Abstract
Background
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is widely used as a curative treatment strategy for most types of hematological diseases. However, strategies for enhancing the graft versus leukemia (GVL) effect without aggravating the graft versus host disease (GVHD) effect are still being pursued.
Methods
A retrospective cohort study was performed to compare the outcomes between combined unrelated umbilical cord blood (UCB-haplo HSCT) and haplo HSCT.
Results
The results showed that neither acute GVHD (aGVHD) nor chronic GVHD (cGVHD) was increased in the UCB-haplo HSCT group, and the engraftment and infection rates were similar between the two groups. However, overall survival and progression-free survival were significantly improved, while transplantation-related mortality and relapse were significantly decreased in the UCB-haplo HSCT group by both univariate and multivariate analyses.
Conclusion
Our results indicated that the addition of a UCB unit could improve the prognosis of haplo-HSCT and enhance the GVL effect without increasing the incidence of GVHD.
Trial registration
The cohort study was retrospectively registered at https://www.chictr.org.cn as ChiCTR2100046681.
Similar content being viewed by others
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) offers a curative treatment strategy for most types of hematological diseases [1, 2]. Haploidentical HSCT (haplo-HSCT) is now widely used, which allows nearly everyone to have suitable donors. With the improvement of the conditioning regimen and graft versus host disease (GVHD) prophylaxis (Beijing protocol), haplo-HSCT has achieved outcomes comparable to those of human leukocyte antigen (HLA)-matched identical sibling HSCT [3]. Cho BS et al. confirmed this conclusion, and their results showed that the 3-year overall survival (OS) rates for HLA-matched-HSCT and haplo-HSCT were 57% [95% confidence interval (CI) 42–69%] and 73% (95% CI 59–83%) in acute myeloid leukemia (AML) patients, respectively [1]. The results of the Beijing protocol also showed the benefit of haplo-HSCT in leukemia, myelodysplastic syndrome (MDS) and aplastic anemia (AA) [4,5,6]. Despite great improvements achieved in haplo-HSCT, strategies for enhancing the graft versus leukemia (GVL) effect without aggravating the GVHD effect are still being pursued [7].
A previous study combined an unrelated umbilical cord blood (UCB) unit with haplo-HSCT, which reduced the relapse rate of recurrent and refractory acute leukemia [8], indicating that additional UCB infusion played an important role in haplo-HSCT. However, whether unrelated UCB could improve outcomes in patients with different statuses of hematologic malignancy and AA is still unknown. Thus, the cohort study was designed to compare the outcomes of patients between UCB-haplo HSCT and haplo HSCT. The primary endpoints were GVL effects [including OS, progression-free survival (PFS), relapse rate and transplantation-related mortality (TRM)] and GVHD incidence. The secondary endpoints were engraftment and infections. The study was registered at https://www.chictr.org.cn as ChiCTR2100046681.
Patients and methods
Patient eligibility
Patients who were eligible to receive haplo-HSCT between April 2016 and October 2020 were enrolled in this retrospective cohort study. All the patients were screened and grouped as shown in Fig. 1. The protocols were approved by the institutional review board of Shengjing Hospital of China Medical University. Informed consent for treatment was obtained from all the patients and their donors.
HLA typing and donor selection
HLA typing was detected by high-resolution DNA techniques for HLA-A, HLA-B, HLA-C, HLA-DQB1 and HLA-DRB1. Donors were selected from family members who shared only one HLA haplotype with the patient. Technically, male donors (especially fathers or sons) were selected. Being a female, mother or second-degree donor was not an ideal choice. In addition, the physical status and willingness of donors were also important matters that physicians should consider.
UCB selection
The selection of UCB units was based on HLA typing results and dose assessment before cell freezing. The UCB units were obtained from Liaoning and Shandong UCB banks certified by the Ministry of Health. All units were qualified clinical grade, normal in volume with depleted red blood cells, and transferred by cold-chain transportation. The selection strategy was as follows: First, HLA matching with patients required 3-6/6 with high-resolution HLA typing for HLA-A, HLA-B and HLA-DRB1. Second, minimum HLA matching required 7-9/10 high-resolution HLA typing with patients for HLA-A, HLA-B, HLA-C, HLA-DQB1 and HLA-DRB1. Third, blood type and sex were compared between UCB units and recipients, and UCB cell numbers were evaluated and considered.
