Abstract
Background
Controversial results still existed on the clinical utility of bone marrow-derived cells (BMCs) for cardiomyopathy (CMP). This study aims to reveal the true power of this promising approach by synthesizing all the available data on this subject matter.
Methods
Twenty studies including 1418 patients were identified from systematic search. Weighted mean differences for changes in left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), 6-min walk distance, and NYHA functional class were estimated with a random-effects model. Major adverse cardiovascular event (MACE), rehospitalization, all-cause mortality, and patients’ quality of life were also calculated.
Results
Compared with the control group, BMC therapy resulted in greater LVEF (3.72%, 95% CI 2.31 to 5.13, P < 0.0001), 6-min walk distance (53.16, 95% CI 25.17 to 81.10, P = 0.0002), NYHA functional class (− 0.48, 95% CI − 0.65 to − 0.31, P < 0.0001), and smaller LVESV (− 16.79, 95% CI − 27.21 to − 6.38, P = 0.002). BMC treatment significantly reduced the mortality rate and improved patients’ quality of life. No significant difference was found between the BMCs and control group in LVEDV, MACE, and rehospitalization rate. However, the outcomes showed a clear trend in favor of the BMC group. Subgroup analysis showed that LVEF improved greater in a subgroup of intracoronary infusion, BMSC, or higher cell dose.
Conclusion
The results of the current meta-analysis suggest that BMC treatment for CMP is safe and feasible. This therapy was associated with persistent improvements in LV function, LV remodeling, functional class, patients’ survival, and quality of life. Intracoronary infusion of high-dose (> 108) BMSC might be a better therapeutic option for CMP patients. Further evidences are needed to verify our results.
Similar content being viewed by others
Introduction
Cardiomyopathy (CMP) is a group of diseases that affect the heart muscle. The common types of CMP are hypertrophic cardiomyopathy, dilated cardiomyopathy, and restrictive cardiomyopathy. Heart failure, most often develops as a result of CMP, is the leading cause of mortality worldwide. Many former treatments have been applied to cure CMP, including medical therapy, cardiac pacemaker implantation, and heart transplantation. However, these traditional therapies cannot fundamentally solve the problems of cardiomyocyte regeneration and cardiac function reconstruction, making the therapeutic effect of cardiomyopathy unsatisfactory [1, 2]. In recent studies, with the newly emerged stem cell therapy, myocardial regeneration and cardiac function reconstruction have become possible [3, 4].
Bone marrow-derived cells (BMCs), mainly consists of hematopoietic stem cells and mesenchymal stem cells, under appropriate conditions can differentiate into mesoderm and ectoderm tissues [5]. Because of its convenience and safety, and it is easy to culture in vitro and its autologous replantation, BMCs have become an important cell source for tissue engineering [6]. A number of studies have shown that BMC transplantation can be successful in homing and colonizing pathological myocardial tissue, derived into different cells which help enhance myocardial contractility, promote myocardial angiogenesis, and prevent adverse ventricular remodeling [7, 8]. It has been performed in the treatment of cardiac pain, myocardial infarction, heart failure, and other cardiac diseases.
Multiple clinical trials have shown that BMC transplantation is safe and feasible in the treatment of cardiomyopathy [9, 10], while others remain controversial. Some of the researches indicated that BMCs improve heart function, increase activity tolerance, and reduce malignant arrhythmia morbidity and mortality [11,12,13]. Other studies suggested that BMC transplantation does not prevent progression, nor reduce mortality. On the contrary, it has the potential to trigger complications such as arrhythmias, myocardial infarction, embolism, and tumor [14]. The inconsistency of these research findings may be on account of the small sample size, the cause of the cardiomyopathy, the condition of the disease, the type of cell use in the transplantation, the number and route of the cell transplantation, and the length of treatment and follow-up duration.
In order to evaluate the efficacy and safety of BMC transplantation in the treatment of cardiomyopathy more objectively and accurately, we have conducted this meta-analysis. By comparing and analyzing the result of the currently published researches related to BMC transplantation for cardiomyopathy, we aim to provide more reliable evidence for clinical use.
