Abstract
Considering the high prevalence and the lack of targeted pharmacological management of acute kidney injury (AKI), the search for new therapeutic approaches for it is in urgent demand. Mesenchymal stem cells (MSCs) have been increasingly recognized as a promising candidate for the treatment of AKI. However, clinical translation of MSCs-based therapies is hindered due to the poor retention and survival rates as well as the impaired paracrine ability of MSCs post-delivery. To address these issues, a series of strategies including local administration, three-dimensional culture, and preconditioning have been applied. Owing to the emergence and development of these novel biotechnologies, the effectiveness of MSCs in experimental AKI models is greatly improved. Here, we summarize the different approaches suggested to optimize the efficacy of MSCs therapy, aiming at promoting the therapeutic effects of MSCs on AKI patients.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI), defined as a rapid increase in serum creatinine, decrease in urine output, or both, is a common clinical syndrome caused by multiple factors, including renal ischemia, sepsis, toxic effects from drugs, and pigment-related injury from myoglobin or hemoglobin [1, 2]. According to a recent statistical report, AKI occurs in approximately 13.3 million people per year worldwide and the number is still increasing [1, 3]. Except for several acute symptoms, AKI is strongly associated with subsequent chronic kidney disease (CKD) and end-stage kidney disease (ESKD) requiring necessary renal replacement therapy (RRT) or transplantation [4,5,6]. These sequelae place a significant financial burden not only on the patients and their families but also on the public healthcare services [7]. Current therapeutic approaches for AKI remain predominantly supportive and preventive, lacking in targeted pharmacological management [8]. In addition, since AKI often coexists with other syndromes such as heart failure, liver failure, and sepsis, AKI patients usually receive concomitant medications [1, 9], which may consequently result in the increased risk of adverse effects. It is therefore imperative to develop more safe and effective strategies to treat and prevent the progression of AKI.
Mesenchymal stem cells (MSCs) are a kind of cells with robust self-renewal and multi-lineage differentiation potential, existing in many tissues including bone marrow, adipose tissue, umbilical cord blood, and placenta [10]. MSCs secret diverse cytokines, chemokines, growth factors, exosomes, and microvesicles (MVs), which exert cell proliferative, anti-fibrotic, anti-inflammatory, anti-apoptosis, angiogenic, regenerative, and immunomodulatory effects [11,12,13,14]. Besides the release of paracrine or endocrine factors, the therapeutic mechanism of MSCs therapy also involves direct cell-to-cell interactions [15]. Increasing evidence has shown the promising renal protective effects of MSCs in AKI [16,17,18,19,20]. However, there are still some limitations hindering the clinical success of this MSCs-based therapy, for example, the low engraftment, poor survival, and the impaired paracrine ability of MSCs after administration [21]. To overcome these obstacles, many innovative approaches have been explored in recent years. This review discusses these novel strategies in the setting of AKI.
Strategies for improving the therapeutic effects of MSCs
Advances in MSCs biology and bioengineering have shed light on new strategies that have the potential to address many of the limitations related to MSCs-based therapy. These strategies include the improvement in administration routes, the application of three-dimensional (3D) approaches, and the use of preconditioning methods.
Improvement in administration routes
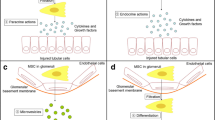
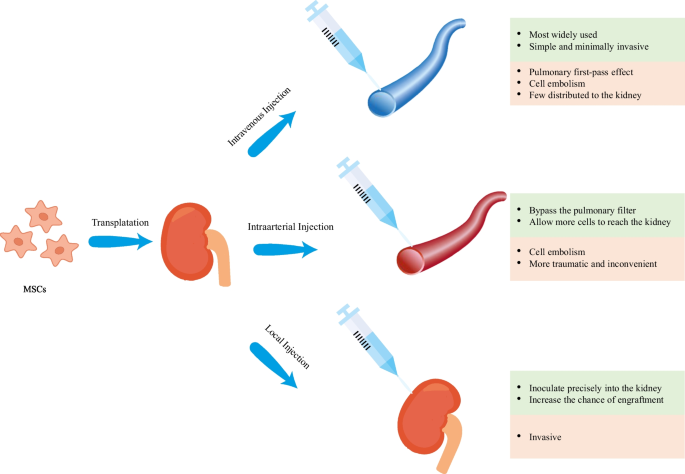
For MSCs to exert their multiple therapeutic functions, a sufficient number of cells are required to be transferred to the sites of injury, which is the basis of MSCs treatment. Therefore, the choice of route of administration appears to be one of the most critical factors influencing the efficacy of MSCs-based therapy. However, no consensus has been reached on the best route for the administration of MSCs. For current preclinical and clinical trials, intravenous, intra-arterial and topical are the three commonly used routes (Fig. 1) [22].
Intravenous (IV) administration is the most widely used route in recent studies [22]. This route is a simple and minimally invasive way to deliver MSCs systematically into the animal models and human bodies. Despite these benefits, there is a great potential for MSCs to be trapped in the lungs, which is known as the pulmonary first-pass effect [23, 24]. In addition to the lungs, liver is also indicated to be a concentrated gathering place of MSCs after IV injection [25], resulting in an inadequate therapeutic concentration in the kidney. Investigators have attempted to employ a higher dose of MSCs to solve this problem but this may increase the risk of adverse events such as pulmonary embolism and thrombotic complications like sinusoidal obstructive syndrome [26,27,28,29]. Intra-arterial (IA) administration has been proved to be more efficacious than IV administration and has been used as an alternative in some treatment indications [22]. Selective IA delivery of MSCs can bypass the pulmonary filter, improving the homing of MSCs and allowing more cells to reach the targeted tissue. However, this route also has the limitation of entailing the risk of cell embolism and is more traumatic as well as inconvenient in clinical practice [22, 30]. Local administration of MSCs is more advantageous compared with IV and IA delivery and thus has become a new focus of current research [31]. MSCs can be inoculated precisely into the kidney via this route, increasing the chance of engraftment and enhancing their therapeutic potential. In addition, local injection avoids the lung barrier, decreasing the risk of lung infarction and mortality [24, 30]. This injection method may be especially beneficial for kidney regeneration as it allows vast amounts of cells to be located at the site of interests. Several local delivery methods have been tested in the animal models of AKI and have shown encouraging results in recent years [31,32,33,34,35,36,37,38,39,40,41,42,43] (Table 1). Among them, renal cortex injection is more widely researched (Table 1), possibly because of its relatively strong operability and reversible injury to the kidney [44]. As shown in Table 1, renal function recovery has been observed and the tubular injury has been ameliorated, with no identifiable safety concerns. However, since few studies have directly compared these different routes of MSC transplantation, additional research is needed to further explore the optimal routes of MSCs administration.