Transplantation regimen
The transplantation regimen and GVHD prophylaxis strategy for malignant hematologic disease and AA were performed according to “Beijing protocols” reported previously, which includes the transplantation of granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood stem cells (G-PBSCs) and G-CSF-primed bone marrow (G-BM) from HLA-mismatched/ haploidentical related donors without in vitro T-cell depletion. [9, 10]. The conditioning therapy for malignant hematologic disease consisted of cytarabine (Ara-C, 4 g/m2/day) administered intravenously on days − 10 to − 9, busulfan (BU, 3.2 mg/kg/day) administered intravenously on days − 8 to − 6, cyclophosphamide (CTX, 1.8 g/m2/day) administered intravenously on days − 5 to − 4, semustine (250 mg/m2) administered orally on day − 3 and anti-thymocyte globulin (ATG, 2.5 mg/kg/day, rabbit) administered intravenously on days − 5 to − 2. The conditioning regime for AA consisted of BU (3.2 mg/kg/day) administered intravenously on days − 7 and − 6, CTX (50 mg/kg/day) administered intravenously on days − 5 to − 2, and ATG (2.5 mg/kg/day, rabbit) administered intravenously on days − 5 to − 2. All transplantation recipients were given cyclosporin A (CsA), mycophenolate mofetil (MMF) and short-term methotrexate (MTX) for GVHD prophylaxis. The regimen of prevention, monitoring, intervention and treatment of relapse was in accordance with previous studies [11].
All patients received G-BM and G-PBSCs from haploidentical donors on Days 0 and 1, respectively. In addition to haploid grafts, unrelated UCB units in the UCB-haplo group were infused on day 0 at least 4 h before haploidentical bone marrow infusion.
Implant chimerism monitoring was determined by short tandem repeat polymerase chain reaction (STR-PCR) among recipient, donor and unrelated UCB.
Definition and assessments
Neutrophil engraftment was defined as the first day when the absolute neutrophil count was > 0.5 × 109/L for 3 consecutive days. Platelet engraftment was defined as a platelet count > 20 × 109/L for 7 consecutive days without platelet transfusion. Criteria for response in AA: (a) none: still fulfil severe disease criteria; (b) partial: transfusion independent and no longer meet criteria for severe disease; and (c) complete: hemoglobin concentration normal for age and gender; ANC > 1.5 × 109/L and platelet count > 150 × 109/L. The diagnosis of GVHD was in accordance with the common international criteria [12,13,14]. CMV-related disease was defined according to reported criteria [15]. Overall survival (OS) time was defined as the time from hematopoietic stem cell transfusion to death by any cause. Progression-free survival (PFS) time was defined as the time from hematopoietic stem cell transfusion to disease progression or death. Relapse was defined by morphologic evidence of disease in peripheral blood, bone marrow and extramedullary sites or by the recurrence and sustained presence of pretransplantation chromosomal abnormalities. For AA, the loss of complete response was defined as relapse. Transplant-related mortality (TRM) was defined as death due to causes unrelated to the underlying disease.
Statistical analysis
Data were censored at the time of death or the last available follow-up on July 01, 2021. Data were collected from the institutional database and verified by the primary investigators and staff of the HSCT team.
Result
Patient characteristics
A total of 79 patients who were eligible to receive HSCT were enrolled in the study. All patients were suggested to receive an additional third-party UCB infusion, and 24 patients did not agree to use UCB. Fifty-five patients were enrolled to search the suitable UCB units as a previous scheme in Liaoning and Shandong UCB banks. However, two patients had no suitable UCB, and 53 patients had suitable UCB were recruited into the experimental group (UCB-haplo group) and provided informed consent for UCB treatment. The left 26 patients were recruited into the control group (haplo group) (Fig. 1). The patients’ characteristics are given in Table 1.
