Abstract
Background
Magnesium (Mg2+)-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells (DPSCs), but the regulatory mechanisms remain undefined. The aim of this work was to assess magnesium’s function in the above process and to explore the associated signaling pathway.
Methods
DPSCs underwent culture in odontogenic medium with the addition of 0, 1, 5, or 10 mM MgCl2. Intracellular Mg2+ levels in DPSCs were evaluated flow cytometrically using Mag-Fluo-4-AM. Mg2+-entry was inhibited by TRPM7 inhibitor 2-aminoethoxydiphenyl borate (2-APB). RNA-Sequencing was carried out for assessing transcriptome alterations in DPSCs during odontogenic differentiation associated with high extracellular Mg2+. KEGG pathway analysis was performed to determine pathways related to the retrieved differentially expressed genes (DEGs). Immunoblot was performed for assessing magnesium’s role and exploring ERK/BMP2/Smads signaling.
Results
Mg2+-enriched microenvironment promoted odontogenic differentiation in DPSCs via intracellular Mg2+ increase. Consistently, the positive effect of high extracellular Mg2+ on odontogenic differentiation in DPSCs was blocked by 2-APB, which reduced Mg2+ entry. RNA-sequencing identified 734 DEGs related to odontogenic differentiation in DPSCs in the presence of high extracellular Mg2+. These DEGs participated in many cascades such as MAPK and TGF-β pathways. Consistently, ERK and BMP2/Smads pathways were activated in DPSCs treated with high extracellular Mg2+. In agreement, ERK signaling inhibition by U0126 blunted the effect of high extracellular Mg2+ on mineralization and odontogenic differentiation in DPSCs. Interestingly, BMP2, BMPR1, and phosphorylated Smad1/5/9 were significantly decreased by U0126, indicating that BMP2/Smads acted as downstream of ERK.
Conclusions
Mg2+-enriched microenvironment promotes odontogenic differentiation in DPSCs by activating ERK/BMP2/Smads signaling via intracellular Mg2+ increase. This study revealed that Mg2+-enriched microenvironment could be used as a new strategy for dental pulp regeneration.
Similar content being viewed by others

Introduction
Dental pulp regeneration may be a potential treatment for managing permanent teeth undergoing necrosis [1]. Regenerative endodontic therapy has been assayed with multiple mesenchymal stem cells (MSCs), growth factors, and biological materials [2, 3]. Magnesium-containing scaffolds are considered to be ideal biomaterials for such treatment, with the properties of releasing Mg2+ to enhance dentin regeneration in MSCs [4].
As reported, Mg2+ acts as an intracellular second messenger connecting cell-surface receptor induction and cytosolic effectors [5]. Mg2+ is the fourth most abundant cation in the human body and is critical for ATP-dependent phosphorylation of DNA, RNA, and enzymes, and the average Mg2+ concentration in dentin is about 1% (wt/wt) [6,7,8]. Mg2+ is involved in the bio-mineralization of bones and teeth and directly affects crystallization and pattern generation of the inorganic mineral phase [8,9,10]. Evidence indicates mutations of the Mg2+ transporters TRPM7 and CNNM4 result in deficient dentin mineralization, confirming Mg2+ involvement in tooth development [11, 12]. Animals fed low Mg2+ diets show deficient dentin and enamel mineralization [13]. Our previous study also demonstrates that the Mg2+ transporter Magt1 plays an important role in odontogenic differentiation of bone marrow MSCs by regulating intracellular Mg2+ [14]. Studies have found high extracellular Mg2+ and its transporter regulate odontogenic differentiation in human DPSCs, with involvement in dentin mineralization [4, 15].
However, the regulatory mechanisms of Mg2+ effects on odontogenic differentiation in DPSCs remain undefined. Till now, the altered transcriptome of DPSCs undergoing odontogenic differentiation induced by an Mg2+-enriched microenvironment has not been assessed; thus, we determine the alteration of transcriptome using RNA sequencing. Moreover, we aim to clarify the role of Mg2+-enriched microenvironment in odontogenic differentiation of DPSCs and determine the associated signaling pathways.