3D stem cell approaches for both culture and delivery
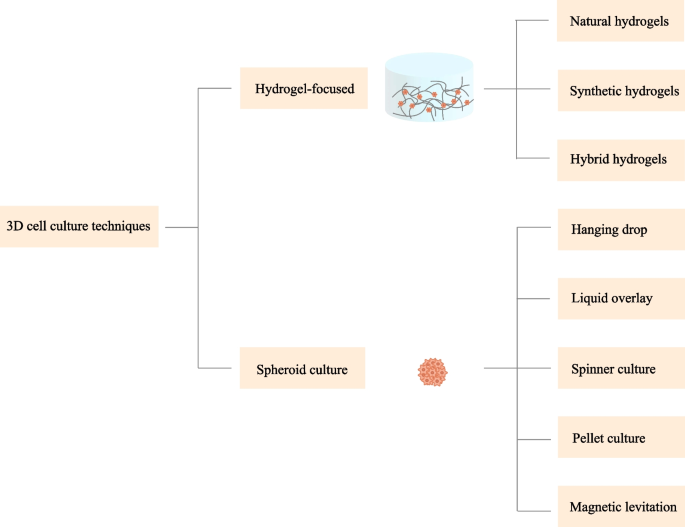
Aside from the delivery mode, several other factors such as the cell growth environment also impact the retention and viability of the stem cells [21, 45]. Conventional approaches for MSCs culture are based on two-dimensional (2D) systems. Cells culture on these platforms often quickly undergoes senescence and a loss of cell functions [46,47,48]. To address these drawbacks, a beneficial 3D microenvironment is necessary to be designed to bridge the gap between the traditional culture system and the complex architecture in vivo. In addition, it is also worth noting that 3D approaches have become a frontier in stem cell delivery by virtue of their superior capability of promoting the survival and function of the transplanted cells [49]. Various methods have been developed to meet the demand for 3D cell culture and delivery over recent years. Broadly, these approaches can be categorized into scaffold-based (hydrogel-focused) and scaffold-free (spheroid culture) strategies (Fig. 2).
Hydrogel-focused strategies
Hydrogels have been considered as one of the most promising candidates for 3D stem cell culture and delivery [50, 51]. Hydrogels are chemically or physically cross-linked 3D porous polymeric networks characterized by high water content and tailorable mechanical, physical, and chemical properties [51,52,53]. The high moisture content and porous structure of hydrogels make it possible for the nutrients and metabolites to be transported in the networks [54, 55]. Hydrogels in this way, can act as an artificial extracellular matrix (ECM) surrounding the cells, providing necessary conditions for cell–cell and cell–matrix interactions, which as a result influence the behaviors and functions of MSCs [21]. To date, a large number of hydrogels have been investigated to mimic the native microenvironment where the cells reside in vivo, ranging from natural to synthetic origin [21]. The utility of hydrogels as scaffolds in supporting the growth and function of the cells has also been demonstrated in many preclinical AKI models [32, 39, 42, 56]. Below, we outline the categories of hydrogels and their corresponding properties.
Natural polymer hydrogels
Natural polymer-based hydrogels have attracted considerable attention over the past decade due to their good biocompatibility, biodegradability, and environmental friendliness [57]. Widely used natural polymers include agarose [58], alginate [59], chitosan [60], hyaluronic acid [61], collagen [62], gelatin [63], and fibrin [64], which can be further sub-classified into polysaccharide-based and protein-based hydrogels [48].
Agarose Agarose is a prominent marine polysaccharide extracted from agar or red seaweeds [58], possessing great biocompatibility, tunable mechanical characteristics, non-toxicity, non-immunogenicity, and thermo-reversible gelling properties [65]. This gel is made up of alternating residues of 1,3-linked β-d-galactopyranose and 1,4-linked 3,6-anhydro-α-l-galactopyranose [66]. Research works have shown that agarose can be used as culture scaffolds to enhance cell attachment and proliferation [55]. In addition, its thermally reversible characteristics offer great opportunities for its injection into the kidney with minimal invasiveness. However, agarose may be related to less enhanced cell functionality [65]. As agarose is seldom employed in current research of AKI, its applicability in renal tissues needs to be further studied.
Alginate The natural polymer alginate is a hydrophilic linear polysaccharide isolated from brown algae and certain bacteria, consisting of β-d-mannuronic acid (M) and α-l-guluronic acid (G) [59]. With the benign nature of cost-effectiveness, high biocompatibility, low cytotoxicity, and appropriate rheological properties, this soluble biopolymer is nowadays one of the most commonly employed bioinks in 3D bioprinting [67]. Prior to its use as a bioink, alginate has been extensively explored as a culture system and delivery vehicle for MSCs in the fields of regenerative medicine and tissue engineering [68,69,70,71,72]. Alginate also plays a significant role in the controlled release of the paracrine factors derived from MSCs [71, 73, 74]. However, alginate offers poor biodegradability and cell adhesive properties, which limit its potential applications [67, 70]. Investigators are trying to overcome these limitations, and a recent work has indicated that hydrogels composed of alginate reinforced with hyaluronic acid may be an exquisite candidate for AKI intervention [75].
Chitosan Chitosan, a linear polysaccharide composed of randomly dispersed β- [1,2,3,4]-linked d-glucosamine (deacetylated unit) and N-acetyl-d-glucosamine (acetylated unit), derived from chitin, is the second most abundant natural biopolymer available on earth [70]. Chitosan can be found in the exoskeleton of crustaceans and the cell envelope of fungi [60]. Aside from having structural similarity to glycosaminoglycans contained in the ECM, chitosan owns the features of biocompatibility, biodegradability, microbial resistance, non-toxicity and low cost [76]. This type of hydrogel is also sensitive to pH and temperature [65], which make it amenable to modification and be used in different kinds of conditions. Recent in vivo studies have highlighted the potency of chitosan-based hydrogels in improving the retention and survival as well as the therapeutic benefits of MSCs in AKI [39, 42]. Though seemingly quite promising, the poor mechanical strength and water solubility of chitosan under physiological conditions limit its use in biomedical applications. Conjugation with peptides or other hydrogels may assist to overcome some of these drawbacks [48, 65].
Hyaluronic acid Hyaluronic acid (HA) is a relatively high molecular weight non-sulfated glycosaminoglycan (GAG) containing repeated units of (β-1,4)-linked d-glucuronic acid and (β-1,3)-linked N-acetyl-d-glucosamine [77]. It is widely distributed throughout the body of adult mammals including connective tissue, synovial fluid, and vitreous humor [61, 78]. HA is an important component of ECM and is essential for cell growth, angiogenesis, embryonic development, wound healing, matrix organization, and morphogenesis [79]. HA has some remarkable properties such as non-adhesiveness, native bio-functionality, hydrophilia, and biodegradability [61, 65]. These advantages make hydrogels built from HA increasingly versatile for a myriad of biomedical applications. Evidence has shown that HA hydrogels can facilitate cell migration and adhesion by binding to the transmembrane receptor CD44 [80]. The implantation of stem cells into the HA hydrogels affects the release of cytokines/chemokines, counterbalancing the secretion of proinflammatory mediators from the immune cells, thereby influencing the immune response and ameliorating the renal damage [81]. Moreover, the highly reversible thermal properties of the HA hydrogels offer great conditions for their use as an injectable scaffold for the culture and delivery of MSCs or an implant material for the repair and reconstruction of the soft and hard tissues [82]. However, for the lack of stability at the body temperature and the ability of controlled release of the bioactive molecules, necessary methods such as chemical modification and covalent crosslink are needed to improve the performance of HA hydrogels [83].
Collagen Collagen, a fiber-like structure, is the most abundant structural protein present in the mammalian ECM [62]. Collagen exhibits a unique triple-helical structure with a repeating amino acid sequence (Gly-X–Y)n [84]. It can be easily manipulated through chemical and physical cross-linking or by blending with other polymers [85]. Owing to its biocompatibility, biodegradability, elasticity as well as structural similarity to the tissues, collagen hydrogels have been frequently investigated as a biomimetic 3D culture scaffold to support cell growth [70]. Previous studies have demonstrated that collagen-based scaffolds can enhance cell retention, cell functionality, cell proliferation, and phenotype maintenance, which thereby increases the therapeutic effects of MSCs for AKI [31, 37, 70]. Nevertheless, pure collagen scaffolds have weak structural stability and mechanical strength [86]. Recent advances in scaffold formulation have contributed to the improvement in the collagen-based hydrogel system.