Methods
Data source and search strategy
A database search of PubMed and EMBASE was performed to retrieve relevant publications up to March 2019. Search terms were chosen to link stem cell therapy with cardiomyopathy and its treatment effect. The following search terms were used: “cardiomyopathy,” “non-ischemic cardiomyopathy,” “dilated cardiomyopathy,” “idiopathic cardiomyopathy,” “stem cell,” “bone marrow cells,” “mesenchymal stem cell.” We also hand searched the reference lists of identified articles, review, and editorial for additional studies. There was no restriction on studies in terms of year and language of publication.
Study selection
Prospective randomized controlled trials (RCT) assessing the left ventricular functions and clinical outcomes in CMP patients treated with BMC transplantation were enrolled in our meta-analysis. Besides, the eligible studies need to fulfill the following criteria: (1) patients with diagnostic CMP receiving stem cell therapy, including bone marrow mononuclear cells (BMNC) and bone marrow mesenchymal stem cells (BMSC); (2) studies that had at least 1 month of follow-up; (3) studies provided proper functional outcomes such as left ventricular ejection fraction (LVEF), left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), 6-min walk distance, New York Heart Association (NYHA) functional class, and exercise capacity or clinical outcomes regarding major adverse cardiovascular events (MACE), mortality rate, rehospitalization rate, and quality of life; (4) studies included a control group which did not receive cell therapy. We excluded articles which were reviews, editorial, and abstracts presented at a conference. Duplicate reports and ongoing or unpublished studies were also excluded.
Data extraction and quality assessment
Investigators independently screened all titles and abstracts to identify whether the studies met our inclusion criteria and extracted relevant data using a standardized form. For each eligible study, we extracted information regarding characteristics of the study (first author, year of publication, study design, sample size), patients (age, gender, type of disease, baseline LVEF), and intervention (dosage, route of administration, imaging modality, timing of follow-up). The outcome measurement included changes in LVEF, LVEDV, LVESV, 6-min walk distance, NYHA functional class, VO2 peak, and patients’ quality of life. MACE, all-cause mortality, and rehospitalization rate were also collected. Outcomes measured by different modes of imaging included echocardiography (ECHO), cardiac magnetic resonance imaging (CMR), electrocardiogram (ECG), and single-photon emission-computed tomography (SPECT) were all extracted. When multiple measuring tools were used in one study, CMR and ECHO data were preferentially selected. Clinical trials with multiple publications with sequential follow-up duration or different outcomes were considered as one study.
Study quality was evaluated using the Cochrane Collaboration’s risk of bias tool [15]. Each study was judged by seven domains, concerning random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. For each domain, studies were rated as high (red), unclear (yellow), or low (green) risk of bias. Any disagreement was resolved by discussion.
Data analysis
All analyses were performed using the Cochrane Collaboration Review Manager (version 5.3) software. Data extracted from each study were pooled by the use of the DerSimonian-Laird random-effects model. For continuous variables, we used the weighted mean difference (WMD) and 95% confidence intervals (CI) to estimate the treatment effects of each outcome. For dichotomous data, such as MACE rate and mortality rate, risk ratio (RR) with its 95% CI was calculated. Heterogeneity between studies was assessed by the I2 statistic (low 25–50%, intermediate 50–75%, high > 75%), and sensitivity analysis was conducted if significant heterogeneity was found (I2 > 50%) in any one of the outcomes. Sensitivity analysis was performed by removing one study at a time to reveal if one particular study could affect the overall result. Also, subgroup analysis stratifying studies according to follow-up duration, cell type, injection route, and dosage of BMCs was performed to discover the source of heterogeneity. Pooled outcomes were displayed using forest plots, and outcomes were considered as statistically significant if P value < 0.05.