Materials and methods
DPSC isolation and identification
Studies involving patients had approval from the Ethics Committee of Sun Yat-Sen University. DPSCs were collected from non-diseased pulp tissues of caries-free third molars of patients. DPSCs were isolated according to a previous report [16] and maintained in α-MEM containing 10% FBS (GIBCO, USA) and 10 mg/mL streptomycin and 10 U/mL penicillin (Sigma, USA). DPSCs at passages 3 to 7 were used in experiments. Flow cytometry analysis was performed to investigate DPSCs for surface markers. DPSCs were incubated with conjugated human antibodies, including CD45-PE, CD73-PE, CD90-APC, and CD166-PE (BD, USA), and assessed flow cytometrically (BD, USA). For confirming the adipogenic differentiation potential of stem cells, DPSCs underwent induction for 28 days with adipogenic medium containing 0.5 μM isobutyl-methylxanthine, 50 μM indomethacin, 0.5 μM dexamethasone, and 5 μg/mL insulin (Sigma, USA). To evaluate osteoblastic differentiation, DPSCs underwent culture for 14 days in osteogenic medium containing 100 nM dexamethasone, 10 mM β-glycerophosphate, and 0.2 mM ascorbic acid (Sigma, USA).
Determination of DPSC proliferation
DPSCs seeded in 96-well plates at 1000 cells/well underwent culture in growth medium after addition of 0, 1, 5, or 10 mM MgCl2 for 1, 2, and 3 days, respectively. Cell Counting Kit-8 (Dojindo, Japan) was applied as directed by the manufacturer.
Odontogenic differentiation of DPSCs induced by the Mg2+-enriched microenvironment
DPSCs seeded in 12-well plates at 3 × 104 cells/well underwent culture in α-MEM containing 10% FBS at 37 °C. At 80% confluency, DPSC culture was performed in odontogenic medium with 100 nM dexamethasone, 10 mM β-glycerophosphate, and 0.2 mM ascorbic acid, with the addition of 0, 1, 5, or 10 mM MgCl2, respectively. After 7 days, the protein amounts of RUNX2, DSP, and DMP-1 were determined by automated immunoblot. After 14 days, mineralization nodules were assessed by Alizarin red S staining.
Alizarin red S staining
Alizarin red S (Sigma, USA) staining was carried out as proposed by the manufacturer. Destaining was performed with 10% cetylpyridinium chloride monohydrate buffer for 30 min, and optical density was obtained at 575 nm. Relative extracellular matrix mineralization was calculated for each sample.
Inhibition of Mg2+ entry by 2-aminoethoxydiphenyl borate (2-APB)
Given that TRPM7 was reported to transport Mg2+ across the cell membrane, we inhibited Mg2+-entry by treatment with 100 μM of the TRPM7 inhibitor 2-APB (Aladdin, China) [17].
Automated immunoblot
Total protein from DPSCs was obtained with RIPA (Cell Signaling, USA). Automated immunoblot was carried out with Simple Wes (Protein Simple, USA) as directed by the manufacturer. In brief, 2.5 μg total protein samples were mixed with the standard fluorescent master mix and added to prefilled Wes assay plates, alongside antibody diluent (Protein Simple); primary antibodies targeting BMP2 (Affinity, USA), BMPR1 (Affinity), p-Smad1/5/9 (Affinity), RUNX2 (Affinity), DSP (Santa Cruz, USA), DMP-1 (Genetex, USA), ERK, p-ERK, JNK, p-JNK, p38, p-p38 (Cell Signaling), and β-Tublin (Affinity, USA) were probed; anti-rabbit and anti-mouse secondary antibodies conjugated to Streptavidin-HRP (Protein Simple) were used for detection. The compass software (Protein Simple) was utilized for analysis.