Matrigel Matrigel, a basement–membrane matrix generated from Engelbreth–Holm–Swarm (EHS) mouse sarcomas, is a widely used collagen-containing hydrogel in tissue engineering applications [87]. The primary components of Matrigel are laminin, collagen IV, entactin, and the heparin sulfate proteoglycan [88]. Matrigel also contains a series of growth factors such as transforming growth factor (TGF) family peptides, fibroblast growth factors (FGFs), and insulin-like growth factors (IGFs), as well as enzymes [89]. Collectively, these components contribute to the excellent biological function of Matrigel. Although Matrigel has been tested as a cell culture tool for several decades [88], its applicability is severely limited due to its ill-defined, complex, and variable constituent [90]. The undefined compositions and antigenicity of Matrigel may lead to batch-to-batch differences in mechanical and biochemical characteristics in cell culture experiments, making it hard to characterize cell behavior and reproduce, which are major hurdles in fundamental research [87].
ECM Kidney ECM hydrogels, obtained through decellularization process, have attracted substantial attention in recent years as new solutions to kidney injuries [91]. By removing the cellular components and retaining the proteins, glycosaminoglycans, as well as growth factors present in the native tissue, these hydrogels are nonimmunogenic, biocompatible, and biologically active [92, 93]. Notably, in contrast to hydrogels composed of individual ECM components, kidney ECM hydrogels reserve the full biochemical complexity of the kidney tissue and, unlike Matrigel, do not consist of proteins originated from tumorigenic cells [94]. Kidney ECM hydrogels are currently being evaluated as an injectable scaffold to facilitate the repair and reconstruction of the renal tissue, and the results are encouraging [91, 95]. Nevertheless, like all natural materials, the properties of kidney ECM hydrogels and the effects of these properties upon cell behaviors are neither well understood nor controlled [94]. Future studies should further elucidate these issues, providing further insight into the management of AKI.
Gelatin As a hydrolytic product of natural collagen, typically of bovine or porcine origin, gelatin is a biocompatible and biodegradable polypeptide containing 18 different kinds of amino acids [70, 96]. In contrast to collagen, gelatin does not elicit any noticeable antigenicity under physiological conditions [96]. In addition to the above advantages, gelatin also has some other desirable properties such as commercial availability, cost economy, water solubility, adhesiveness, and easy processability, making it attractive in the applications of biomedicine [63]. MSCs-laden gelatin-based hydrogels have been shown to prolong the survival of MSCs and thus promote the repair of injured tissues in experimental AKI models [32]. Still, there are disadvantages existed in gelatin hydrogels, including poor mechanical strength, rapid enzymatic degradation, and inferior heat stability [97]. It is well known that pure gelatin has a solgel transition point around body temperature [98]. Therefore, pure gelatin could be injected as a low-viscosity fluid at 37 °C, but failed to form a stable hydrogel in vivo. Further modification is required to help improve the overall properties of native gelatin.
Fibrin Fibrin is a kind of natural polymer derived from key proteins involved in the blood clotting process [64]. In other words, it comes from fibrinogen and thrombin. The morphology, mechanical properties, and stability of fibrin hydrogels can be easily modulated by controlling the ratio of fibrinogen and thrombin in the hydrogels [99]. Fibrin-based hydrogels have been widely utilized for culturing and delivering MSCs due to their unique viscoelastic behavior, biocompatibility, biodegradability, and hemostasis [100,101,102]. When used as cell delivery systems, fibrin hydrogels have the advantage of being able to be implanted through injection without invasive surgery [51]. However, fibrin hydrogels face the challenges of weak mechanical strength and fast degradation speed, which limit their applications in renal diseases [48]. Researchers have tried to combine fibrin with other molecules or biomaterials to enhance its inherent biological properties. The usability and validity of these fibrin-based hydrogels in AKI have yet to be clarified.
Synthetic polymer hydrogels
Synthetic hydrogels are constructed with industrially manufactured polymers. Unlike natural hydrogels, synthetic hydrogels provide researchers with highly versatile materials that can be precisely controlled and designed [103, 104]. In addition, many synthetic hydrogels are essentially bioinert, allowing engineers to specifically modulate the cell–material interactions [77]. In this perspective, more predictable results can be achieved. Poly (ethylene glycol) (PEG) is the most widely implemented bioinert synthetic polymer in 3D cell culture [103]. Other commonly used synthetic hydrogels include poly (vinyl alcohol) (PVA) and poly [2-hydroxyethyl methacrylate] (PHEMA) [103].
Poly (ethylene glycol) (PEG) Poly (ethylene glycol) (PEG), sometimes referred to as poly (ethylene oxide) (PEO) depending on its molecular weight, is a very popular synthetic hydrophilic polymer used for hydrogel formation [85]. The basic structure of PEG is PEG diol with hydroxyl groups at each terminus, which can be converted into other functional groups like methyloxyl, carboxyl, amine, thiol, azide, vinyl sulfone, acetylene, and acrylate [105]. PEG has been considered as an ideal candidate for cell culture due to its non-toxicity to living tissues, superior resistance to protein adsorption, and ease of modification [106]. Its relatively low protein absorption prevents undesired cell–matrix reactions on the one hand, while, on the other hand, it also precludes this kind of material from having any interactions with the cells, which plays an important instructive role in mediating cell growth and functions [107]. Consequently, this type of hydrogel needs to be further modified with peptides or proteins, allowing individual control over each property of the matrix. Although recent work has shown that PEG-based hydrogels could increase stem cell attachment and proliferation [108,109,110], few are applied in the treatment of AKI. Thus, additional studies are required to further evaluate their effect on the kidneys.
Poly (vinyl alcohol) (PVA) Poly (vinyl alcohol) (PVA) is a water-soluble semicrystalline synthetic polymer with a backbone composed only of carbon atoms [111]. PVA is also a type of protein-resistant hydrogel and offers great flexibility in terms of precursor design [112]. PVA has received great attention in biomedical fields because of its advantages such as biodegradability, non-toxicity, non-carcinogenicity, and excellent mechanical properties [113,114,115]. Although some previous studies have indicated the facilitating effect of PVA on MSCs proliferation and its safety for in vivo use [111, 116, 117], evidence is still lacking in renal application.
Poly [2-hydroxyethyl methacrylate] (PHEMA) Poly [2-hydroxyethyl methacrylate] (PHEMA), one of the most important members of the methacrylate polymers, is the first successfully employed hydrogel in biological fields [118]. The presence of free hydroxyl group in PHEMA leads to the highly hydrophilic nature of this hydrogel, which facilitates the transportation of solutes and oxygen [119, 120]. This property in conjunction with other properties like cytocompatibility, non-toxicity, and ease of tuning makes hydrogel fabricated from PHEMA a fit candidate for biomedical use, especially for controlled drug release [120,121,122]. However, PHEMA is relatively weak in mechanical strength and is considered nonbiodegradable, limiting its application in vivo. In this regard, modifications have to be made by incorporating some enzymatically susceptible monomers or cross-linking agents into the PHEMA hydrogels [103]. Similar to PEG and PVA, although modified PHEMA hydrogels have been shown to promote the attachment, spread, and proliferation of MSCs, their effects on the kidney have not been validated yet [123, 124].