UCB and haploid graft characteristics
Following the previously described protocol, UCB units were chosen by the same physician and rechecked by another transplantation group physician. The characteristics of UCB units and haploid grafts are listed in Table 2. In addition, the quantities of MNC and CD34+ cells in haploid grafts were not significantly different between the UCB-haplo group and the haplo group. For HLA matching between the UCB unit and recipient, 10 units were 9/10 matched, 26 units were 8/10 matched, and 17 units were 7/10 matched. Before UCB infusion, an anti-allergy regimen was performed. There was no obvious transfusion reaction observed.
Engraftment
All surviving patients underwent chimerism analysis at Day 30 after HSCT, and they all achieved full donor chimerism. Chimerism analyses in these patients were continued regularly until disease relapse. During follow-up, in UCB-haplo group, there were two patients experienced mixed chimerism (unrelated UCB and haplo-identical donor). One patient was found to have mixed chimerism at the six-month visit with a normal range of routine blood tests and died at Day 235 after HSCT because of a serious fungal infection. The other patient was found to have mixed chimerism at Day 60 after HSCT and turned to full donor chimerism at Day 90 after HSCT.
The day of neutrophil and platelet engraftment was not significantly different between the UCB-haplo group and the haplo group. The median day of neutrophil engraftment was at day 12 (range, 10–24) and day 13 (range, 10–42) for the UCB-haplo group and haplo group, respectively (P = 0.349). The cumulative incidence of neutrophil engraftment on day 30 was 100% in the UCB-haplo group and 96% (95% CI 72.7–99.4%) in the haplo group (P = 0.52). Meanwhile, the median day of platelet engraftment was at day 14 (range, 9–69) and day 13 (range, 8–96) for the UCB-haplo group and haplo group, respectively (P = 0.974). The cumulative incidence of platelet engraftment on day 100 was 100% in both the UCB-haplo group and haplo group (P = 0.55).
GVHD
Both aGVHD and cGVHD were considered in the present study. In the UCB-haplo group, the 100-day cumulative incidences of grade II-IV aGVHD and grade III-IV aGVHD were 24.53% (95% CI 12.01–35.27%) and 5.67% (95% CI 0–11.68%), respectively. However, in the haplo group, the 100-day cumulative incidences of grade II–IV aGVHD and grade III–IV aGVHD were 15.38% (95% CI 0.31–28.18%) and 7.69% (95% CI 0–17.39%), respectively. There was no significant difference between the two groups (P = 0.36 and 0.73, respectively). The 1-year cumulative incidence rates of cGVHD in both the UCB-haplo group and the haplo group were 30.19% (95% CI 16.67–41.52%) and 38.46% (95% CI 16.61–54.59%), respectively. There was also no significant difference between the two groups (P = 0.45).
CMV and EBV infection
The 100-day cumulative incidence of CMV viremia was 47.17% (95% CI 31.87–59.04%) in the UCB-haplo group versus 50.00% (95% CI 26.56–65.96%) in the haplo group (P = 0.78).
The 100-day cumulative incidence of EBV viremia was 39.62% (95% CI 24.9–51.45%) in the UCB-haplo group versus 19.23% (95% CI 2.57–33.04%) in the haplo group (P = 0.062).
OS, PFS, TRM and relapse rate
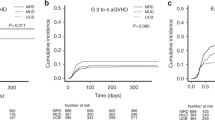
The probability of OS in the UCB-haplo and haplo groups was 79.92% (95% CI 69.39–92.04%) and 61.54% (95% CI 45.41–83.39%), respectively (P = 0.035). (Fig. 2) PFS in the UCB-haplo and haplo groups was 74.92% (95% CI 63.25–88.74%) and 53.85% (95% CI 37.72–76.86%), respectively (P = 0.011). (Fig. 2).
The cumulative incidence of TRM was 18.13% (95% CI 6.47–28.34%) and 35.38% (95% CI 13.78–51.58%) in the UCB-haplo and haplo groups, respectively (P = 0.045). (Fig. 2) The cumulative incidence of relapse was 8.10% (95% CI 0.00–16.87%) and 28.57% (95% CI 6.38–45.50%) in the UCB-haplo and haplo groups, respectively (P = 0.004). (Fig. 2).
