Abstract
Background
Aspirin has been demonstrated to promote osteoblast-mediated bone formation and inhibit osteoclast (OC)-mediated bone resorption. However, it remains unclear whether aspirin influences other immune cells during bone resorption. Dendritic cells (DCs), the most potent antigen-presenting cells, can also transdifferentiate into active OCs in the presence of receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). The effects of aspirin on DC-derived OCs (DDOCs) were investigated in the current study.
Methods
Flow cytometry and mixed lymphocyte reaction (MLR) assays were used for DC identification. The proliferative capacity of DCs was determined by BrdU assays. Apoptosis was examined by flow cytometry. The osteoclastic potential of DCs was tested using tartrate-resistant acid phosphatase (TRAP) staining, western blotting, and reverse transcription polymerase chain reaction (RT-PCR). Western blotting was also used to examine signaling pathways. A mandibular bone defect model was established to assess the effect of aspirin on bone resorption.
Results
Aspirin had no influence on the surface phenotype, proliferation, or apoptosis of DCs, though aspirin significantly inhibited osteoclast differentiation in RANKL-stimulated DCs. DC osteoclast differentiation was modulated by aspirin via the nuclear factor kappa B (NF-κB)/nuclear factor of activated T cell, cytoplasmic 1 (NFATc1) signaling pathway. Aspirin treatment also had favorable therapeutic effects on bone regeneration in the bone defect model, and the number of osteoclasts was decreased.
Conclusions
Aspirin inhibited RANKL-induced OC differentiation in DCs via the NF-κB pathway, downregulating expression of NFATc1. Aspirin treatment promoted bone regeneration by inhibiting DDOC activation in the early stages of inflammation in a rat mandibular bone defect model.
Similar content being viewed by others
Background
Bone homeostasis is maintained through the balance between the activity of osteoclasts (OCs) and that of osteoblasts. However, in some inflammatory diseases, such as osteoporosis and periodontitis, bone resorption exceeds bone formation, creating an imbalance caused by abnormal activation of OCs [1].
OCs, unique cells capable of dissolving bone matrix, are derived from the monocyte/macrophage lineage of hematopoietic precursors. OC differentiation and activation is regulated by two critical cytokines: macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kB ligand (RANKL) [2]. During differentiation, the fusion of mononuclear precursors is indispensable for the formation of mature multinucleated OCs, which play an essential role in resorbing bone [3].
In addition to the major involvement of OCs in bone homeostasis, the involvement of other immune cells in bone resorption has been shown in several studies. Dendritic cells (DCs), the major antigen-presenting cells, also share several features with OCs; they are derived from the same myeloid precursor, and the functions of both cell types are closely dependent upon RANKL [4]. Interestingly, there is increasing evidence that immature DCs (imDCs) have osteoclastogenic potential in some inflammatory pathological conditions because imDCs can transdifferentiate into active OCs after stimulation with RANKL and M-CSF [5, 6]. These findings clearly highlight a potential link between DCs and OCs.
Because of the major involvement of OCs in bone homeostasis, a feasible strategy is to inhibit osteoclastogenesis and/or decrease bone resorption activity in active OCs to prevent or treat bone resorption-related disorders. Aspirin is a classic nonsteroidal anti-inflammatory drug (NSAID) that is well known for its antipyretic, analgesic, and anti-inflammatory effects. Aspirin irreversibly inhibits cyclooxygenase-1(COX-1) and cyclooxygenase-2 (COX- 2) activities via covalent modification [7]. Moreover, multiple animal and clinical studies have reported that COX-2 inhibition negatively affects bone healing [8,9,10,11], though a small minority of these studies have demonstrated no lasting negative effects [11,12,13]. Recent studies have shown that aspirin can regulate the bone balance in ovariectomy-induced osteoporosis [7]. Furthermore, aspirin was found to inhibit RANKL-induced osteoclastogenesis in RAW264.7 cells [14]. However, the effects of aspirin on bone healing remain highly debated. The main factors that may underlie the discrepancies between studies are the mode of administration, the variability of the drug dose, the dosing duration, and the type of bone defect model [11]. According to our previous studies, aspirin is able to promote bone marrow mesenchymal stem cell (BMSC)-based skull bone regeneration in mini-pigs after release in a local, sustained, and controlled manner [15]. In the present study, we examined whether aspirin regulates dendritic cell-derived osteoclast (DDOC) formation and activation.
Materials and methods
Animals
C57BL/6J mice (male, aged 8–10 weeks) and rats were sourced from the Institute of Vital River (Beijing, China). This study was performed according to institutionally set guidelines for animal research.