Hybrid hydrogels
Both natural and synthetic hydrogels have their own advantages and disadvantages. To overcome the inherent drawbacks of the traditional single-component hydrogels, researchers have been devoting efforts to combine multiple kinds of polymers to form hybrid hydrogels [125]. In this regard, hydrogels can be endowed with some particularly desirable characteristics to better mimic the native microenvironment. Generally, these combinations can be classified into three types: [1] a mixture of two or more ingredients that can form hydrogels alone; [2] a cocktail of the materials in which at least one of them cannot form hydrogels alone; and [3] a mix of the functional groups and the biomaterials. Recent studies [32, 34, 37, 39, 42, 81] have reported that hybrid hydrogels displayed wonderful potency in enhancing the engraftment as well as the survival of MSCs, thereby accelerating the renal functional recovery (Table 2). However, as the use of these hydrogels for AKI is relatively new and there is only a modest amount of data about their performance on the kidneys, further research regarding their in vivo efficacy and safety is still needed.
Spheroid culture
Spheroid culture is also a promising method for 3D cell culture. Through this method, 3D aggregations of MSCs and their secreted ECM could be obtained without the involvement of a scaffold mimicking the real tissues. Several techniques have been used for spheroid fabrication, including hanging drop, liquid overlay, spinner culture, pellet culture, and magnetic levitation [126, 127].
The hanging drop technique was the earliest described method used for spheroid fabrication [127]. In this method, cells gather at the bottom of the droplet and spontaneously aggregate to form spheroids. Hanging drop culture has many advantages such as controllable spheroid size and no need for professional equipment [126]. Alternatively, liquid overlay also enables cell aggregation and is suitable for large-scale production. It allows cells to grow in plates with substrates that limit cell adhesion. Typically, the non-adherent substrate is composed of agarose or PEG [128]. Another popular method for spheroid formation is spinner culture. In this system, cell suspension is put into a flask which is continuously stirred. This approach is especially amenable for long-term culture and intensive cell expansion in addition to mass production [129]. Spheroids can also be generated by centrifugation, which is often referred to as the method of pellet culture. This method is commonly used to induce the differentiation of MSCs [126]. A more recently developed technique for spheroid culture is magnetic levitation. The resultant spheroids can be easily manipulated and tracked via this means [130]. In general, with the help of 3D spheroid culture, MSCs could better maintain their distinct phenotypic and functional properties as well as secrete higher levels of cytokines or other factors, which as a result, improves the therapeutic effects of MSCs for AKI [131, 132].
Preconditioning methods
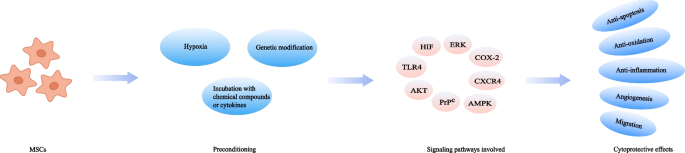
Another critical bottleneck in the field of MSCs therapy is the harsh endogenous environment where the cells are located after transplantation [133]. This chokepoint has sparked the creation of preconditioning strategy (Fig. 3). Currently, researchers have attempted to pretreat MSCs with various physical, chemical, or biological factors to improve their efficacy in preclinical AKI models and the results are promising (Table 3).
Hypoxia
Hypoxia preconditioning has been frequently applied to improve the therapeutic potential of MSCs. MSCs are cultured in the environment with a 21% oxygen level generally, but once transplanted into the injured tissues, they often encounter hypoxic conditions with oxygen concentrations ranging from 1 to 6% [134]. The changed culture oxygen tension could affect a wide variety of cellular activities, including proliferation, differentiation, senescence, and metabolism, which may consequently compromise the cell ability for repairing dysfunctional organs [135]. Pre-exposure of MSCs to hypoxia could help conquer this obstacle. As reported in previous studies, hypoxic pretreated MSCs remarkably accelerate the functional and histological recovery in ischemic AKI models [40, 136, 137], which hypoxia-inducible factor-1α (HIF-1α) is thought to play a crucial role in this process [138].
Incubation with chemical compounds or cytokines
Pre-incubation of MSCs with various chemical compounds or cytokines has also been proved as an effective tool to improve the therapeutic efficacy of MSCs. Currently documented chemicals and biomacromolecules used for MSCs pretreatment in preclinical AKI models include melatonin, atorvastatin, and insulin growth factor-1 (IGF-1) (Table 3).
Melatonin is a neurohormone secreted by the pineal gland, having a variety of functions such as circadian rhythms regulation, anti-inflammation, and anti-oxidation [139, 140]. It was documented that MSCs pretreated with melatonin led to an enhanced therapeutic outcome in AKI models. The underlying mechanism might be that melatonin could suppress reactive oxygen species (ROS) generation and oxidative stress in either a receptor-dependent manner through ERK1/2, AMPK/ACC, and PrPC/PINK1 signaling pathways, or receptor-independent manner [141, 142]. The HMG-CoA reductase inhibitor atorvastatin has also been tested for treating AKI because of its anti-apoptotic, antioxidant, and anti-inflammatory effects [143]. Incubation of MSCs with atorvastatin prior to transplantation increased the viability of MSCs, resulting in the promotion of renal recovery. HMGB1/TLR4 pathway is considered to play a pivotal role during this process [144]. Another potential cell protective reagent is S‐nitroso N‐acetyl penicillamine (SNAP). It is a nitric oxide (NO) donor with the ability to regulate hemodynamics. In a model of renal ischemia/reperfusion (I/R) injury, MSCs preconditioned with SNAP were found more effective than those untreated, which was accompanied by an increase in the expression of PI3K/AKT pathway-related proteins [145]. 14S,21Rdihydroxy-docosa4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14S,21R-diHDHA) is also a cytoprotective agent exerting its beneficial effects on MSCs via PI3K/AKT pathway. A study demonstrated that preconditioning of MSCs with 14S,21R-diHDHA was able to ameliorate renal dysfunction and renal histological injury [146]. Similarly, the administration of MSCs primed with hyaluronan monoesters with butyric acid (HB), a differentiating agent, helped decrease the level of inflammation, which consequently reinforced the effectiveness of MSCs-based treatment in ischemic AKI [43].
The interaction between cytokines and their receptors can activate signaling cascades relevant to cell survival, proliferation, and migration. Therefore, cytokines preconditioning may have an impact on the fate of MSCs in vitro and in vivo. Interleukin-17A (IL-17A) pretreatment protected MSCs from harmful immune response, which thereby consolidated the therapeutic utility of MSCs. This improved effect was proved to be due to the increase in Treg percentages through the COX-2/PGE2 pathway [147]. Likewise, preconditioning with IGF-1 enhanced the migration of MSCs, leading to an improvement in the therapeutically relevant effects. An overexpression of CXCR4 was observed in this preconditioning method, which was considered to be associated with the increased migratory capacity [148].
Genetic modification
Another approach employed to increase the therapeutic potency of MSCs is genetic manipulation. Recent data have indicated that several genes are related to the function of MSCs and thus could be targets for modification. For example, heme oxygenase-1 (HO-1) is highly correlated with anti-oxidative activity and vascular endothelial growth factor (VEGF) is responsible for angiogenesis [149]. By overexpressing these specific factors, the migration ability, vasculotropic action, as well as the anti-inflammatory and survival capacities of MSCs could be boosted, contributing to the better recovery of renal function. PI3K/Akt, MEK/ERK, and other signaling pathways are involved in this cytoprotective process [150,151,152,153,154,155,156,157,158,159,160,161,162,163]. Furthermore, it should also be mentioned that in clinical practice, the application of genetic modification needs to be more prudent as consistent activation of some specific genes might be a risk factor for stem cell-derived tumors.