The probability of PFS excluding AA in the UCB-haplo and haplo groups was 66.72% (95% CI 51.76–85.99%) and 50.00% (95% CI 32.92–75.94%), respectively (P = 0.049). The cumulative incidence of relapse excluding AA patients was 12.36% (95% CI 0.00–25.39%) and 29.41% (95% CI 4.06–48.06%) in the UCB-haplo and haplo groups, respectively (P = 0.029).
Univariate analysis
Univariate analysis was performed to determine the factors for predicting survival and relapse. The basic characteristics of patients and donors were used to identify predictive factors, and unrelated UCB, GVHD and infection were also included. The results showed that older age, poor disease status, high EBMT risk score, accompanying cGVHD, infection and the absence of a UCB unit were associated with poor OS and PFS as measured by univariate analysis (P < 0.10, shown in Table 3). Per univariate analysis, disease status, EBMT risk score, cGVHD, infection and the combination of UCB were also related to TRM (P < 0.10, shown in Table 3). However, poor disease status, high EBMT risk score and no combination of UCB were related to relapse (P < 0.10, shown in Table 3).
Multivariate analysis
In multivariate analysis (Table 4), the combination of UCB units (HR 0.331; 95% CI 0.130–0.843; P = 0.020) was an independent factor for improving OS. Infection (HR 2.964, 95% CI 1.172–7.496; P = 0.022) and cGVHD (HR 0.199; 95% CI 0.054–0.729; P = 0.015) were independent factors associated with poor OS. Meanwhile, the combination of UCB units (HR 0.338; 95% CI 0.144–0.795; P = 0.013) was an independent factor for improving PFS. However, cGVHD (HR 0.232; 95% CI 0.074–0.72; P = 0.012) was an independent factor associated with poor PFS. For TRM, the combination of UCB units (HR 0.329; 95% CI 0.121–0.889; P = 0.028) was an independent factor for reducing TRM. Infection (HR 4.104, 95% CI 1.506–11.186; P = 0.006) and cGVHD (HR 0.228; 95% CI 0.061–0.850; P = 0.028) were independent factors associated with increased TRM. The only independent factor for relapse was the combination of UCB units (HR 0.243; 95% CI 0.061–0.973; P = 0.046), which could reduce relapse.
Discussion
Until recently, improving the outcomes of haplo-HSCT and enhancing the GVL effect without aggravating GVHD effects were the main concerns. The biology of grafts was the first thing needing to be known. Currently, grafts for transplantation mainly include peripheral blood, bone marrow and UCB hematopoietic cells. The grafts of classical haplo-HSCT did not include UCB units. Previous laboratory research showed that UCB was a rich source of hematopoietic stem (HSCs) and progenitor (HPC) cells [16]. Studies also confirmed that the hematopoietic reconstitution capacity of UCB-derived HSCs in immune-deficient mice was superior to that of adult marrow cells in vivo [17]. Furthermore, UCB possesses great proliferation and expansion potential [18]. Therefore, umbilical cord blood was also used as an HSCT graft. Initially, UCB transplantation (UCBT) was used in children and achieved satisfactory outcomes [19]. Because UCB has few CD34+ cells, new approaches, such as the use of double UCBT (dUCBT), have been used in adult patients to avoid a prolonged delay in immune reconstitution [20]. The progress of UCBT was also summarized in a review [21]. In a previous study of UCBT, the incidence of severe GVHD was found to be lower than that of HLA-matched HSCT, especially HLA-matched unrelated donor HSCT [22]. The cause of GVHD was possibly attributable to the reactivity of donor T cells with recipient minor histocompatibility antigens. The reason why UCB grafts could reduce GVHD after UCBT is mainly as follows. First, UCB T lymphocytes are typically CD45RA+ naïve T cells with low levels of activation markers [23]. Second, altered recognition of recipient self-antigens by UCB donor T cells may result upon interaction with the recipient’s antigen presenting cells (APC) [24]. Third, there is a limited response of these naïve donor