Results
Search results
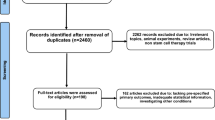
The systematic search identified 887 articles from PubMed and EMBASE. After reviewing all the titles and abstracts, 686 studies were excluded due to non-related topic and duplication, and 201 remained as potential candidates for our meta-analysis. We further excluded 145 studies since they did not meet our eligibility. Fifty-six studies were under full-text reviews and data extraction, of which 11 studies lacked of raw data, 7 articles did not include a control group, another 7 trials did not provide sufficient data for outcome analysis, 3 reports were an animal experiment, and 8 present repetitive data from an author with additional studies. Thus, the final 20 studies were included in our meta-analysis. Figure 1 presented the flowchart of a literature search.
Study characteristics
The included 20 studies consisted of a total of 1418 patients, with 705 patients receiving BMC therapy and 713 patients served as control [14, 16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. We selected studies published between 2004 and 2017, which included the latest study of this subject matter. Studied size ranged from 20 to 258 patients, and the majority of trials used a 1:1 randomization scheme. Among the chosen studies, 8 trials included a BMSC treatment group, 14 used BMNC as seed cells, and 2 studies applied Ixmyelocel-T as their treatment cells. Patients’ age was varied widely among the included studies, ranging from 30 to 80. Male patients took up around 71% of the population. Detailed information regarding baseline LVEF, dosage of BMCs, and administration route was presented in Table 1. The imaging modality of the enrolled studies included CMR, ECHO, ECG, and SPECT, and data of cardiac parameters measured by the above appliances were considered equivalent. Table 1 summarized the characteristic of the included studies.
The methodological quality of the included trials was reckoned to be acceptable, as each domain was mostly ranked as low or unclear risk of bias. Low risk of bias mostly occurred in performance bias, detection bias, and attrition bias. Unclear risk of bias was mostly detected in selection bias, because many of the studies only mentioned that the study was RCT without describing the specific randomization and allocation concealment method. Summary of risk of bias analysis was presented in Fig. 2.
Cardiac parameters of LV function
The overall result revealed a significant improvement of LVEF by 3.72 (95% CI 2.31 to 5.13, P < 0.0001, I2 = 90%) (Fig. 3) in the BMC group compared with the control group. However, heterogeneity was high and many of the studies provided LVEF data at different follow-up period. It is less rigorous to only assess the combined effect. Thus, we estimated the BMC efficacy according to different time duration. Subgroup results showed that the beneficial effect of BMC therapy on LVEF exerted in 1-month (3.57, [95% CI 2.09 to 5.05], P < 0.0001, I2 = 0%), 3-month (4.60, [95% CI 3.27 to 5.94], P < 0.0001, I2 = 48%), 6-month (3.37, [95% CI 0.27 to 6.46], P = 0.03, I2 = 89%), and 12–60-month (3.59, [95% CI, 0.74 to 6.44], P = 0.01, I2 = 98%) follow-up. Sensitivity analysis was conducted in all subgroups. After removing each study from the analysis, no contrary result was found in 1-month, 3-month, and 12–60-month follow-up. However, when respectively excluding the data of Bartolucci, Chang, Chen, Perin, and Vrtoves, the significant effect of BMCs on LVEF in 6-month follow-up disappeared. Interestingly, after permanently removed the study of Martino, the positive effect retained in a sensitivity analysis. From the above analysis, whether BMC therapy improves LVEF at 6-month follow-up still need to be further discussed.
Six studies provided data of LVESV. The overall result showed a significant difference in LVESV between BMC group and controls, which was − 16.79 (95% CI − 27.21 to − 6.38, P = 0.002, I2 = 57.4%) (Fig. 4). Subgroup analysis revealed that BMC treatment significantly reduced LVESV in 12–60-month follow-up (− 21.29, [95% CI − 33.20 to − 9.39], P = 0.0005, I2 = 0%) compared with controls, whereas no significant effect was found in 3-month (− 2.05, [95% CI − 23.59 to 19.50], P = 0.85, I2 = 0%) follow-up period. The results of the sensitivity analysis were consistent with the original results.
Data of LVEDV was measured in six trials. Results indicated that BMC therapy did not possess superior effect in improving LVEDV compared with the control group, with overall assessment (2.35, [95% CI − 6.42 to 11.12], P = 0.60, I2 = 18%), 3-month (4.82, [95% CI − 18.80 to 28.44], P = 0.40, I2 = 0%) and 12- to 60-month (1.37, [95% CI − 12.47 to 15.21], P = 0.85, I2 = 47%) follow-up (Fig. 5).