Isolation and culture of CD11c+ DCs
Murine DCs were generated from bone marrow precursors. Bone marrow single-cell suspensions were generated from tibias and femurs and filtered through a 70-μm strainer (BD Bioscience, CN). The cells were then incubated with Red Blood Cell Lysis Buffer (APPLYGEN, China) and washed. The cells were then seeded in six-well plates (106 cells/ml) in RPMI-1640 medium (Gibco, USA) containing 10% heat-inactivated fetal bovine serum (FBS), 100 μg/ml streptomycin, 100 U/ml penicillin, and 2 mM l-glutamine and supplemented with 25 ng/mL recombinant mouse GM-CSF (rmGM-CSF; R&D Systems, USA). The cells were induced for 3–7 days, and then imDCs were induced to mature by the addition of TNF-alpha (PeproTech, USA, 20 ng/ml) for 24 h before harvesting on the 7th day.
Surface markers of DCs after aspirin treatment
DCs cultured with or without aspirin were prepared for surface marker expression analysis by FACS staining. Cells were incubated on ice for 15–30 min with specific monoclonal antibodies (mAbs): FITC-labeled anti-CD11c, PerCP/Cy5.5-labeled anti-MHC class II, PE-labeled anti-CD80, and APC-labeled anti-CD86 (BD Biosciences, USA). After staining, the labeled cells were analyzed with a BD FACSCanto II flow cytometer (BD Biosciences).
Isolation and culture of CD4+ T cells
A mixed lymphocyte reaction (MLR) assay was employed to determine the functional activity of DCs; T lymphocytes were used as responder cells. CD4+ T cells were isolated from splenocyte suspensions and sorted by MIDI magnetic cell sorting (MACS; Miltenyi Biotec, Germany). Naive CD4+ T cells were incubated in T cell culture medium (RPMI-1640 medium containing 10% FBS, 2 mmol/l-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin, and 20 mol/L HEPES; Invitrogen, USA). The naive CD4+ T cells were activated using soluble anti-mouse CD28 (2.5 μg/ml) and plate-bound anti-mouse CD3 (5 μg/ml) antibodies.
MLR assay
To analyze T cell proliferation, cells (1 × 106) were labeled with 5 mM carboxyfluorescein succinimidyl ester (CFSE; Life Technologies, USA) for 15 min in PBS (1% BSA). The cells were seeded in round-bottom 96-well plates to ensure effective DC/T cell interaction, after which DCs were added in gradient doses to activate the T cells (1 × 105 cells per well). The cells were cultured for 72 h, and the proliferation of the CD4+ T cells in response to priming by the DCs was assessed by FACS. Data were analyzed using ModFit LT.
BrdU assay for cell proliferation
ImDC proliferation was evaluated with BrdU Cell Proliferation Assay Kit (BioVision, USA). DCs (5.0 × 103 cells/well) were seeded in triplicate in a 96-well flat-bottom plate (Costar, USA) and incubated in 200 μl of standard culture medium with or without aspirin for 24 h. The cultures were then incubated with a BrdU solution for 4 h. Absorbance at 450 nm was measured using an enzyme-linked immunosorbent assay (ELISA) reader (ELx800; Bio-Tek Instruments Inc., USA), with the optical density (OD) value at 450 nm representing proliferating cells.
Apoptosis assay
To detect apoptotic cells in the presence or absence of aspirin, apoptosis was assessed using Annexin V Apoptosis Detection Kit FITC (eBioscience, USA).
OC differentiation and the tartrate-resistant acid phosphate (TRAP) assay
imDCs were further differentiated with α-minimum essential medium (α-MEM; Gibco, USA) containing 100 ng/ml RANKL and 25 ng/ml M-CSF to obtain OCs.
A leukocyte acid phosphatase kit (Sigma-Aldrich) was utilized to examine the TRAP activity of adherent OC cultures. If TRAP-positive cells had three or more nuclei, they were considered OCs. TRAP-positive cells were analyzed by cell counting in at least five random fields under a light microscope (OLYMPUS, Japan).
Reverse transcription polymerase chain reaction (RT-PCR)
imDCs (106 cells/ml) were plated in a six-well plate with various concentrations of aspirin and cultured for 5–7 days in culture medium. Total RNA was isolated from the cultured cells with TRIzol reagent (Invitrogen, USA) and reverse transcribed into cDNA using oligo (dT) according to the manufacturer’s protocol (Invitrogen, USA). Real-time PCR was performed using iCycler iQ Multi-Color Real-time PCR Detection System and a QuantiTect SYBR Green PCR kit (Qiagen, Germany). The specific primer sequences used are listed in Table 1.
Western blot analysis
imDCs were plated in six-well plates and treated with aspirin for 24 h prior to treatment with RANKL and M-CSF. The cells were lysed in RIPA Lysis Buffer (Beyotime, China). Nuclear proteins were extracted using an EpiQuik Nuclear Extraction kit (EpiGentek, USA). Protein extracts were separated by 10% SDS polyacrylamide gel electrophoresis (Applygen, China), and subsequent processes were performed following a standard protocol.