T cells activated by the recipient alloantigen. In primary mixed lymphocyte culture, UCB T cells demonstrate proliferative responses to allogeneic stimulation but less cytotoxic effector function, less proliferation and greater activation-induced cell death (AICD) [25]. Fourth, these changes result in impaired cytokine production, limited cellular activation and lack of clonal expansion of alloreactive T cells. UCB immune tolerance includes altered toll-like receptor and adhesion molecule expression on donor graft APCs [26]. Studies have also suggested that UCB graft T cells display reduced expression of nuclear factor of activated T cells-1 (NFAT1), which may be one important molecular mechanism underlying their reduced capacity for cytokine production [27, 28]. Taken together, the lower incidence and severity of GVHD found in UCB recipients is a direct consequence of the reduced proliferation, cytokine production, and cytotoxicity to alloantigens displayed by UCB lymphocytes. On the other hand, because of its biology, UCB permits a greater degree of HLA mismatching with an acceptable incidence of GVHD, without compromising the GVL effect. In addition, significant immunity against leukemia and viral antigens was provided by lymphocytes in the UCB graft. As previously mentioned, dUCBT improved the hematopoietic reconstitution in adult patients. Furthermore, studies also showed that dUCBT enhanced the GVL effect through the graft-versus-graft (GVG) effect because CD4+ T cells from the predominant UCB could rapidly reject nonengrafting UCB [29]. The enhanced GVL effect through the GVG effect during dUCBT is also mediated by specific CD8+ T-cell responses [30]. Another important concern for UCBT is hematopoietic and immune reconstitution. The TNC and CD34+ cells in the UCB unit were limited. However, UCB had a less mature phenotype of CD34+ progenitors compared to adult marrow and peripheral blood grafts, which might have a higher proliferation potential than adult CD34+ cells [31]. Taken together, the UCB unit, as one of the grafts for allo-HSCT, could reduce the GVHD effect, enhance the GVL effect and promote engraftment. The UCB unit as a transplantation graft might be considered a supplement for haploidentical HSCT.
In the present cohort study, we compared the outcomes between UCB-haplo HSCT and haplo HSCT. The result confirmed our postulation that the third-party UCB unit could enhance the GVL effect for reducing the risk of relapse and elevating the OS and PFS in haplo-HSCT. The risks of TRM and relapse were significantly decreased in the UCB-haplo group. Disease relapse was the primary cause of transplantation-related failure and death. In our study, the cumulative incidence of relapse was also decreased in the UCB-haplo group. This result was consistent with previous studies on the combination of UCB units in haplo-UCBT [8, 32]. GVHD was also a concern in HSCT. We analyzed aGVHD, including II-IV aGVHD and III-IV aGVHD, and cGVHD, including extensive cGVHD, in both groups. The results showed that the combination of third-party UCB did not increase any GVHDs. The results were also consistent with previous studies [8, 32]. As previously reported, UCBT might cause an increase in infection [33], especially CMV and EBV infection [34, 35]. In our study, the 100-day cumulative incidences of CMV viremia and EBV viremia were not significantly different between the UCB-haplo group and the haplo group. This result was consistent with that of Liu et al. [36]. However, they found that the combination of haploidentical and UCB HSCT resulted in rapid engraftment, yielding a different conclusion from our study. Our study showed that there were no significant differences between the UCB-haplo group and the haplo group. However, the transplantation regimen was different from that used by Liu et al. with reduced-intensity conditioning and our study with the “Beijing protocol,” which was a myeloablative conditioning regimen.