Conclusion and future perspective
In conclusion, MSCs hold a considerable promise for the treatment of AKI. Nevertheless, the major outcomes of MSCs therapy in clinical trials of AKI have fallen far short of the theoretical effects of MSCs in preclinical studies. Challenges remain with respect to the clinical translation of this stem cell-based therapy. To address these challenges, various regimens including local administration, 3D cell culture as well as preconditioning have been exploited. In addition, considering the heterogeneity among patients, it is also important to realize that “one-size-fits-all” approach is clinically outdated. The characteristics of the patients such as age, genetics, and overall health status should be taken into consideration when applying the aforementioned strategies. Further research focused on the optimization of MSCs-based therapy is still needed to achieve the maximum therapeutic efficiency of MSCs in AKI patients.
Availability of data and materials
Not applicable.
Abbreviations
- AKI:
-
Acute kidney injury
- CKD:
-
Chronic kidney disease
- ECM:
-
Extracellular matrix
- ESKD:
-
End-stage kidney disease
- HA:
-
Hyaluronic acid
- HB:
-
Hyaluronan monoesters with butyric acid
- HO-1:
-
Heme oxygenase-1
- IA:
-
Intra-arterial
- IGF-1:
-
Insulin growth factor-1
- IL-17A:
-
Interleukin-17A
- I/R:
-
Ischemia/reperfusion
- IV:
-
Intravenous
- MSCs:
-
Mesenchymal stem cells
- MVs:
-
Microvesicles
- NO:
-
Nitric oxide
- PEG:
-
Poly(ethylene glycol)
- PHEMA:
-
Poly(2-hydroxyethyl methacrylate)
- PVA:
-
Poly(vinyl alcohol)
- ROS:
-
Reactive oxygen species
- RRT:
-
Renal replacement therapy
- SNAP:
-
S‐nitroso N‐acetyl penicillamine
- VEGF:
-
Vascular endothelial growth factor
- 14S,21R-diHDHA:
-
14S,21Rdihydroxy-docosa4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
References
Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–64.
Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170–9.
Mehta RL, Cerda J, Burdmann EA, Tonelli M, Garcia-Garcia G, Jha V, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–43.
Sato Y, Takahashi M, Yanagita M. Pathophysiology of AKI to CKD progression. Semin Nephrol. 2020;40(2):206–15.
Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66.
See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–72.
Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207.
Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67–75.
Moore PK, Hsu RK, Liu KD. Management of acute kidney injury: core curriculum 2018. Am J Kidney Dis. 2018;72(1):136–48.
Kou M, Huang L, Yang J, Chiang Z, Chen S, Liu J, et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. 2022;13(7):580.
Khubutiya MS, Vagabov AV, Temnov AA, Sklifas AN. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy. 2014;16(5):579–85.
de Almeida DC, Donizetti-Oliveira C, Barbosa-Costa P, Origassa CS, Camara NO. In search of mechanisms associated with mesenchymal stem cell-based therapies for acute kidney injury. Clin Biochem Rev. 2013;34(3):131–44.
Liang XT, Ding Y, Zhang YL, Tse HF, Lian QZ. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–59.
Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22.
Fan M, Zhang J, Xin H, He X, Zhang X. Current perspectives on role of MSC in renal pathophysiology. Front Physiol. 2018;9:1323.
Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15(7):1794–804.
Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68(4):1613–7.
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–67.
Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, et al. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol. 2018;29(1):260–7.
Rodrigues CE, Capcha JM, de Braganca AC, Sanches TR, Gouveia PQ, de Oliveira PA, et al. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res Ther. 2017;8(1):19.
Wechsler ME, Rao VV, Borelli AN, Anseth KS. Engineering the MSC secretome: a hydrogel focused approach. Adv Healthc Mater. 2021;10(7):e2001948.
Sanchez-Diaz M, Quinones-Vico MI, Sanabria de la Torre R, Montero-Vilchez T, Sierra-Sanchez A, Molina-Leyva A, et al. Biodistribution of mesenchymal stromal cells after administration in animal models and humans: a systematic review. J Clin Med. 2021;10(13):2925.
Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–92.
Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transpl Proc. 2007;39(2):573–6.
Schmuck EG, Koch JM, Centanni JM, Hacker TA, Braun RK, Eldridge M, et al. Biodistribution and clearance of human mesenchymal stem cells by quantitative three-dimensional cryo-imaging after intravenous infusion in a rat lung injury model. Stem Cells Transl Med. 2016;5(12):1668–75.
Kyriakou C, Rabin N, Pizzey A, Nathwani A, Yong K. Factors that influence short-term homing of human bone marrow-derived mesenchymal stem cells in a xenogeneic animal model. Haematologica. 2008;93(10):1457–65.
Cui LL, Kerkela E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6:11.
Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE. 2012;7(10):e47559.
Kansu E. Thrombosis in stem cell transplantation. Hematology. 2012;17(Suppl 1):S159–62.
Bagno LL, Salerno AG, Balkan W, Hare JM. Mechanism of action of mesenchymal stem cells (MSCs): impact of delivery method. Expert Opin Biol Ther. 2022;22(4):449–63.
Huang M, Li D, Chen J, Ji Y, Su T, Chen Y, et al. Comparison of the treatment efficacy of umbilical mesenchymal stem cell transplantation via renal subcapsular and parenchymal routes in AKI-CKD mice. Stem Cell Res Ther. 2022;13(1):128.
Fu Z, Chu Y, Geng X, Ma Y, Chi K, Song C, et al. Artificial kidney capsule packed with mesenchymal stem cell-laden hydrogel for the treatment of rhabdomyolysis-induced acute kidney injury. ACS Biomater Sci Eng. 2022;8(4):1726–34.
Yang YJ, Geng XD, Chi K, Liu C, Liu R, Chen XM, et al. Ultrasound enhances the therapeutic potential of mesenchymal stem cells wrapped in greater omentum for aristolochic acid nephropathy. Stem Cell Res Therapy. 2021;12(1):261.
Wang H, Shang Y, Chen X, Wang Z, Zhu D, Liu Y, et al. Delivery of MSCs with a hybrid beta-sheet peptide hydrogel consisting IGF-1C domain and D-form peptide for acute kidney injury therapy. Int J Nanomed. 2020;15:4311–24.
Paglione D, Gatta L, Cavallari G, Perri A, Bonofiglio R, Catalano S, et al. Acute kidney ischemic injury in a rat model treated by human omental mesenchymal stem cells. Transpl Proc. 2020;52(10):2977–9.
Havakhah S, Sankian M, Kazemzadeh GH, Sadri K, Bidkhori HR, Naderi-Meshkin H, et al. In vivo effects of allogeneic mesenchymal stem cells in a rat model of acute ischemic kidney injury. Iran J Basic Med Sci. 2018;21(8):824–31.
Huang S, Li Y, Wang X, Ma X, Zhang X. Injectable co-gels of collagen and decellularized vascular matrix improve MSC-based therapy for acute kidney injury. J Biomater Sci Polym Ed. 2017;28(18):2186–95.
Geng X, Hong Q, Wang W, Zheng W, Li O, Cai G, et al. Biological membrane-packed mesenchymal stem cells treat acute kidney disease by ameliorating mitochondrial-related apoptosis. Sci Rep. 2017;7:41136.