Mandibular bone defect model
We established a mandibular bone defect in rats ranging from the incisors to the molars, 5 mm long, 2 mm wide, and 1 mm high, under general anesthesia (10% pentobarbital, 40 mg/kg). After surgery, all rats were randomly divided into aspirin and control groups. We mixed hydrogel (500 μl, BeaverBio, China) and hydroxyapatite/tricalcium phosphate (HA/TCP) (20 mg) with or without 150 μg/ml aspirin and filled the mandibular bone defect with this mixture, followed by suturing the incisions intermittently with 0–4 absorbable sutures (Fig. 5a). The rats were sacrificed on days 3 and 14 and at 2 months.
Histological evaluations of the bone defect
After rats were sacrificed, the defective area in the rat mandibular bone was observed by stereomicroscopy. After the mandibular bones were fixed in 4% paraformaldehyde (PFA), they were decalcified with buffered 10% EDTA (pH 8.0) and embedded in paraffin. The tissues were sectioned at 5 μm and stained with TRAP and hematoxylin and eosin (H&E).
Immunohistochemical staining
We euthanized rats after surgery to perform immunohistochemical examinations. Paraffin-embedded sections acquired as described above were dewaxed in xylene and dehydrated in alcohol. To reduce nonspecific staining, all sections were incubated with 3% hydrogen peroxide and then blocked with 10% serum. Next, the samples were incubated with a mouse anti-rat CD11c antibody (1200, Abcam, UK) and treated with biotinylated secondary antibodies. After the samples were developed by DAB staining and counterstained, observations were performed with a confocal microscope (OLYMPUS, Japan). Positively stained cells were counted in at least five random fields and quantified using Image-Pro Plus 6.0.
Statistical analysis
GraphPad Prism7 was used for statistical analysis. Unless otherwise noted, statistical significance comparisons of two groups were performed by Student’s t test, and one-way ANOVA was applied for comparisons among multiple groups. For samples with heteroscedasticity, Kruskal-Wallis and Mann-Whitney U tests were used to evaluate differences.
Results
Isolation and characterization of DCs
imDCs can be stimulated to mature by exposure to cytokines. TNF-α induced maturation of DCs, which was characterized by significantly increased expression of CD11c, the costimulatory molecules CD80 and CD86, and the antigen-presenting molecule MHC class II (Fig. 1a, b).
Dendritic cells (DCs) have greater T cell proliferation promotion ability. a, b DCs generated from wild-type mice expressed high levels of CD11c, MHC class II, CD80, and CD86 surface molecules. c DCs induced proliferation of allogeneic CD4+ T cells in a mixed lymphocyte reaction (P < 0.01). No significant differences were detected in the levels of T cell proliferation when different ratios of DC/T cells were cultured and harvested (P > 0.05). All experiments are representative of three replicates. *P < 0.05, **P < 0.01
The DCs were then tested for their ability to promote the proliferation of allogeneic CD4+ T cells in an MLR assay. CFSE-labeled naive CD4+ T cells were cocultured with different numbers of DCs. The cells were harvested on day 3, and the number of proliferating cells had increased (Fig. 1c; P < 0.01).
Effect of aspirin on imDC proliferation and apoptosis
The effects of aspirin on imDCs were evaluated by culturing imDCs with aspirin at different concentrations, followed by assessment of the rates of cell proliferation and apoptosis. Aspirin did not have different effects on cell proliferation at the concentrations tested (Fig. 2a; P > 0.05). Additionally, aspirin did not dramatically induce apoptosis in imDCs (Fig. 2c; P > 0.05).
Aspirin has no influence on DC apoptosis, proliferation, or immunophenotype. a Aspirin was not found to exert different effects on cell proliferation at the concentrations tested (P > 0.05). b Immature DCs (imDCs) expressed low levels of CD11c, MHC class II, CD80, and CD86 surface molecules; mature DCs highly expressed these surface molecules. Expression of surface markers on DCs with or without aspirin treatment showed no obvious differences. c The addition of aspirin had no influence on DC apoptosis (P > 0.05). All experiments are representative of three replicates. *P < 0.05, **P < 0.01
Aspirin was inefficient at modulating the DC surface phenotype
Cells were treated with aspirin overnight before maturation with TNF-α. The aspirin-treated DCs displayed a surface expression pattern similar to that of untreated cells, as the levels of CD11c, CD80, CD86, and MHC-II expression on the cell surface were higher than those on the imDC cell surface (Fig. 2b).