Flow cytometry evaluation of intracellular magnesium
Intracellular magnesium amounts in DPSCs were assessed with Mag-Fluo-4-AM (Invitrogen, USA) as directed by the manufacturer. Briefly, Mag-Fluo-4-AM was added at 2 μM and incubated for 30 min. This was followed by three cell washes. Fluorescence intensities were obtained by flow cytometry (BD, USA) in triplicate assays.
RNA sequencing (RNA-seq)
RNA-seq was carried out according to our previous work [14]. Briefly, DNase I treated total RNA specimens were enriched for mRNA using Oligo (dT) magnetic beads. Random-hexamer primers were employed for reverse transcription, and second-strand cDNA synthesis was carried out with Second Strand cDNA Synthesis Kit (Beyotime, China) as directed by the manufacturer. Short fragment purification used the QiaQuick PCR extraction kit (Qiagen, the Netherlands). Sequencing adapters were next added to these short fragments. Suitable fragments were amplified, and an Illumina HiSeq™ 2000 (Illumina Inc., USA) was employed for the cDNA sequencing. False discovery rate (FDR) adjusted p < 0.05 was obtained by the Benjamini-Hochberg method.
GO and KEGG pathway analyses
Gene Ontology (GO, http://www.geneontology.org) and Kyoto Encyclopedia of Genes and Genomes pathway (KEGG, http://www.genome.jp/kegg/pathway.html) analyses were carried out for determining biological functions and enriched metabolic or signal transduction pathways associated with the differentially expressed genes (DEGs), respectively.
MAPK signaling assessment
To assess MAPK signaling involvement, DPSCs underwent culture in 12-well plates in odontogenic medium with the addition of 0, 1, 5, or 10 mM MgCl2 for 7 days, respectively. Then, total protein from DPSCs was isolated to measure the protein amounts of ERK, p-ERK, JNK, p-JNK, p38, and p-p38 by automated immunoblot. During odontogenic differentiation of DPSCs administered 5 mM MgCl2 for 7 days, ERK/MAPK signaling was suppressed with 50 μM U0126 (AbMole BioScience, USA) for 2 h every 2 days.
Statistical analysis
Experiments were performed three times. Data were expressed as mean ± SD and were assessed by ANOVA test with SPSS 17.0 (SPSS, USA); p < 0.05 indicated statistical significance.
Results
Effects of the Mg2+-enriched microenvironment on proliferation and differentiation in DPSCs
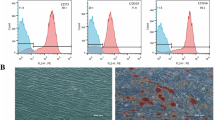
Passage 3 DPSCs were examined. As shown in Fig. 1a, DPSCs could differentiate into osteoblasts and adipocytes, suggesting their multi-lineage differentiation potential. Flow cytometry analysis showed DPSCs had low CD45 (hematopoietic cell marker) amounts (0.60%), but elevated amounts of the mesenchymal stem cell markers CD73 (99.02%), CD90 (99.09%), and CD166 (98.13%) (Fig. 1b).
To evaluate the impact of high extracellular Mg2+ on their proliferation, DPSCs underwent incubation in Mg2+-enriched media at different concentrations (0 mM, 1 mM, 5 mM, and 10 mM) for 1, 2, and 3 days, respectively. Cell Counting Kit-8 analysis showed that high extracellular Mg2+ did not affect DPSC proliferation (Fig. 2).
To analyze the impact of high extracellular Mg2+ on their differentiation, DPSCs were cultured in Mg2+-enriched odontogenic medium at different concentrations (0 mM, 1 mM, 5 mM, and 10 mM). After 7 days, RUNX2, DMP-1, and DSP protein amounts were markedly elevated in DPSCs treated with 1 mM, 5 mM, and 10 mM Mg2+, respectively, compared with 0 mM Mg2+ (Fig. 3a). Consistently, mineralization nodules were also markedly increased (Fig. 3b). However, there was no difference between the 5 and 10 mM Mg2+ groups (Fig. 3). These results showed that the Mg2+-enriched microenvironment promoted the odontogenic differentiation of DPSCs.