The present study showed that combining the third-party UCB unit with haploid grafts to perform UCB-haplo HSCT achieved better outcomes due to the GVL effect without increasing GVHD or infections. To determine the function of the UCB unit in HSCT, the comparison between single-UCBT and dUCBT studies was also reviewed. The outcomes of previous studies reached conflicting conclusions. Verneris et al. performed a randomized study in 2009 that found that dUCBT could decrease the risk of relapse, indicating the enhancement of the GVL effect, but increased the II–IV aGVHD effect [37]. Gérard Michel et al. found that dUCBT did not decrease transplantation strategy failure and even caused extensive cGVHD more frequently through a prospective randomized study in 2016 [38]. The conflicting results might be due to the mismatch between UCB units and receipts. Tozatto-Maio et al. found that a lower number of HLA mismatches with the recipient was indicated in dUCBT for acute leukemia patients [39]. Wang et al. compared the outcomes between cord-haplo and haplo-HSCT in refractory acute leukemia and established a mutual haploidentical donor mismatched antigen (MHMA) algorithm. The results showed that MHMA influenced both relapse and TRM in patients in the cord-haplo group. Patients with 1 MHMA had the most favorable PFS rate [8]. Lamers et al. suggest that cytotoxicity exerted by CD4+ T cells from the predominant UCB toward HLA class II alleles drives the rapid rejection of the nonengrafting UCB, whose alloreactive effect might also contribute to the GVL effect. In our study, we selected the UCB units with the strategy previously mentioned. We reviewed our data and found that the selection of mismatched UCB units had no significant effect on OS, PFS, relapse or TRM. First, the comparison between UCB units and patients on HLA-A, -B and -DR was performed as Gérard Michel et al. mentioned [38]. The results showed that more mismatched loci might predict longer PFS and OS and a lower risk of TRM, but the difference was not significant. There was nearly the same risk of relapse between groups in this algorithm. Second, the comparison was performed with the MHMA algorithm as Wang et al. established [8]. More mismatched loci represented longer PFS and a lower risk of relapse, but there was also no significant difference. There was no difference in TRM or OS between the groups. Third, HLA class II allele mismatch comparisons were performed as Lamers et al. suggested [29]. Furthermore, mismatches of HLA-A, -B and -DR comparisons were also calculated between UCB units and haploid donors. The results showed that more mismatched loci indicated better outcomes, while there was also no significant difference. The HLA-C and HLA-DQ loci were also compared between groups. The result also provided clues to us that the more mismatched loci there were, the better the outcomes, also without any significant difference.
There were several limitations in the present study. The result came from a single center with limited patients. However, we found that the combination of UCB units and haploid grafts significantly improved patient outcomes during HSCT. The conclusions remain to be validated in further independent and more extensive studies. We found clinical results that indicated that third-party UCB could enhance the prognosis, while there are still many experimental studies to confirm our conclusion.
Conclusion
Above all, the combination of third-party UCB units in haplo-HSCT increased the GVL effect without enhancing the GVHD effect. This result might be attributed to the biology of UCB and the GVG effect between the two grafts.
Availability of data and materials
The data was availability. All the data could ask for corresponding author to provide by email.
Abbreviations
- haplo-HSCT:
-
Haploidentical hematopoietic stem cell transplantation
- GVL:
-
Graft versus leukemia
- GVHD:
-
Graft versus host disease
- UCB-haplo HSCT:
-
Unrelated umbilical cord blood
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- TRM:
-
Transplantation-related mortality
- allo-HSCT:
-
Allogeneic hematopoietic stem cell transplantation
- HLA:
-
Human leukocyte antigen
- AML:
-
Acute myeloid leukemia
- MDS:
-
Myelodysplastic syndrome
- AA:
-
Aplastic anemia
- GVL:
-
Graft versus leukemia
- UCB:
-
Umbilical cord blood
- STR-PCR:
-
Short tandem repeat polymerase chain reaction
- HSCs:
-
Hematopoietic stem cells
- HPC:
-
Progenitor cells
- UCBT:
-
UCB transplantation
- dUCBT:
-
Double UCBT
- APC:
-
Antigen presenting cells
- AICD:
-
Activation-induced cell death
- NFAT1:
-
Nuclear factor of activated T cells1
- GVG:
-
Graft-versus-graft
- MHMA:
-
Mutual haploidentical donor mismatched antigen
References
Cho B-S, Min G-J, Park S, Park S-S, Shin S-H, Yahng S-A, Jeon Y-W, Yoon J-H, Lee S-E, Eom K-S, Kim Y-J, Lee S, Min C-K, et al. Haploidentical vs matched unrelated donor transplantation for acute myeloid leukemia in remission: a prospective comparative study. Am J Hematol. 2021;96:98–109.
Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018; 2. https://pubmed.ncbi.nlm.nih.gov/30108110/. Cited 14 Jul 2021.
Chang Y-J, Zhao X-Y, Huang X-J. Granulocyte colony-stimulating factor-primed unmanipulated haploidentical blood and marrow transplantation. Front Immunol. 2019;10:2516.