Aspirin inhibited OC differentiation in RANKL-stimulated imDCs
We then examined whether aspirin treatment can inhibit the osteoclastic potential of imDCs. imDCs were induced by RANKL and M-CSF as well as aspirin (0, 50, 100, 150, or 200 μg/ml) and then stained after 7 days of culture (Fig. 3a; P < 0.01). The results demonstrated that aspirin suppressed the formation of OC-like cells following stimulation with RANKL, which was verified by the downregulation of OC-specific marker gene (cathepsin K (CTSK), TRAP, and calcitonin receptor (CTR)) expression in imDCs cultured under OC-inducing conditions with aspirin for 5 days (Fig. 3b; P < 0.01). As the reduced gene expression was most stable and significant in the 150 μg/ml aspirin group, we used this dose for treating imDCs in subsequent assays. Western blotting results showed that expression of CTSK was inhibited by aspirin treatment, which was in accordance with RT-PCR results (Fig. 3c).
Aspirin inhibits osteoclast differentiation in RANKL-stimulated imDCs. a TRAP+ multinuclear cells (MNCs) with more than three nuclei were considered mature osteoclasts (OCs). TRAP staining showed that aspirin significantly suppressed the formation of RANKL-induced osteoclast-like cells among imDCs (P < 0.01). b RT-PCR showed that aspirin treatment downregulated expression of tartrate-resistant acid phosphatase (TRAP), calcitonin receptor (CTR), and cathepsin K (CTSK) (P < 0.01). c Consistent with the RT-PCR results, western blot analysis showed that CTSK expression levels were increased during osteopetrosis induction but that aspirin treatment partially suppressed this increase. All experiments are representative of three replicates. *P < 0.05, **P < 0.01. Scale bar = 20 μm in a
Aspirin inhibited RANKL-induced OCs by directly inhibiting the IκK/IκB/NF-Κb/NFATc1 pathway
NF-κB is a dimeric transcription factor complex that plays a critical role in osteoclastogenesis [16, 17]. NFATc1 expression, which has an integral role in OC differentiation, is dependent on RANKL-NF-κB pathways. Additionally, previous studies have shown that aspirin can suppress the activity of IκB kinase (IκK)-β in the NF-κB pathway [18]. Thus, we detected the activities of NF-κB-related proteins. The results showed that IκK, p-IκK, IκB, and p-IκB expression increased after RANKL treatment and that aspirin reduced this upregulation (Fig. 4a, c). In addition, RANKL promoted P65 and NFATc1 nuclear translocation, whereas aspirin treatment suppressed this effect (Fig. 4a, c). Subsequent experiments were carried out for verification purposes. To this end, we blocked the IκK/IκB/NF-κB pathway with the IκK-β inhibitor (IMD 0354; Selleck, USA) before RANKL treatment and found that the RANKL-induced upregulation of IκB and p-IκB protein expression was suppressed. In addition, the IκK-β inhibitor blocked NFATc1 nuclear translocation (Fig. 4b).
Aspirin inhibits RANKL-induced OCs via the IκK/IκB/NF-κB pathway. a, b Western blot showing that RANKL treatment upregulated IKK, p-IKK, IKB, p-IKB, and p-p65 and that IKK inhibitor treatment significantly reduced this upregulation. The expression levels of NFATc1 showed the same tendency. c Components of the IκK/IκB/NF-κB pathway were overexpressed after RANKL induction, which was decreased by aspirin pretreatment, with the results for NFATc1 expression being consistent. d Schematic representation of the timing of aspirin and IKK inhibitor treatment in our experiments. e Based on all of the results described above, aspirin inhibits RANKL-induced OCs via the NF-κB pathway, ultimately suppressing NFATc1 synthesis. All experiments are representative of at least three replicates
Importantly, these results indicated that the NF-Κb/ NFATc1 pathway is involved in aspirin-mediated inhibition of the differentiation of DCs into OCs (Fig. 4d).
Aspirin treatment showed favorable therapeutic effects on bone regeneration
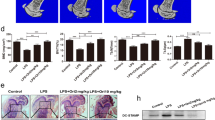
To identify the abilities of aspirin-pretreated DDOCs in a bone defect, we generated a defect (5 mm × 2 mm × 1 mm) in the rat mandible (Fig. 5a). Defect healing in the aspirin group occurred more rapidly than in the control group at 2 weeks (Fig. 5b; P < 0.05). Two months later, the aspirin group showed a favorable defect-to-healing ratio (Fig. 5c; P < 0.01). In addition, H&E staining of bone defect sections acquired from both groups showed that aspirin promoted bone healing (Fig. 6g; P < 0.05). In the control group, new bone had formed over the bone defect surface with a moderate number of new blood vessels (Fig. 6a–c). HA/TCP particles were found in the mineralized tissue. However, numerous new bones were observed in the aspirin group, which exhibited almost complete repair of the bone defect, and new bone formation was still active around the surface of the HA/TCP particles (Fig. 6d–f).