Functional class, exercise capacity, and quality of life
BMC treatment significantly improved 6-min walk distance (53.16, [95% CI 25.17 to 81.10], P = 0.0002, I2 = 94%) and NYHA functional class (− 0.48, [95% CI − 0.65 to − 0.31], P < 0.0001, I2 = 45%) compared with controls, and the benefit of BMCs could be seen in all 3-month, 6-month, and 12- to 60-month follow-up period. For peak VO2, a significant difference was not detected between BMCs and control group (0.94, [95% CI − 3.15 to 5.02], P = 0.65, I2 = 67%) (Table 2). Only four studies measured this parameter [14, 19, 23, 35], subgroup analysis was not available, and the conclusion could not be drawn until more data were acquired.
Patients’ quality of life was measured using the Minnesota Living with Heart Failure Questionnaire (MlHFQ). MlHFQ scores range between 0 and 105 with higher scores indicate a worst quality of life. The pooled outcome of 4 trials revealed that MlHFQ scores significantly decreased in BMC group compared with controls (− 18.41, [95% CI − 29.90 to − 6.92], P = 0.002, I2 = 0%) (Table 2).
Clinical outcomes
Compared with controls, BMC therapy had a tendency to reduce the incidence of MACE and rehospitalization; however, the results did not reach statistical significance, with RR of 0.79 (95% CI 0.59 to 1.04, P = 0.09, I2 = 0%) and 0.71 (95% CI 0.49 to 1.04, P = 0.08, I2 = 0%), respectively (Table 2). Overall estimates showed a significant reduction in all-cause mortality in the BMC group compared with controls (0.74, [95% CI 0.56 to 0.98], P = 0.04, I2 = 0%). The same trend was observed in cardiovascular death analysis (Table 2).
Subgroup analysis
Subgroup analysis stratifying studies based on the route of administration (intracoronary vs intramyocardial), cell types (BMNC vs BMSC), and dosage (106 vs 108) was performed with LVEF outcome owing to limited data. Comparison of administration route revealed that intracoronary injection of BMCs significantly improved LVEF in subgroups of 3, 6, and 12–60 months, while the effect of intramyocardial injection only manifested at 6-month follow-up (Table 3). For cell types, BMSC therapy appeared to have a superior effect than BMNC in improving LVEF throughout all follow-up period (Table 3). The different dosage might also affect the therapeutic efficacy of cell therapy. According to the subgroup analysis, patients treated with an injection of up to 108 cells benefit more than patients received a lower cell dose in the improvement of LVEF at 3-month, 6-month, and 12–60-month follow-up (Table 3).
Discussion
We believed that this is the largest meta-analysis on the subject of BMCs in treating CMP which included 20 studies with the most latest trials. The overall estimates of the meta-analysis demonstrated that BMC transplantation resulted in significant improvements in LVEF, LVESV, 6-min walk distance, NYHA functional class, MIHFQ scores, and all-cause mortality. Although significant differences were not observed in LVEDV, peak VO2, MACE, and rehospitalization rate, these results tended to in favor the BMC group, indicating that cell therapy was safe and feasible in patients with CMP without worsening the LV function and patients’ survival. However, a lot is still unknown or in a dispute about BMC therapy. Thus, several subgroup analyses were also conducted in an attempt to reveal its true effect.
Whether BMC therapy has a short-term or long-term effect is still the biggest argument. Subgroup analysis based on follow-up time showed that BMCs therapy had both short-term and long-term benefit on LVEF, 6-min walk distance, and NYHA functional class, whereas the effect on LVESV only emerged at 12–60-month follow-up. LVEF improved in short-term follow-up (3–6 month) without reduction of LVESV and LVEDV, suggesting that BMCs cause little changes in the remodeling process at the beginning and might exert its effect through a paracrine manner [37]. In long-term (12–60 month) period, the improvement of LVEF might be because of amelioration in LVESV, that is BMCs could contribute to the regeneration and differentiation of cardiomyocytes and as a consequence improve cardiac function by inhibiting LV remodeling [29, 38, 39]. The improvement in functional parameters at both short- and long-term follow-up indicated that BMC transplantation accelerated cardiac function recovery and the beneficial effects manifested soon after cell delivery and sustained.
