Bitker L Sutton 1998 Posttransplant lymphoproliferative disorders not associated with Epstein-Barr virus: a distinct entity? J Clin Oncol 16 2052 2059
T Menter M Dickenmann D Juskevicius J Steiger S Dirnhofer A Tzankov 2017 Comprehensive phenotypic characterization of PTLD reveals potential reliance on EBV or NF-κB signalling instead of B-cell receptor signalling Hematol Oncol 35 187 197
FE Craig LR Johnson SAK Harvey MA Nalesnik JH Luo SD Bhattacharya 2007 Gene expression profiling of Epstein–Barr virus-positive Diagn Mol Pathol 16 158 168
BP Nelson MA Nalesnik DW Bahler J Locker JJ Fung SH Swerdlow 2000 Epstein-Barr virus-negative post-transplant lymphoproliferative disorders: a distinct entity? Am J Surg Pathol 24 375 385
R Boellaard K Tatsch LC Pike G Testanera J Kotzerke MM Graham 2014 FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0 Eur J Nucl Med Mol Imaging 42 328 354
C-A Keerberghen Van K Goffin V Vergote T Tousseyn G Verhoef A Laenen 2019 Role of interim and end of treatment positron emission tomography for response assessment and prediction of relapse in posttransplant lymphoproliferative disorder Acta Oncol (Madr) 58 1041 1047
H Zimmermann T Denecke MH Dreyling C Franzius P Reinke M Subklewe 2018 End-of-treatment positron emission tomography after uniform first-line therapy of B-cell posttransplant lymphoproliferative disorder identifies patients at low risk of relapse in the prospective German PTLD registry Transplantation 102 868 875
Acknowledgements
Not applicable.
Funding
There was no external funding for this study.
Author information
Authors and Affiliations
Contributions
FMJ—Conception and design, acquisition of data, literature search, analysis/interpretation of data and writing of the manuscript. WN, AWJMG, TCK—Conception and design, analysis/interpretation of data, supervision and writing of the manuscript. DD, VV, OG—Conception and design, acquisition of data, interpretation of data, writing of the manuscript. RAJOD—Revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the approval of the Medical Ethics Review Board of the University Medical Center Groningen (201700855, 07-12-2017) and the Ethical Committee of the University Hospitals Leuven (S-62132, 12-04-2019). No additional informed consent was required for this retrospective study.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montes de Jesus, F., Dierickx, D., Vergote, V. et al. Prognostic superiority of International Prognostic Index over [18F]FDG PET/CT volumetric parameters in post-transplant lymphoproliferative disorder. EJNMMI Res 11, 29 (2021). https://doi.org/10.1186/s13550-021-00769-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-021-00769-8