Aspirin treatment improves bone healing in the rat mandibular defect model. a The diagram above shows the establishment process of the alveolar bone defect model in a rat. b Two weeks after the surgery, the rate of bone defect healing in the aspirin group was much faster than that in the control group (P < 0.05). c Two months later, aspirin showed a clear benefit on the defect healing ratio compared to the control group (P < 0.01). The blue boxes in b and c are the mandibular defect area generated at the beginning of surgery. All experiments are representative of three replicates. *P < 0.05, **P < 0.01
Aspirin treatment promotes HA/TCP-mediated new bone formation in the rat mandibular defect model. a Two months after surgery, new bone and blood vessels were observed in the control group. HA/TCP was also detected in the mineralized tissues. (Black lines show the margins of the bone defect generated during surgery. Blue lines show the area of new bone formation.) b The area of a green rectangle in a shows new bone around HA/TCP particles under a high-magnification microscope, and new blood vessels can be seen between the bone. c Large volumes of connective tissues remain at the edge of the bone defect, which surround the HA/TCP particles. (The area enclosed with a yellow rectangle in a.) d New bone formation almost completely repaired the bone defect in the aspirin group after 2 months. (Black lines show the margins of the bone defect generated during surgery. Blue lines show the area of new bone formation.) e The area in a green rectangle of d shows new bone formation and a large number of new surrounding blood vessels. f Higher magnification of the area in a yellow rectangle in d shows osteoblasts surrounding the surface of HA/TCP particles, with new bone formation still being active. g Two months later, aspirin treatment accelerated bone regeneration compared to the control group (P < 0.05). HA = HA/TCP particles, CT = connective tissue, BV = blood vessel, NB = new bone. Scale bar = 200 μm in a, d and scale bar = 50 μm in b, c, e, f
Aspirin inhibited OC differentiation in the bone defect model
The number of local OCs was examined in the bone defect model. According to TRAP staining, OCs remained around the bone defect in both groups showed on the third day (Fig. 7a–d). Additionally, the aspirin group exhibited a significant reduction in the number of OCs (Fig. 7i; P < 0.05). Most studies have indicated that immature CD11c+ DCs can act as OC precursors and thus become functional OCs in the inflammation-induced bone environment [19]. Thus, we adopted immunohistochemical staining to verify that the number of CD11c+ cells increased in both groups (Fig. 7e–h) and found a lower number of CD11c+ cells in the aspirin group than in the control group (Fig. 7j; P < 0.05).
Aspirin inhibits osteoclasts during early inflammation in the rat mandibular bone defect model. a–d TRAP staining showed that by the 5th day after surgery, osteoclasts were increased in both the control group and aspirin group. e–h We performed immunohistochemical staining on sections, and the results indicated overexpression of CD11c (brown-yellow color) in both the control and aspirin groups. i However, compared to the control group, the aspirin group maintained lower osteoclast numbers (P < 0.05). j After surgery, CD11c+ cells showed a lower ratio in the aspirin group (P < 0.05). All experiments are representative of three replicates. *P < 0.05, **P < 0.01. Scale bar = 200 μm in a, c; scale bar = 100 μm in e, g; scale bar = 50 μm in b, d, f, h
Discussion
Under physiological conditions, bone mass maintenance is achieved through a delicate balance between bone formation by osteoblasts and bone resorption by OCs. However, in certain inflammatory diseases, this balance is disrupted, and the activation of OCs predominates, resulting in localized bone erosion. Thus, it is important to understand the mechanisms underlying the relative activity of OCs to minimize bone injury in these inflammatory diseases [2].
Osteoclastogenesis depends on progenitor cells of the monocyte hematopoietic lineage, and these progenitor cells have been demonstrated to fuse to form OCs under the influence of M-CSF and RANKL [2, 4]. In addition, human DCs can differentiate into mature OCs [20]. In agreement with this, several researchers have generated OCs from splenic or bone marrow-derived mouse DCs in a RANKL-dependent manner [21]. Moreover, DCs are considered to be more efficient at differentiating into OCs than are monocytes in terms of fusion efficiency and the number of nuclei per OC, suggesting that DCs might have an unexpected direct role in osteoclastogenesis [22, 23]. Accordingly, downregulation of DDOC differentiation or function may be an ideal target for the treatment of pathological bone diseases.