Effects of Mg2+-enriched microenvironment on DPSC differentiation. a After 7 days, RUNX2, DMP-1, and DSP protein amounts were markedly elevated in DPSCs treated with 1 mM, 5 mM, and 10 mM Mg2+, compared with the 0 mM Mg2+ group. b Consistently, mineralization nodules were also markedly increased after 14 days. However, there was no difference between the 5 and 10 mM Mg2+ groups
Mg2+-enriched microenvironment promotes odontogenesis in DPSCs by increasing intracellular Mg2+
Intracellular magnesium levels in DPSCs treated with Mg2+-enriched odontogenic media at different concentrations (0 mM, 1 mM, 5 mM, and 10 mM) were assessed flow cytometrically using Mag-Fluo-4-AM. Figure 4a suggests that intracellular Mg2+ was starkly increased in DPSCs treated with 1 mM, 5 mM, and 10 mM Mg2+, compared with the 0 mM Mg2+ group. However, there was no difference in intracellular magnesium of DPSCs treated with 5 mM and 10 mM Mg2+, which is consistent with their comparable effects on odontogenic differentiation.
Mg2+-enriched microenvironment promotes odontogenic differentiation in DPSCs by increasing intracellular Mg2+. a Intracellular Mg2+ was significantly increased in DPSCs exposed to high extracellular Mg2+ as assessed flow cytometrically using Mag-Fluo-4-AM; however, there was no difference between the 5 and 10 mM Mg2+ groups. b Mg2+-entry was significantly inhibited by the TRPM7 inhibitor 2-APB. c The positive effect of Mg2+-enriched microenvironment on odontogenic differentiation in DPSCs was also blocked by 2-APB
Given that TRPM7 was reported to transport Mg2+ across the cell membrane [17], we next examined the effects of its blocker 2-APB. The results showed that Mg2+ entry was significantly blunted by 2-APB (100 μM, inhibiting TRPM7 [17]) (Fig. 4b). The positive effect of high extracellular Mg2+ (5 mM Mg2+) on odontogenic differentiation of DPSCs was also blocked by 2-APB (Fig. 4c). These results indicated that high extracellular Mg2+ promoted odontogenic differentiation in DPSCs via intracellular Mg2+ increase.
High extracellular Mg2+ alters the transcriptome of DPSCs
Compared with control cells (0 mM Mg2+), exposure to high extracellular Mg2+ (5 mM Mg2+) for 7 days resulted in 734 DEGs in DPSCs, including 250 upregulated and 484 downregulated (Fig. 5a, b).
RNA-Seq of DPSCs undergoing odontogenic differentiation upon induction by high extracellular Mg2+. a, b There were734 genes significantly altered, with 250 upregulated and 484 downregulated. c GO analysis of differentially expressed genes (DEGs). d KEGG pathway analysis revealed that the DEGs were involved in many signaling pathways, including MAPK and TGF-β pathways
GO and KEGG pathway analyses of DEGs
In GO analysis, DEGS were mostly involved in developmental process, anatomical structure development, organism development, system development, channel activity, metal ion binding, integral component of plasma membrane, etc. (Fig. 5c). KEGG pathway analysis indicated that the DEGs retrieved participated in many pathways, e.g., MAPK and TGF-β pathways (Fig. 5d).
ERK signaling is activated by high extracellular Mg2+ during odontogenic differentiation of DPSCs
As shown above, KEGG pathway analysis revealed MAPK signaling activation by high extracellular Mg2+during odontogenic differentiation of DPSCs. To verify this finding, DPSCs were incubated in odontogenic medium with the addition of 0, 1, 5, and 10 mM MgCl2 for 7 days, respectively. The results showed that ERK phosphorylation was markedly enhanced in DPSCs treated with 1 mM, 5 mM, and 10 mM Mg2+ compared with the 0 mM Mg2+ group, but p38 and JNK phosphorylation showed no change (Fig. 6a). In accordance, the positive effect of high extracellular Mg2+ on ERK phosphorylation was blocked by 2-APB (100 μM, inhibiting TRPM7) (Fig. 6b), which reduced intracellular Mg2+amounts (Fig. 4b).