Feng G, Zhang J, Li Y, Nie Y, Zhu D, Wang R, et al. IGF-1 C domain-modified hydrogel enhances cell therapy for AKI. J Am Soc Nephrol. 2016;27(8):2357–69.
Zhang W, Liu L, Huo Y, Yang Y, Wang Y. Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. Biomed Res Int. 2014;2014:462472.
Cheng K, Rai P, Plagov A, Lan X, Kumar D, Salhan D, et al. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Exp Mol Pathol. 2013;94(3):466–73.
Gao J, Liu R, Wu J, Liu Z, Li J, Zhou J, et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials. 2012;33(14):3673–81.
La Manna G, Bianchi F, Cappuccilli M, Cenacchi G, Tarantino L, Pasquinelli G, et al. Mesenchymal stem cells in renal function recovery after acute kidney injury: use of a differentiating agent in a rat model. Cell Transplant. 2011;20(8):1193–208.
Yang WY, Chen LC, Jhuang YT, Lin YJ, Hung PY, Ko YC, et al. Injection of hybrid 3D spheroids composed of podocytes, mesenchymal stem cells, and vascular endothelial cells into the renal cortex improves kidney function and replenishes glomerular podocytes. Bioeng Transl Med. 2021;6(2):e10212.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89.
Tietze S, Krater M, Jacobi A, Taubenberger A, Herbig M, Wehner R, et al. Spheroid Culture of Mesenchymal Stromal Cells Results in Morphorheological Properties Appropriate for Improved Microcirculation. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2019;6(8):1802104.
Wong KU, Zhang A, Akhavan B, Bilek MM, Yeo GC. Biomimetic culture strategies for the clinical expansion of mesenchymal stromal cells. ACS Biomater Sci Eng. 2021.
Hu X, Xia Z, Cai K. Recent advances in 3D hydrogel culture systems for mesenchymal stem cell-based therapy and cell behavior regulation. J Mater Chem B. 2022;10(10):1486–507.
Zhao Y, Song S, Wang D, Liu H, Zhang J, Li Z, et al. Nanozyme-reinforced hydrogel as a H(2)O(2)-driven oxygenerator for enhancing prosthetic interface osseointegration in rheumatoid arthritis therapy. Nat Commun. 2022;13(1):6758.
Liu XY, Liu J, Lin ST, Zhao XH. Hydrogel machines. Mater Today. 2020;36:102–24.
Huang Q, Zou Y, Arno MC, Chen S, Wang T, Gao J, et al. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem Soc Rev. 2017;46(20):6255–75.
Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124–8.
Vedadghavami A, Minooei F, Mohammadi MH, Khetani S, Rezaei Kolahchi A, Mashayekhan S, et al. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017;62:42–63.
Bai RB, Yang JW, Suo ZG. Fatigue of hydrogels. Eur J Mech a-Solid. 2019;74:337–70.
Yap JX, Leo CP, Mohd Yasin NH, Show PL, Chu DT, Singh V, et al. Recent advances of natural biopolymeric culture scaffold: synthesis and modification. Bioengineered. 2022;13(2):2226–47.
Zhang C, Shang Y, Chen X, Midgley AC, Wang Z, Zhu D, et al. Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) Peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano. 2020;14(9):12133–47.
Jiang Y, Wang Y, Li Q, Yu C, Chu W. Natural polymer-based stimuli-responsive hydrogels. Curr Med Chem. 2020;27(16):2631–57.
Khodadadi Yazdi M, Taghizadeh A, Taghizadeh M, Stadler FJ, Farokhi M, Mottaghitalab F, et al. Agarose-based biomaterials for advanced drug delivery. J Control Release. 2020;326:523–43.
Zhang M, Zhao X. Alginate hydrogel dressings for advanced wound management. Int J Biol Macromol. 2020;162:1414–28.
Kou SG, Peters LM, Mucalo MR. Chitosan: a review of sources and preparation methods. Int J Biol Macromol. 2021;169:85–94.
Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23(12):H41-56.
Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221(1–2):1–22.
Bello AB, Kim D, Kim D, Park H, Lee SH. Engineering and functionalization of gelatin biomaterials: from cell culture to medical applications. Tissue Eng Part B Rev. 2020;26(2):164–80.
Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods. 2016;13(5):405–14.
Wagenbrenner M, Mayer-Wagner S, Rudert M, Holzapfel BM, Weissenberger M. Combinations of hydrogels and mesenchymal stromal cells (MSCs) for cartilage tissue engineering—a review of the literature. Gels. 2021;7(4):217.
Firouzabadi H, Iranpoor N, Gholinejad M, Kazemi F. Agarose hydrogel as an effective bioorganic ligand and support for the stabilization of palladium nanoparticles. Application as a recyclable catalyst for Suzuki-Miyaura reaction in aqueous media. RSC Adv. 2011;1(6):1013–9.
Axpe E, Oyen ML. Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci. 2016;17(12):1976.
Khatab S, Leijs MJ, van Buul G, Haeck J, Kops N, Nieboer M, et al. MSC encapsulation in alginate microcapsules prolongs survival after intra-articular injection, a longitudinal in vivo cell and bead integrity tracking study. Cell Biol Toxicol. 2020;36(6):553–70.
Ghanta RK, Aghlara-Fotovat S, Pugazenthi A, Ryan CT, Singh VP, Mathison M, et al. Immune-modulatory alginate protects mesenchymal stem cells for sustained delivery of reparative factors to ischemic myocardium. Biomater Sci. 2020;8(18):5061–70.
Troy E, Tilbury MA, Power AM, Wall JG. Nature-based biomaterials and their application in biomedicine. Polymers (Basel). 2021;13(19):3321.
Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–26.
Dhamecha D, Movsas R, Sano U, Menon JU. Applications of alginate microspheres in therapeutics delivery and cell culture: past, present and future. Int J Pharm. 2019;569:118627.
Mohammadi M, Luong JC, Rodriguez SM, Cao R, Wheeler AE, Lau H, et al. Controlled release of stem cell secretome attenuates inflammatory response against implanted biomaterials. Adv Healthc Mater. 2020;9(12):e1901874.
Bari E, Scocozza F, Perteghella S, Sorlini M, Auricchio F, Torre ML, et al. 3D bioprinted scaffolds containing mesenchymal stem/stromal lyosecretome: next generation controlled release device for bone regenerative medicine. Pharmaceutics. 2021;13(4):515.
Amirian J, Van TTT, Bae SH, Jung HI, Choi HJ, Cho HD, et al. Examination of In vitro and In vivo biocompatibility of alginate-hyaluronic acid microbeads As a promising method in cell delivery for kidney regeneration. Int J Biol Macromol. 2017;105(Pt 1):143–53.
Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials. 2005;26(36):7616–27.
Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32–33):3307–29.
Ho TC, Chang CC, Chan HP, Chung TW, Shu CW, Chuang KP, et al. Hydrogels: properties and applications in biomedicine. Molecules. 2022;27(9):2902.
Arifka M, Wilar G, Elamin KM, Wathoni N. Polymeric hydrogels as mesenchymal stem cell secretome delivery system in biomedical applications. Polymers (Basel). 2022;14(6):1218.
Bhattacharya D, Svechkarev D, Souchek JJ, Hill TK, Taylor MA, Natarajan A, et al. Impact of structurally modifying hyaluronic acid on CD44 interaction. J Mater Chem B. 2017;5(41):8183–92.
Zullo JA, Nadel EP, Rabadi MM, Baskind MJ, Rajdev MA, Demaree CM, et al. The secretome of hydrogel-coembedded endothelial progenitor cells and mesenchymal stem cells instructs macrophage polarization in endotoxemia. Stem Cells Transl Med. 2015;4(7):852–61.