Aspirin is an NSAID commonly used in clinical applications because of its various antipyretic, analgesic, antirheumatic, and anti-platelet aggregation effects [19, 24]. The properties of aspirin involve a variety of pathways, such as inhibition of COX-2 and COX-1 and prostaglandin E2 (PGE2) activities [7, 25, 26]. PGE2 is a multifunctional regulator of bone metabolism that affects bone resorption and formation [27], and previous studies have shown that treatment with COX-2-specific NSAIDS reduce levels of PGE2 while negatively affecting bone healing [28]. The inhibitory effect of aspirin on bone healing has also been demonstrated in four animal studies and a human in vitro study [8, 29,30,31,32]. Nonetheless, a recent study indicated that aspirin has positive effects on cranial bone regeneration in miniature pigs, partly by promoting bone marrow mesenchymal stem cell-based osteogenesis [8]. Moreover, epidemiological studies have demonstrated that frequent use of aspirin may have a moderate beneficial effect on bone mineral density in postmenopausal women [33]. In general, the controversial results may depend on the preparations of the drug, modes of administration, doses, and ability to inhibit COX-2. Aspirin inhibits OCs [34] by promoting telomerase activity and also dramatically activates osteoblasts [26, 35]. In addition, exogenous PGE2 may strongly stimulate bone resorption in bone organ cultures and osteoclast differentiation in bone marrow cultures. PGE2 has biphasic effects on RANKL-induced OC formation in bone marrow cultures [27]. For example, PGE2 increases RANKL-induced OC differentiation in RAW264.7 cells [36] but inhibits differentiation in cultured human peripheral blood mononuclear cells (PBMCs) [37]. Furthermore, aspirin was shown to inhibit RANKL-induced osteoclastogenesis in RAW264.7 cells [14] and to decrease bone resorption by downregulating PGE2 both in vivo and in vitro [38]. Regardless, the inhibitory potential and mechanisms of aspirin with regard to the differentiation of DCs into OCs have not been elucidated.
In this study, we observed that DCs were able to induce TRAP+ OC formation in response to RANKL and that this process was inhibited by aspirin. We further investigated the underlying molecular mechanisms involving aspirin in OC differentiation and function. The RANKL/RANK/osteoprotegerin (OPG) axis is the classic regulatory system of bone metabolism, and it can regulate OC differentiation [39, 40]. Binding of RANKL to its receptor RANK results in rapid recruitment of multiple intracellular signaling molecules, such as MAPK, NF-κB, AP-1, TRAFs, and NFATc1, with NF-κB being the most important factor [41]. Indeed, NF-κB signaling has been shown to play an essential role in osteoclastogenesis, and its suppression can inhibit NFATc1 expression [42]. Previous research demonstrated that the NF-κB inhibitor (−)-dehydroxymethylepoxyquinomicin suppresses RANKL-induced osteoclastogenesis by downregulating NFATc1 expression [43]. In the present study, we found that treatment with aspirin suppressed RANKL-induced NF-κB signaling pathways.
Multiple previous studies have established that during osteoclastogenesis, NFATc1 is the specific transcription factor that regulates OC-specific genes, such as cathepsin K, TRAP, and CTR, involved in the regulation of RANKL-mediated OC differentiation, fusion, and activation [39, 44]. The present data suggest that stimulation of imDCs with RANKL leads to expression of NFATc1 and that aspirin treatment reduces RANKL-induced NFATc1 activation. Collectively, these results indicate that aspirin effectively inhibits OC differentiation in imDCs by regulating NF-κB induction and NFATc1 following RANKL/RANK interaction.
Conclusion
In summary, our results demonstrate that aspirin downregulates the RANKL-mediated induction of NF-κB and NFATc1. This effect then participates in downregulating expression of the downstream nuclear transcription factor NFATc1 and inhibiting RANKL-induced OC differentiation in preosteoclastic imDCs. However, further investigations into the role of imDCs in bone resorption and the feasibility of using aspirin in clinical applications are still required.
Availability of data and materials
All data analyzed during the current study are included in this published article.
Abbreviations
- α-MEM:
-
α-minimum essential medium
- BMSC:
-
Bone marrow mesenchymal stem cell
- CTR:
-
Calcitonin receptor
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- CTSK:
-
Cathepsin K
- COX-1:
-
Cyclooxygenase-1
- COX-2:
-
Cyclooxygenase-2
- DCs:
-
Dendritic cells
- DDOCs:
-
Dendritic cell-derived osteoclasts
- H&E:
-
Hematoxylin and eosin
- imDCs:
-
Immature dendritic cells
- M-CSF:
-
Macrophage colony-stimulating factor
- MLR:
-
Mixed lymphocyte reaction
- mAbs:
-
Monoclonal antibodies
- NSAID:
-
Nonsteroidal anti-inflammatory drug
- NF-κB:
-
Nuclear factor kappa B
- NFATc1:
-
Nuclear factor of activated T cell,
cytoplasmic 1
- OC:
-
Osteoclast
- OPG:
-
Osteoprotegerin
- PFA:
-
Paraformaldehyde
- PBMC:
-
Peripheral blood mononuclear cell
- PGE2 :
-
Prostaglandin E2
- RANKL:
-
Receptor activator of nuclear factor-κB ligand
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- TRAP:
-
Tartrate-resistant acid phosphatase
References
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42.