The ERK and BMP2 signaling pathway is activated by high extracellular Mg2+ in DPSCs during odontogenic differentiation. a ERK phosphorylation was significantly enhanced in DPSCs treated with 1 mM, 5 mM, and 10 mM Mg2+ compared with the 0 mM Mg2+ group, but p38 and JNK phosphorylation amounts were unchanged. b ERK phosphorylation was reduced by 2-APB. c Consistently, the protein levels of BMP2, BMPR1, and phosphorylated Smad1/5/9 were significantly increased in DPSCs exposed to high extracellular Mg2+. d BMP2, BMPR1, and phosphorylated Smad1/5/9 protein amounts were decreased by 2-APB
BMP2/Smads signaling is activated by high extracellular Mg2+during odontogenic differentiation of DPSCs
As reported previously, BMP2 is a TGF-β family member controlling odontogenic differentiation of stem cells [18, 19]. To verify the above KEGG pathway data, we performed Western blot to determine whether BMP2/Smads signaling is activated by high extracellular Mg2+. The results showed that BMP2, BMPR1, and phosphorylated Smad1/5/9 were significantly increased in DPSCs cultured in odontogenic medium containing high extracellular Mg2+(Fig. 6c). In accordance, the positive effects of high extracellular Mg2+ on BMP2, BMPR1, and phosphorylated Smad1/5/9 were blocked by 2-APB (100 μM, inhibiting TRPM7) (Fig. 6d).
Mg2+-enriched microenvironment promotes odontogenic differentiation in DPSCs via ERK/BMP2/Smads signaling
We inhibited ERK signaling with U0126, and mineralization nodules were markedly decreased, as well as RUNX2, DSP, and DMP-1 protein amounts in DPSCs treated with Mg2+-enriched odontogenic medium (5 mM Mg2+) for 7 days (Fig. 7a,b). Interestingly, BMP2, BMPR1, and phosphorylated Smad1/5/9 amounts were also significantly decreased by U0126, indicating that BMP2/Smads acted as downstream of ERK (Fig. 7c).
Mg2+-enriched microenvironment promotes odontogenic differentiation in DPSCs via ERK/BMP2/Smads signaling. a After treatment with U0126, the protein amounts of RUNX2, DSP, and DMP-1 were markedly decreased in DPSCs treated with high extracellular Mg2+. b Consistently, mineralization nodules were also reduced. c After treatment with U0126, BMP2, BMPR1, and phosphorylated Smad1/5/9 protein amounts were significantly decreased, indicating that BMP2/Smads signaling acted as downstream of ERK
Taken together, these findings suggested that Mg2+-enriched microenvironment promoted odontogenic differentiation in DPSCs by activating ERK/BMP2/Smads signaling via intracellular Mg2+ increase (Fig. 8).
Discussion
Regenerative endodontic therapy involves biological procedures for replacing damaged dental structures such as dentin and root, and cells of the dentin/pulp complex [20]. DPSCs are suitable for such treatment, and their differentiation into odontogenic stem cells associated with biomaterials and growth factors is critical for dental pulp regeneration [1]. It is known that magnesium-containing scaffolds are ideal tools for regenerative endodontic therapy, releasing high extracellular Mg2+ to enhance dentin regeneration in DPSCs [4]. Our previous studies found the Mg2+ transporters TRPM7 and MagT1 regulate odontogenic differentiation in stem cells [14, 15]. Nakano and collaborators [12] reported that TRPM7 silencing in mice results in marked tooth hypomineralization, suggesting TRPM7 to be involved in enamel and dentin biomineralization by supplying enough Mg2+ for ALPL activity. Luder and colleagues [11] demonstrated that mutation of CNNM4, a Mg2+ transporter, caused mineralization abnormalities in both enamel and dentin, in association with starkly reduced magnesium amounts. Consistent with previous studies, the present work determined that Mg2+-enriched microenvironment enhanced odontogenic differentiation in DPSCs by altering the formation of mineralization nodules as well as RUNX2, DMP-1, and DSP protein amounts, through intracellular Mg2+ concentration increase.