Shoma Suresh K, Bhat S, Guru BR, Muttigi MS, Seetharam RN. A nanocomposite hydrogel delivery system for mesenchymal stromal cell secretome. Stem Cell Res Ther. 2020;11(1):205.
Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia X. Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter. 2012;8(12):3280–94.
Mayo KH. NMR and x-ray studies of collagen model peptides. Biopolymers. 1996;40(4):359–70.
Naahidi S, Jafari M, Logan M, Wang Y, Yuan Y, Bae H, et al. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol Adv. 2017;35(5):530–44.
Dong C, Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers (Basel). 2016;8(2):42.
Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater. 2020;5(7):539–51.
Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–86.
Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev. 2014;79–80:3–18.
Talbot NC, Caperna TJ. Proteome array identification of bioactive soluble proteins/peptides in Matrigel: relevance to stem cell responses. Cytotechnology. 2015;67(5):873–83.
Sobreiro-Almeida R, Quinteira R, Neves NM. Renal regeneration: the role of extracellular matrix and current ECM-based tissue engineered strategies. Adv Healthc Mater. 2021;10(14):e2100160.
Kim JW, Nam SA, Yi J, Kim JY, Lee JY, Park SY, et al. Kidney decellularized extracellular matrix enhanced the vascularization and maturation of human kidney organoids. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2022;9(15):e2103526.
Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19(5):646–51.
Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 2017;49:1–15.
Zhou C, Zhou L, Liu J, Xu L, Xu Z, Chen Z, et al. Kidney extracellular matrix hydrogel enhances therapeutic potential of adipose-derived mesenchymal stem cells for renal ischemia reperfusion injury. Acta Biomater. 2020;115:250–63.
Rohanizadeh R, Swain MV, Mason RS. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J Mater Sci Mater Med. 2008;19(3):1173–82.
Helminger M, Wu B, Kollmann T, Benke D, Schwahn D, Pipich V, et al. Synthesis and characterization of gelatin-based magnetic hydrogels. Adv Funct Mater. 2014;24(21):3187–96.
Mushtaq F, Raza ZA, Batool SR, Zahid M, Onder OC, Rafique A, et al. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int J Biol Macromol. 2022;218:601–33.
Tan J, Li L, Wang H, Wei L, Gao X, Zeng Z, et al. Biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair. Mater Sci Eng C Mater Biol Appl. 2021;121:111749.
Heher P, Muhleder S, Mittermayr R, Redl H, Slezak P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv Drug Deliv Rev. 2018;129:134–47.
Goetzke R, Franzen J, Ostrowska A, Vogt M, Blaeser A, Klein G, et al. Does soft really matter? Differentiation of induced pluripotent stem cells into mesenchymal stromal cells is not influenced by soft hydrogels. Biomaterials. 2018;156:147–58.
Ricles LM, Hsieh PL, Dana N, Rybalko V, Kraynak C, Farrar RP, et al. Therapeutic assessment of mesenchymal stem cells delivered within a PEGylated fibrin gel following an ischemic injury. Biomaterials. 2016;102:9–19.
Unal AZ, West JL. Synthetic ECM: bioactive synthetic hydrogels for 3D tissue engineering. Bioconjug Chem. 2020;31(10):2253–71.
Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv Mater. 2010;22(31):3484–94.
Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62(1–2):81–7.
Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639–56.
Browning MB, Russell B, Rivera J, Hook M, Cosgriff-Hernandez EM. Bioactive hydrogels with enhanced initial and sustained cell interactions. Biomacromol. 2013;14(7):2225–33.
Guerra AD, Rose WE, Hematti P, Kao WJ. Minocycline enhances the mesenchymal stromal/stem cell pro-healing phenotype in triple antimicrobial-loaded hydrogels. Acta Biomater. 2017;51:184–96.
Martin JR, Patil P, Yu F, Gupta MK, Duvall CL. Enhanced stem cell retention and antioxidative protection with injectable, ROS-degradable PEG hydrogels. Biomaterials. 2020;263:120377.
Xin S, Gregory CA, Alge DL. Interplay between degradability and integrin signaling on mesenchymal stem cell function within poly(ethylene glycol) based microporous annealed particle hydrogels. Acta Biomater. 2020;101:227–36.
Rivera-Hernandez G, Antunes-Ricardo M, Martinez-Morales P, Sanchez ML. Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int J Pharm. 2021;600:120478.
Jiang S, Liu S, Feng W. PVA hydrogel properties for biomedical application. J Mech Behav Biomed Mater. 2011;4(7):1228–33.
Hassan CM, Peppas NA. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv Polym Sci. 2000;153:37–65.
Luo Q, Shan Y, Zuo X, Liu J. Anisotropic tough poly(vinyl alcohol)/graphene oxide nanocomposite hydrogels for potential biomedical applications. Rsc Adv. 2018;8(24):13284–91.
Rolim WR, Pieretti JC, Reno DLS, Lima BA, Nascimento MHM, Ambrosio FN, et al. Antimicrobial activity and cytotoxicity to tumor cells of nitric oxide donor and silver nanoparticles containing PVA/PEG films for topical applications. ACS Appl Mater Interfaces. 2019;11(6):6589–604.
Shafiee A, Soleimani M, Chamheidari GA, Seyedjafari E, Dodel M, Atashi A, et al. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J Biomed Mater Res A. 2011;99(3):467–78.
Gomide VS, Zonari A, Ocarino NM, Goes AM, Serakides R, Pereira MM. In vitro and in vivo osteogenic potential of bioactive glass-PVA hybrid scaffolds colonized by mesenchymal stem cells. Biomed Mater. 2012;7(1):015004.
Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960;185(4706):117–8.
Peng W, Lu X, Wu J, Wang Y, Zhu X, Ouyang H, et al. Autoclaving pHEMA-based hydrogels immersed in deionized water has no effect on physicochemical properties and cell behaviors. ACS Omega. 2022;7(36):32038–45.
Li J, Chee HL, Chong YT, Chan BQY, Xue K, Lim PC, et al. Hofmeister effect mediated strong PHEMA-gelatin hydrogel actuator. ACS Appl Mater Interfaces. 2022.
Lu SX, Anseth KS. Photopolymerization of multilaminated poly(HEMA) hydrogels for controlled release. J Control Release. 1999;57(3):291–300.
Noble ML, Mourad PD, Ratner BD. Digital drug delivery: on-off ultrasound controlled antibiotic release from coated matrices with negligible background leaching. Biomater Sci. 2014;2(6):839–902.
Kubinova S, Horak D, Sykova E. Cholesterol-modified superporous poly(2-hydroxyethyl methacrylate) scaffolds for tissue engineering. Biomaterials. 2009;30(27):4601–9.
Mohanty S, Alm M, Hemmingsen M, Dolatshahi-Pirouz A, Trifol J, Thomsen P, et al. 3D printed silicone-hydrogel scaffold with enhanced physicochemical properties. Biomacromol. 2016;17(4):1321–9.
Yang J, Zhang YS, Yue K, Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25.
Ryu NE, Lee SH, Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells. 2019;8(12):1620.
Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, de Boer J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013;31(2):108–15.
Costa EC, de Melo-Diogo D, Moreira AF, Carvalho MP, Correia IJ. Spheroids formation on non-adhesive surfaces by liquid overlay technique: considerations and practical approaches. Biotechnol J. 2018;13(1).