Charles JF, Aliprantis AO. Osteoclasts: more than ‘bone eaters’. Trends Mol Med. 2014;20(8):449–59.
Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, et al. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001;98(8):2544–54.
Lapérine O, Blin-Wakkach C, Guicheux J, Beck-Cormier S, Lesclous P. Dendritic-cell-derived osteoclasts: a new game changer in bone-resorption-associated diseases. Drug Discov Today. 2016;21(9):1345–54.
Speziani C, Rivollier A, Gallois A, Coury F, Mazzorana M, Azocar O, et al. Murine dendritic cell transdifferentiation into osteoclasts is differentially regulated by innate and adaptive cytokines. Eur J Immunol. 2007;37(3):747–57.
Tucci M, Stucci S, Savonarola A, Ciavarella S, Cafforio P, Dammacco F, et al. Immature dendritic cells in multiple myeloma are prone to osteoclast-like differentiation through interleukin-17A stimulation. Br J Haematol. 2013;161(6):821–31.
Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS One. 2008;3(7):e2615.
Allen HL, Wase A, Bear WT. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand. 1980;51(4):595–600.
Goodman S, Ma T, Trindade M, Ikenoue T, Matsuura I, Wong N, et al. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002;20(6):1164–9.
Trancik T, Mills W, Vinson N. The effect of indomethacin, aspirin, and ibuprofen on bone ingrowth into a porous-coated implant. Clin Orthop Relat Res. 1989;249:113–21.
Mullis BH, Copland ST, Weinhold PS, Miclau T, Lester GE, Bos GD. Effect of COX-2 inhibitors and non-steroidal anti-inflammatory drugs on a mouse fracture model. Injury. 2006;37(9):827–37.
Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004;86(1):116–23.
Long J, Lewis S, Kuklo T, Zhu Y, Riew KD. The effect of cyclooxygenase-2 inhibitors on spinal fusion. J Bone Joint Surg Am. 2002;84(10):1763–8.
Zeng YP, Yang C, Li Y, Fan Y, Yang HJ, Liu B, et al. Aspirin inhibits osteoclastogenesis by suppressing the activation of NF-κB and MAPKs in RANKL-induced RAW264.7 cells. Mol Med Rep. 2016;14(3):1957–62.
Cao Y, Xiong J, Mei S, Wang F, Zhao Z, Wang S, et al. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res Ther. 2015;6:210.
Shinohara M, Takayanagi H. Novel osteoclast signaling mechanisms. Curr Osteoporos Rep. 2007;5(2):67–72.
de la Rica L, García-Gómez A, Comet NR, Rodríguez-Ubreva J, Ciudad L, Vento-Tormo R, et al. NF-κB-direct activation of microRNAs with repressive effects on monocyte-specific genes is critical for osteoclast differentiation. Genome Biol. 2015;16:2.
Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I (kappa) B kinase-beta. Nature. 1998;396(6706):77–80.
Kristensen DM, Mazaud-Guittot S, Gaudriault P, Lesné L, Serrano T, Main KM, et al. Analgesic use - prevalence, biomonitoring and endocrine and reproductive effects. Nat Rev Endocrinol. 2016;12(7):381–93.
Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104(13):4029–37.
Alnaeeli M, Penninger JM, Teng YT. Immune interactions with CD4+ T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol. 2006;177(5):3314–26.
Alnaeeli M, Park J, Mahamed D, Penninger JM, Teng YT. Dendritic cells at the osteo-immune interface: implications for inflammation-induced bone loss. J Bone Miner Res. 2007;22(6):775–80.
Gallois A, Lachuer J, Yvert G, Wierinckx A, Brunet F, Rabourdin-Combe C, et al. Genome-wide expression analyses establish dendritic cells as a new osteoclast precursor able to generate bone-resorbing cells more efficiently than monocytes. J Bone Miner Res. 2010;25(3):661–72.
Bauer DC, Orwoll ES, Fox KM, Vogt TM, Lane NE, Hochberg MC, et al. Aspirin and NSAID use in older women: effect on bone mineral density and fracture risk. Study of osteoporotic fractures research group. J Bone Miner Res. 1996;11(1):29–35.
Lack WD, Fredericks D, Petersen E, Donovan M, George M, Nepola J, et al. Effect of aspirin on bone healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2013;95(6):488–96.
Shi S, Yamaza T, Akiyama K. Is aspirin treatment an appropriate intervention to osteoporosis? Fut Rheumatol. 2008;3(6):499–502.
Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21(5):294–301.
Simon AM, O'Connor JP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am. 2007;89(3):500–11.
Baratieri A, Deli R. The effect on bone repair of aspirin cones placed in extraction sockets in dogs: a histopathologic study. J Oral Pathol. 1979;8(4):198–206.