However, how high extracellular Mg2+ affects odontogenic differentiation remains unknown. Therefore, RNA-Seq was carried out to assess transcriptome alterations in DPSCs exposed to Mg2+-enriched microenvironment. Interestingly, GO and KEGG pathway analyses revealed 734 DEGs contributing to odontogenic differentiation in DPSCs induced by high extracellular Mg2+. The obtained DEGs participated in many pathways, such as MAPK and TGF-β pathways.
MAPK signaling regulates stem cell proliferation, migration, and differentiation [21,22,23]. Yu and colleagues [24] demonstrated that dentine matrix promoted odontogenic differentiation in bone marrow MSCs by inducing ERK and P38 MAPK pathways. Huang and collaborators [25] revealed that dental pulp cell-derived exosomes promote odontogenic differentiation in bone marrow MSCs via P38/MAPK signaling. As reported in previous studies, MAPK signaling also contributes to odontogenic differentiation in DPSCs [26]. In the present study, ERK phosphorylation was significantly increased in DPSCs treated with high extracellular Mg2+, but p38 and JNK phosphorylation levels remained unchanged, indicating ERK/MAPK signaling is induced by high extracellular Mg2+. Consistently, blunting ERK/MAPK signaling with U0126 blocked the effects of high extracellular Mg2+ on mineralization and odontogenic differentiation in DPSCs.
According to previous studies, intracellular Mg2+ plays a pivotal role in ERK/MAPK signaling induction [27,28,29,30]. For example, Mg2+ deprivation downregulates p-ERK1/2 in MDCK cells, and Mg2+ re-supplementation reverses this effect [29, 30]. Meanwhile, TRPM7 suppression inhibits ERK phosphorylation by reducing intracellular Mg2+ amounts [27, 28]. Our previous studies also revealed that suppression of MagT1 inactivates the ERK/MAPK pathway by decreasing intracellular Mg2+, which results in inhibited odontogenic differentiation of bone marrow MSCs [14]. In the present study, intracellular magnesium in DPSCs exposed to high extracellular Mg2+ was assessed flow cytometrically with Mag-Fluo-4-AM. As shown above, intracellular Mg2+ amounts were starkly increased in DPSCs treated with high extracellular Mg2+. In accordance, the positive effect of high extracellular Mg2+ on ERK phosphorylation was blocked by 2-APB, which reduces intracellular Mg2+ amounts, indicating that high extracellular Mg2+ activates ERK/MAPK signaling by increasing intracellular Mg2+.
BMP2, a member of the TGF-β family of proteins, contributes to odontogenic differentiation in DPSCs [18, 19]. Consistent with KEGG pathway data, BMP2, BMPR1, and phosphorylated Smad1/5/9 amounts were significantly increased in DPSCs incubated in Mg2+-enriched odontogenic media. As expected, the positive effects of high extracellular Mg2+ on BMP2, BMPR1, and phosphorylated Smad1/5/9 were blocked by the Mg2+transporter inhibitor 2-APB, indicating that BMP2/Smads signaling is activated during odontogenic differentiation induced by Mg2+-enriched microenvironment. This finding corroborated Cheng et al. [31] showing that high-purity magnesium scaffolds promote bone regeneration in rabbits via activation of BMP2 signaling by releasing high amounts of extracellular Mg2+.
Interestingly, ERK signaling inhibition by U0126 resulted in significantly decreased BMP2, BMPR1, and phosphorylated Smad1/5/9 amounts, indicating that BMP2/Smads is a downstream signaling of ERK. Taken together, these findings demonstrated that high extracellular Mg2+ promotes odontogenic differentiation in DPSCs by activating ERK/BMP2/Smads signaling via intracellular Mg2+increase.