Chang Y-J, Wang Y, Liu Y-R, Xu L-P, Zhang X-H, Chen H, Chen Y-H, Wang F-R, Han W, Sun Y-Q, Yan C-H, Tang F-F, Mo X-D, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017;10:134.
Mo X-D, Zhang X-H, Xu L-P, Wang Y, Yan C-H, Chen H, Chen Y-H, Han W, Wang F-R, Wang J-Z, Liu K-Y, Huang X-J. Haploidentical hematopoietic stem cell transplantation for myelodysplastic syndrome. Biol Blood Marrow Transplant. 2017;23:2143–50.
Xu Z-L, Huang X-J. Haploidentical stem cell transplantation for aplastic anemia: the current advances and future challenges. Bone Marrow Transplant. 2021;56:779–85.
Chang Y-J, Zhao X-Y, Huang X-J. Strategies for enhancing and preserving anti-leukemia effects without aggravating graft-versus-host disease. Front Immunol. 2018; 9. https://pubmed.ncbi.nlm.nih.gov/30619371/. Cited 23 Jul 2021.
Wang J, Wang Z, Wei W, Zhang W, Zhang T, Cheng H, Fei X, Yin Y, Gu J, Yuan L. Cord haploidentical non-in vitro T cell depletion allogeneic hematopoietic stem cell transplantation reduces relapse of refractory acute leukemia. Biol Blood Marrow Transplant. 2019;25:121–8.
Wang Y, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, Ma X, Fan Z-P, Wu D-P, Huang X-J. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125:3956–62.
Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, Liu D, Liu Q, Liu T, Jiang M, Ren H, Song Y, Sun Z, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol. 2018;11:33.
Wang H-T, Chang Y-J, Xu L-P, Liu D-H, Wang Y, Liu K-Y, Huang X-J. EBMT risk score can predict the outcome of leukaemia after unmanipulated haploidentical blood and marrow transplantation. Bone Marrow Transplant. 2014;49:927–33.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, Ciceri F, Cornelissen J, Malladi R, Duarte RF, Giebel S, Greinix H, Holler E, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–67.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng G-S, Kerr H, Stratton P, Duarte RF, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389-401.e1.
Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–7.
Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–24.
Vormoor J, Lapidot T, Pflumio F, Risdon G, Patterson B, Broxmeyer HE, Dick JE. Immature human cord blood progenitors engraft and proliferate to high levels in severe combined immunodeficient mice. Blood. 1994;83:2489–97.
Lansdorp PM, Dragowska W, Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med. 1993;178:787–91.
Mo XD, Tang BL, Zhang XH, Zheng CC, Xu LP, Zhu XY, Wang Y, Liu HL, Yan CH, Chu XD, Chen H, Geng L-Q, Liu K-Y, et al. Comparison of outcomes after umbilical cord blood and unmanipulated haploidentical hematopoietic stem cell transplantation in children with high-risk acute lymphoblastic leukemia. Int J Cancer. 2016; 139. https://pubmed.ncbi.nlm.nih.gov/27356906/. Cited 15 Jul 2021.
Fatobene G, Volt F, Moreira F, Mariano L, Chevallier P, Furst S, Labussière-Wallet H, de la Tour RP, Deconinck E, Cluzeau T, Russell N, Karakasis D, Forcade E, et al. Optimizing selection of double cord blood units for transplantation of adult patients with malignant diseases. Blood Adv. 2020;4:6327–35.
Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122:491–8.
Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–61.
Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–8.
Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, Bina M, Panoskaltsis-Mortari A, Rubinstein P, Van Rooijen N, Golovina TN, Suhoski MM, Miller JS, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4–1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–57.
Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127:286–97.
Sawitzki B, Brunstein C, Meisel C, Schumann J, Vogt K, Appelt C, Curtsinger JM, Verneris MR, Miller JS, Wagner JE, Blazar BR. Prevention of graft-versus-host disease by adoptive T regulatory therapy is associated with active repression of peripheral blood Toll-like receptor 5 mRNA expression. Biol Blood Marrow Transplant. 2014;20:173–82.
Kadereit S, Mohammad SF, Miller RE, Woods KD, Listrom CD, McKinnon K, Alali A, Bos LS, Iacobucci ML, Sramkoski MR, Jacobberger JW, Laughlin MJ. Reduced NFAT1 protein expression in human umbilical cord blood T lymphocytes. Blood. 1999;94:3101–7.