Carroll PB, Melfi RC. The histologic effect of topically applied acetylsalicylic acid on bone healing in rats. Oral Surg Oral Med Oral Pathol. 1972;33(5):728–835.
Mizuno H, Liang RF, Kawabata A. Effects of oral administration of various non-steroidal anti-inflammatory drugs on bone growth and bone wound healing in mice. Meikai Daigaku Shigaku Zasshi. 1990;19(2):234–50.
Guida L, Annunziata M, Passaro I, Buonaiuto C, Rullo R, Tetè S, et al. Acetylsalicylic acid inhibits proliferation of human bone marrow stromal cells and matrix mineralization. Int J Immunopathol Pharmacol. 2008;21(4):921–8.
Carbone LD, Tylavsky FA, Cauley JA, Harris TB, Lang TF, Bauer DC, et al. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res. 2003;18(10):1795–802.
Lussana F, Squizzato A, Permunian ET, Cattaneo M, et al. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res. 2014;134(3):599–603.
Chen C, Akiyama K, Yamaza T, You YO, Xu X, Li B, et al. Telomerase governs immunomodulatory properties of mesenchymal stem cells by regulating FAS ligand expression. EMBO Mol Med. 2014;6(3):322–34.
Kobayashi Y, Mizoguchi T, Take I, Kurihara S, Udagawa N, Takahashi N. Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J Biol Chem. 2005;280(12):11395–113403.
Take I, Kobayashi Y, Yamamoto Y, Tsuboi H, Ochi T, Uematsu S, et al. Prostaglandin E2 strongly inhibits human osteoclast formation. Endocrinology. 2005;146(12):5204–14.
Norrdin RW, Jee WS, High WB. The role of prostaglandins in bone in vivo. Prostaglandins Leukot Essent Fatty Acids. 1990;41(3):139–49.
Ahern E, Smyth MJ, Dougall WC, Teng MWL. Roles of the RANKL-RANK axis in antitumour immunity - implications for therapy. Nat Rev Clin Oncol. 2018;15(11):676–93.
Xiong J, Cawley K, Piemontese M, Fujiwara Y, Zhao H, Goellner JJ, et al. Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss. Nat Commun. 2018;9(1):2909.
Tan EM, Li L, Indran IR, Chew N, Yong EL, et al. TRAF6 mediates suppression of osteoclastogenesis and prevention of ovariectomy-induced bone loss by a novel prenylflavonoid. J Bone Miner Res. 2017;32(4):846–60.
Zhang Y, Xu S, Li K, Tan K, Liang K, Wang J, et al. mTORC1 inhibits NF-κB/NFATc1 signaling and prevents osteoclast precursor differentiation, in vitro and in mice. J Bone Miner Res. 2017;32(9):1829–40.
Takatsuna H, Asagiri M, Kubota T, Oka K, Osada T, Sugiyama C, et al. Inhibition of RANKL-induced osteoclastogenesis by (−)-DHMEQ, a novel NF-κB inhibitor, through downregulation of NFATc1. J Bone Miner Res. 2005;20(4):653–62.
Lorenzo J. The many ways of osteoclast activation. J Clin Invest. 2017;127(7):2530–2.
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (81470751 to Y. L, 81800968 to ZH. L, 81600891 to L.G), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201703 to Y.B), the Beijing Baiqianwan Talents Project (2017A17 to Y.L), Beijing Municipal Education Commission and Science Technology Project of the (KM201910025008 to ZH.L).
Funding
This work was supported by grants from the National Nature Science Foundation of China 81470751 to Y. L (supporting the research), 81800968 to ZH. L (supporting experiments process), and 81600891 to L. G (supporting the manuscript preparation), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201703 to Y. B, supporting data analysis), the Beijing Baiqianwan Talents Project (2017A17 to Y. L, supporting interpretation of data), Beijing Municipal Education Commission and Science Technology Project of the (KM201910025008 to ZH. L, supporting the design of the study).
Author information
Authors and Affiliations
Contributions
LW performed the research with the aid of YitL, LJ, and YJ and drafted the manuscript. ZhL participated in the design of the study and helped draft the manuscript. JD participated in the statistical analysis. YB performed the experimental facility and coordination. LG helped to analyze the preliminary data. YiL conceived of the study and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We conducted the study following the informed guidelines approved by the Animal Ethical Committee of the School of Stomatology, Capital Medical University (Beijing, China#2012-x-53). All participants gave informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wu, L., Luo, Z., Liu, Y. et al. Aspirin inhibits RANKL-induced osteoclast differentiation in dendritic cells by suppressing NF-κB and NFATc1 activation. Stem Cell Res Ther 10, 375 (2019). https://doi.org/10.1186/s13287-019-1500-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-019-1500-x