Chaicharoenaudomrung N, Kunhorm P, Noisa P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J Stem Cells. 2019;11(12):1065–83.
Lee SY, Lee JW. 3D Spheroid cultures of stem cells and exosome applications for cartilage repair. Life (Basel). 2022;12(7):939.
Cesarz Z, Tamama K. Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016;2016:9176357.
Xu Y, Shi T, Xu A, Zhang L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J Cell Mol Med. 2016;20(7):1203–13.
Zhao L, Hu C, Zhang P, Jiang H, Chen J. Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury. J Cell Mol Med. 2019;23(2):720–30.
Stuart JA, Fonseca J, Moradi F, Cunningham C, Seliman B, Worsfold CR, et al. How Supraphysiological oxygen levels in standard cell culture affect oxygen-consuming reactions. Oxid Med Cell Longev. 2018;2018:8238459.
Buravkova LB, Andreeva ER, Gogvadze V, Zhivotovsky B. Mesenchymal stem cells and hypoxia: where are we? Mitochondrion. 2014;19(Pt A):105–12.
Yu X, Lu C, Liu H, Rao S, Cai J, Liu S, et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS ONE. 2013;8(5):e62703.
Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS ONE. 2012;7(4):e34608.
Silva LHA, Antunes MA, Dos Santos CC, Weiss DJ, Cruz FF, Rocco PRM. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther. 2018;9(1):45.
Vasey C, McBride J, Penta K. Circadian rhythm dysregulation and restoration: the role of melatonin. Nutrients. 2021;13(10):3480.
Carrascal L, Nunez-Abades P, Ayala A, Cano M. Role of melatonin in the inflammatory process and its therapeutic potential. Curr Pharm Des. 2018;24(14):1563–88.
Chen HH, Lin KC, Wallace CG, Chen YT, Yang CC, Leu S, et al. Additional benefit of combined therapy with melatonin and apoptotic adipose-derived mesenchymal stem cell against sepsis-induced kidney injury. J Pineal Res. 2014;57(1):16–32.
Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem cells (Dayton, Ohio). 2008;26(7):1749–57.
Yang YJ, Qian HY, Huang J, Geng YJ, Gao RL, Dou KF, et al. Atorvastatin treatment improves survival and effects of implanted mesenchymal stem cells in post-infarct swine hearts. Eur Heart J. 2008;29(12):1578–90.
Cai J, Yu X, Zhang B, Zhang H, Fang Y, Liu S, et al. Atorvastatin improves survival of implanted stem cells in a rat model of renal ischemia-reperfusion injury. Am J Nephrol. 2014;39(6):466–75.
Masoud MS, Anwar SS, Afzal MZ, Mehmood A, Khan SN, Riazuddin S. Pre-conditioned mesenchymal stem cells ameliorate renal ischemic injury in rats by augmented survival and engraftment. J Transl Med. 2012;10:243.
Tian H, Lu Y, Shah SP, Wang Q, Hong S. 14S,21R-dihydroxy-docosahexaenoic acid treatment enhances mesenchymal stem cell amelioration of renal ischemia/reperfusion injury. Stem Cells Dev. 2012;21(7):1187–99.
Bai M, Zhang L, Fu B, Bai J, Zhang Y, Cai G, et al. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 2018;93(4):814–25.
Xinaris C, Morigi M, Benedetti V, Imberti B, Fabricio AS, Squarcina E, et al. A novel strategy to enhance mesenchymal stem cell migration capacity and promote tissue repair in an injury specific fashion. Cell Transplant. 2013;22(3):423–36.
Xu H, Chen C, Hu L, Hou J. Gene-modified mesenchymal stem cell-based therapy in renal ischemia- reperfusion injury. Curr Gene Ther. 2017;17(6):453–60.
Ni W, Zhang Y, Yin Z. The protective mechanism of Klotho gene-modified bone marrow mesenchymal stem cells on acute kidney injury induced by rhabdomyolysis. Regen Ther. 2021;18:255–67.
Roudkenar MH, Halabian R, Tehrani HA, Amiri F, Jahanian-Najafabadi A, Roushandeh AM, et al. Lipocalin 2 enhances mesenchymal stem cell-based cell therapy in acute kidney injury rat model. Cytotechnology. 2018;70(1):103–17.
Yan X, Cheng X, He X, Zheng W, Yuan X, Chen H. HO-1 overexpressed mesenchymal stem cells ameliorate sepsis-associated acute kidney injury by activating JAK/stat3 pathway. Cell Mol Bioeng. 2018;11(6):509–18.
Liu N, Wang H, Han G, Cheng J, Hu W, Zhang J. Enhanced proliferation and differentiation of HO-1 gene-modified bone marrow-derived mesenchymal stem cells in the acute injured kidney. Int J Mol Med. 2018;42(2):946–56.
Mori da Cunha MG, Zia S, Beckmann DV, Carlon MS, Arcolino FO, Albersen M, et al. Vascular endothelial growth factor up-regulation in human amniotic fluid stem cell enhances nephroprotection after ischemia-reperfusion injury in the rat. Crit Care Med. 2017;45(1):e86–96.
Zhaleh F, Amiri F, Mohammadzadeh-Vardin M, Bahadori M, Harati MD, Roudkenar MH, et al. Nuclear factor erythroid-2 related factor 2 overexpressed mesenchymal stem cells transplantation, improves renal function, decreases injuries markers and increases repair markers in glycerol-induced Acute kidney injury rats. Iran J Basic Med Sci. 2016;19(3):323–9.
Liu N, Wang H, Han G, Tian J, Hu W, Zhang J. Alleviation of apoptosis of bone marrow-derived mesenchymal stem cells in the acute injured kidney by heme oxygenase-1 gene modification. Int J Biochem Cell Biol. 2015;69:85–94.
Mohammadzadeh-Vardin M, Habibi Roudkenar M, Jahanian-Najafabadi A. Adenovirus-mediated over-expression of Nrf2 within mesenchymal stem cells (MSCs) protected rats against acute kidney injury. Adv Pharm Bull. 2015;5(2):201–8.
Yuzeng Q, Weiyang H, Xin G, Qingson Z, Youlin K, Ke R. Effects of transplantation with marrow-derived mesenchymal stem cells modified with survivin on renal ischemia-reperfusion injury in mice. Yonsei Med J. 2014;55(4):1130–7.
Liu N, Patzak A, Zhang J. CXCR4-overexpressing bone marrow-derived mesenchymal stem cells improve repair of acute kidney injury. Am J Physiol Renal Physiol. 2013;305(7):F1064–73.
Yuan L, Wu MJ, Sun HY, Xiong J, Zhang Y, Liu CY, et al. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;300(1):F207–18.
Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye S, et al. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20(1):103–13.
Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008;19(8):807–19.
Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292(5):F1626–35.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFE0126600 and 2018YFA0108803), National Natural Science Foundation of China (No. 82020741, 82204744, 82270758 and 82170686), and the Fostering Fund of Chinese PLA General Hospital for National Distinguished Young Scholar Science Fund (2019-JQPY-002).
Author information
Authors and Affiliations
Contributions
ZNF and QH designed the concept. ZNF, YFZ, XDG, KC, CL, and CCS searched the literature and collected the data. ZNF and YFZ drafted the manuscript. GYC, XMC, and QH revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fu, Z., Zhang, Y., Geng, X. et al. Optimization strategies of mesenchymal stem cell-based therapy for acute kidney injury. Stem Cell Res Ther 14, 116 (2023). https://doi.org/10.1186/s13287-023-03351-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03351-2