Apart from functional markers, we also assessed clinical parameters such as MACE, rehospitalization, mortality rate, and patients’ quality of life. Results showed a trend towards a decrease in MACE and rehospitalization in BMC patients. Meanwhile, a significantly lower mortality rate and a better quality of life were detected in patients receiving stem cell therapy as compared with the controls. This suggests that the improvement in cardiac function after BMC treatment also translates into clinical benefits.
In stem cell therapy, intravenous administration, intracoronary infusion, and intramyocardial injection are the three currently available methods for cell delivery [40]. Yet, after decades of evaluation, no consensus has been reached on the optimal method of application. Some suggest that intramyocardial injection can exert better effect than intracoronary infusion because more cells are retained after intramyocardial injection (about 10% of total cells) compared with intracoronary infusion (about 3%) [41, 42]. Subgroup analysis in this meta-analysis indicated that patients with intracoronary infusion might benefit more than an intramyocardial injection, as patients treated with intracoronary infusion of BMCs demonstrated long-lasting benefit in LVEF whereas patients with intramyocardial injection exhibit improvement in LVEF only at 6-month analysis. However, given the fact that the sample size in the intramyocardial group was relatively small, this outcome needs to be interpreted with caution. Interestingly, at 6-month follow-up, the improvement of LVEF was greater in the intramyocardial group than the intracoronary group (5.29% vs 3.62%). Thus, the retention of more stem cells within the myocardium after injection might provide greater benefit to LV function as the previous investigation suggested. However, more clinical evidences are required until we can draw a definite conclusion.
Different cell types might also bring different outcomes. Unselected mononuclear bone marrow cells are the most commonly used seed cell in BMC therapy. Many consider that the potential beneficial effects might be attributed to the combined effects of all infused mononuclear cells rather than the small amount of progenitor cell or stem cell present in the bone marrow [43]. More recently, more and more investigators have focused on the effect of BMSC for CMP patients. It is suggested that BMSC possesses great potential for proliferation and can be cultured and amplified in vitro, t?>making them an ideal seed cell type for clinical cell therapy [44]. To provide more insights into the choice of cells, we conducted a subgroup analysis to compare the effect between BMNC and BMSC. Results showed that both cell types were effective in enhancing LVEF. At the same time, we noticed that BMSC produced more pronounced improvement in LVEF compared with BMNC data in all time groups, indicating that BMSC might be a more suitable seed cell for stem cell therapy.
Our analysis also included two studies using ixmyelocel-T in the treatment group. Ixmyelocel-T is an expanded multi-cellular therapy cultured from autologous BMNC. Previous studies have demonstrated that ixmyelocel-T therapy has the potential in decreasing secretion of proinflammatory cytokines after inflammatory stimuli and could efficiently remove apoptotic cells. This subpopulation of cells may have a potential role in tissue repair and regeneration [45,46,47]. The two studies enrolled in our meta-analysis, Henry et al. and Patel et al., concluded that although delivery of ixmyelocel-T in patients with ischemic dilated cardiomyopathy did not have a profound influence on LVEF, it resulted in a significant reduction in a major adverse cardiovascular event [21, 22]. This discovery might provide a new approach for cardiomyopathy treatment. Further studies should be conducted to evaluate the efficacy of this treatment option.
Poor cell engraftment and low survival of the transplanted cells are two major problems affecting the development of cell-based treatment [48]. Evidences show that most of the infusion cells die after a short amount of time, leaving only a small proportion that remains in the heart [49, 50]. Thus, clinicians hypothesized that the efficacy of cell-based therapy might improve in a dose-dependent manner. Several studies demonstrated that BMC therapy is associated with favorable effects on cardiac function, with a greater benefit observed with a higher cell dose [51,52,53]. Results of our subgroup analysis supported the previous finding, which found higher cell dose imparting greater benefit in the improvement of LVEF.