Weitzel RP, Lesniewski ML, Haviernik P, Kadereit S, Leahy P, Greco NJ, Laughlin MJ. microRNA 184 regulates expression of NFAT1 in umbilical cord blood CD4+ T cells. Blood. 2009;113:6648–57.
Lamers CHJ, Wijers R, van Bergen CAM, Somers JAE, Braakman E, Gratama JW, Debets R, Falkenburg JHF, Cornelissen JJ. CD4+ T-cell alloreactivity toward mismatched HLA class II alleles early after double umbilical cord blood transplantation. Blood. 2016;128:2165–74.
Gutman JA, Turtle CJ, Manley TJ, Heimfeld S, Bernstein ID, Riddell SR, Delaney C. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115:757–65.
Vanegas D, Galindo C-C, Páez-Gutiérrez I-A, González-Acero L-X, Medina-Valderrama P-T, Lozano J-C, Camacho-Rodríguez B, Perdomo-Arciniegas A-M. Human leukocyte antigen and red blood cells impact umbilical cord blood CD34+ cell viability after thawing. Int J Mol Sci. 2019;20:4875.
Chen J, Wang R-X, Chen F, Sun A-N, Qiu H-Y, Jin Z-M, Tang X-W, Han Y, Fu Z-Z, He G-S, Miao M, Ma X, Wu D-P. Combination of a haploidentical SCT with an unrelated cord blood unit: a single-arm prospective study. Bone Marrow Transplant. 2014;49:206–11.
Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang M-J, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, Marks DI, van Rood JJ, Scaradavou A, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–75.
Ballen K, Woo Ahn K, Chen M, Abdel-Azim H, Ahmed I, Aljurf M, Antin J, Bhatt AS, Boeckh M, Chen G, Dandoy C, George B, Laughlin MJ, et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:1636–45.
Servais S, Hannon M, Peffault de Latour R, Socie G, Beguin Y. Reconstitution of adaptive immunity after umbilical cord blood transplantation: impact on infectious complications. Stem Cell Investig. 2017;4:40.
Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, Kline J, Nguyen V, Cunningham J, Larson RA, del Cerro P, Schroeder L, Pape L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–45.
Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, Burke MJ, Blazar BR, Miller JS, McGlave PB, Weisdorf DJ, Wagner JE. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–9.
Michel G, Galambrun C, Sirvent A, Pochon C, Bruno B, Jubert C, Loundou A, Yakoub-Agha I, Milpied N, Lutz P, Marie-Cardine A, Gandemer V, Blaise D, et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood. 2016;127:3450–7.
Tozatto-Maio K, Giannotti F, Labopin M, Ruggeri A, Volt F, Paviglianiti A, Kenzey C, Hayashi H, Cornelissen J, Michallet M, Karakasis D, Deconinck E, Rohrlich P-S, et al. Cord blood unit dominance analysis and effect of the winning unit on outcomes after double-unit umbilical cord blood transplantation in adults with acute leukemia: a retrospective study on behalf of Eurocord, the Cord Blood Committee of Cellular Therapy, Immunobiology Working Party, and the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24:1657–63.
Acknowledgements
The authors thank all the patients recruited from Shengjing Hospital of China Medical University for participating in the research that made this study possible.
Funding
This work was supported by the 345 Talent Project in Shengjing Hospital of China Medical University.
Author information
Authors and Affiliations
Contributions
HTW and YY conceived and designed the study. MZ and MQL sign the informed consents and collected the data. YCL performed the protocol. WY performed statistical analysis. YY, HTW, and ZGL revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocols were approved by institutional review board of Shengjing Hospital of China Medical University. Informed consents were obtained from all the patients and their donors.
Consent for publication
We consent for publication.
Competing interests
There was no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Y., Zhang, M., Li, M. et al. Unrelated umbilical cord blood can improve the prognosis of haploidentical hematopoietic stem cell transplantation. Stem Cell Res Ther 13, 485 (2022). https://doi.org/10.1186/s13287-022-03170-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-03170-x