Conclusions
Overall, significant transcriptome alterations were detected in DPSCs during odontogenic differentiation associated with high extracellular Mg2+. In addition, Mg2+-enriched microenvironment enhanced odontogenic differentiation in DPSCs by activating ERK/BMP2/Smads signaling via intracellular Mg2+ increase.
Availability of data and materials
The authors confirm the availability of all data generated or analyzed in this manuscript.
Abbreviations
- RNA-Seq:
-
RNA-Sequencing
- MAPK:
-
Mitogen-activated protein kinase
- ERK:
-
Extracellular signal-related kinase
- JNK:
-
c-Jun NH2-terminal kinase
- DSP:
-
Dentin sialophosphoprotein
- DMP-1:
-
Dentin matrix protein 1
- GO:
-
Gene Ontology
- RUNX2:
-
Runt-related transcription factor 2
- BMP2:
-
Bone morphogenetic protein 2
- BMPR1:
-
Bone morphogenetic protein receptor 1
References
Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10(455). https://doi.org/10.1126/scitranslmed.aaf3227.
Ravindran S, Huang CC, George A. Extracellular matrix of dental pulp stem cells: applications in pulp tissue engineering using somatic MSCs. Front Physiol. 2014;4:395.
Yang JW, Zhang YF, Sun ZY, Song GT, Chen Z. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. J Biomater Appl. 2015;30(2):221–9.
Qu T, Jing J, Jiang Y, Taylor RJ, Feng JQ, Geiger B, Liu X. Magnesium-containing nanostructured hybrid scaffolds for enhanced dentin regeneration. Tissue Eng A. 2014;20(17–18):2422–33.
Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475(7357):471–6.
Maguire ME, Cowan JA. Magnesium chemistry and biochemistry. Biometals. 2002;15(3):203–10.
Wolf FI, Cittadini A. Chemistry and biochemistry of magnesium. Mol Asp Med. 2003;24(1–3):3–9.
Wiesmann HP, Tkotz T, Joos U, Zierold K, Stratmann U, Szuwart T, Plate U, Hohling HJ. Magnesium in newly formed dentin mineral of rat incisor. J Bone Miner Res. 1997;12(3):380–3.
Todorovic T, Vujanovic D. The influence of magnesium on the activity of some enzymes (AST, ALT, ALP) and lead content in some tissues. Magnes Res. 2002;15(3–4):173–7.
Scibior A, Adamczyk A, Mroczka R, Niedzwiecka I, Golebiowska D, Fornal E. Effects of vanadium (V) and magnesium (Mg) on rat bone tissue: mineral status and micromorphology. Consequences of V-Mg interactions. Metallomics. 2014;6(12):2260–78.
Luder HU, Gerth-Kahlert C, Ostertag-Benzinger S, Schorderet DF. Dental phenotype in Jalili syndrome due to a c.1312 dupC homozygous mutation in the CNNM4 gene. PloS One. 2013;8(10):e78529.
Nakano Y, Le MH, Abduweli D, Ho SP, Ryazanova LV, Hu Z, Ryazanov AG, Den Besten PK, Zhang Y. A critical role of TRPM7 as an ion channel protein in mediating the mineralization of the craniofacial hard tissues. Front Physiol. 2016;7:258.
Trowbridge H, Louchard W, Jenks P. Histochemical studies on the dentin of magnesium-deficient rats. J Periodontal Res. 1971;6(2):130–9.
Zheng JM, Kong YY, Li YY, Zhang W. MagT1 regulated the odontogenic differentiation of BMMSCs induced byTGC-CM via ERK signaling pathway. Stem Cell Res Ther. 2019;10(1):48.
Cui L, Xu SM, Ma DD, Wu BL. The effect of TRPM7 suppression on the proliferation, migration and osteogenic differentiation of human dental pulp stem cells. Int Endod J. 2014;47(6):583–93.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–30.
Zhang Y, Xu J, Ruan YC, Yu MK, O'Laughlin M, Wise H, Chen D, Tian L, Shi D, Wang J, et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160–9.
Zhang W, Zhang X, Li J, Zheng J, Hu X, Xu M, Mao X, Ling J. Foxc2 and BMP2 induce osteogenic/odontogenic differentiation and mineralization of human stem cells from apical papilla. Stem Cells Int. 2018;2018:2363917.
Zhang W, Zhang X, Ling J, Liu W, Zhang X, Ma J, Zheng J. Proliferation and odontogenic differentiation of BMP2 genetransfected stem cells from human tooth apical papilla: an in vitro study. Int J Mol Med. 2014;34(4):1004–12.
Metlerska J, Fagogeni I, Nowicka A. Efficacy of autologous platelet concentrates in regenerative endodontic treatment: a systematic review of human studies. J Endod. 2019;45(1):20–30 e21.
Woo SM, Lim HS, Jeong KY, Kim SM, Kim WJ, Jung JY. Vitamin D promotes odontogenic differentiation of human dental pulp cells via ERK activation. Molecules and cells. 2015;38(7):604–9.
Shilo BZ. The regulation and functions of MAPK pathways in drosophila. Methods. 2014;68(1):151–9.
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83.
Yu Y, Wang L, Yu J, Lei G, Yan M, Smith G, Cooper PR, Tang C, Zhang G, Smith AJ. Dentin matrix proteins (DMPs) enhance differentiation of BMMSCs via ERK and P38 MAPK pathways. Cell Tissue Res. 2014;356(1):171–82.
Huang CC, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103–15.
Wu J, Li N, Fan Y, Wang Y, Gu Y, Li Z, Pan Y, Romila G, Zhou Z, Yu J. The conditioned medium of calcined tooth powder promotes the osteogenic and odontogenic differentiation of human dental pulp stem cells via MAPK signaling pathways. Stem Cells Int. 2019;2019:4793518.
Baldoli E, Maier JA. Silencing TRPM7 mimics the effects of magnesium deficiency in human microvascular endothelial cells. Angiogenesis. 2012;15(1):47–57.
Zeng Z, Leng T, Feng X, Sun H, Inoue K, Zhu L, Xiong ZG. Silencing TRPM7 in mouse cortical astrocytes impairs cell proliferation and migration via ERK and JNK signaling pathways. PLoS One. 2015;10(3):e0119912.
Ikari A, Kinjo K, Atomi K, Sasaki Y, Yamazaki Y, Sugatani J. Extracellular Mg (2+) regulates the tight junctional localization of claudin-16 mediated by ERK-dependent phosphorylation. Biochim Biophys Acta. 2010;1798(3):415–21.
Ikari A, Atomi K, Kinjo K, Sasaki Y, Sugatani J. Magnesium deprivation inhibits a MEK-ERK cascade and cell proliferation in renal epithelial Madin-Darby canine kidney cells. Life Sci. 2010;86(19–20):766–73.
Cheng P, Han P, Zhao C, Zhang S, Wu H, Ni J, Hou P, Zhang Y, Liu J, Xu H, et al. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016;81:14–26.
Acknowledgements
Not applicable.
Funding
The present work was funded by the Natural Science Foundation of Guangdong Province (No.2019A1515011289), National Natural Science Foundation of China (No.81700950), the Youth Teacher Training Project of Sun Yat-Sen University (No. 17ykpy74), Grant-in-Aid from Health and Family Planning Commission of Guangdong Province (No.A2017579), and the Bureau of Health of the Guangzhou Municipality (No.20171A011324).
Author information
Authors and Affiliations
Contributions
JZ and BW conceived this study. JZ, Y-yK, XH, and YZ performed the experiments, as well as data collection and analysis. KX helped carry out the experiments. JZ, BW, and YK wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study had approval from the Ethics Committee of Sun Yat-Sen University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kong, Y., Hu, X., Zhong, Y. et al. Magnesium-enriched microenvironment promotes odontogenic differentiation in human dental pulp stem cells by activating ERK/BMP2/Smads signaling. Stem Cell Res Ther 10, 378 (2019). https://doi.org/10.1186/s13287-019-1493-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-019-1493-5