The scope of our results is limited by some limitations. The high degree of heterogeneity observed among the included studies is one of them. Although sensitivity and subgroup analyses were performed, we still could not completely eliminate the bias brought by different studies. Some of the factors should be taken into consideration, for example, baseline LVEF and BMC cell source. Baseline LVEF was proved to be an independent predictor of functional response to BMC therapy in previous investigation [36, 54]. However, limited data from the included studies hampered us to conduct a certain group of analysis. Furthermore, the comparison between autologous and allogeneic stem cell therapies in the treatment of heart disease is trending. Although autologous stem cells can avoid immunologic reaction during cell delivery, this type of cell is limited in immediate use due to the time-consuming nature of cell culturing and validation, which might miss the optimal window for treatment [55]. Allogeneic cell therapy is suggested to overcome the limitation of autologous cells, and already in clinical trials, allogeneic cells were found to have a superior effect on cardiac function compared with autologous cells without the sight of immunologic profile [56, 57]. Nevertheless, a study evaluating the effectiveness of autologous versus allogeneic cells is often without a control group. Therefore, analysis cannot be performed until more evidences from prospective randomized controlled trials are provided. These factors should be taken into consideration in future analysis.
Conclusion
Overall, BMC therapy for CMP is safe and feasible without causing serious damage to the cardiac function and patients’ survival compared with standard treatment. Cell therapy results in improvement of LV function and amelioration of LV remodeling, and the benefit effect is durable. The enhancement of cardiac function leads to better patients’ survival and quality of life. Based on our subgroup analysis, intracoronary infusion of higher dosage (> 108) of BMSC might bring about greater therapeutic efficacy. Future adequately powered trials with more reliable and more patient-centered outcomes are required to validate our result.
Abbreviations
- BMCs:
-
Bone marrow-derived cells
- BMNC:
-
Bone marrow mononuclear cells
- BMSC:
-
Bone marrow mesenchymal stem cells
- CMP:
-
Cardiomyopathy
- CMR:
-
Cardiac magnetic resonance imaging
- ECG:
-
Electrocardiogram
- ECHO:
-
Echocardiography
- LVEDV:
-
Left ventricular end-diastolic volume
- LVEF:
-
Left ventricular ejection fraction
- LVESV:
-
Left ventricular end-systolic volume
- MACE:
-
Major adverse cardiovascular event
- MlHFQ:
-
Minnesota Living with Heart Failure Questionnaire
- SPECT:
-
Single-photon emission-computed tomography
- WMD:
-
Weighted mean difference
References
Spinarova L, Spinar J. Pharmacotherapy of dilated cardiomyopathy. Curr Pharm Des. 2015;21:449–58.
Kawai C, Matsumori A. Dilated cardiomyopathy update: infectious-immune theory revisited. Heart Fail Rev. 2013;18:703.
Selem SM, Kaushal S, Hare JM. Stem cell therapy for pediatric dilated cardiomyopathy. Curr Cardiol Rep. 2013;15:1–13.
Vrtovec B, Poglajen G, Haddad F. Stem cell therapy in patients with heart failure. Methodist Debakey Cardiovasc J. 2013;9:6.
Ai JW, Liu Y, Liu CF, Pei B. Safety and efficacy of autologous bone marrow mesenchymal stem cells for dilated cardiomyopathy: a meta-analysis; 2017.
Duke CM, Taylor HS. Stem cells and the reproductive system: historical perspective and future directions. Maturitas. 2013;76:284–9.
Alihassansayegh S, Mirhosseini SJ, Lotfaliani MR, Dehghan HR, Sedaghathamedani F, Kayvanpour E, Rezaeisadrabadi M, Ghaffari N, Vahabzadeh V, Jebran AF. Transplantation of bone marrow stem cells during cardiac surgery. Asian Cardiovasc Thorac Ann. 2015;23:363–74.
Sampson S, Bottovan BA, Aufiero D. Autologous bone marrow concentrate: review and application of a novel intra-articular orthobiologic for cartilage disease. Phys Sportsmed. 2013;41:7.
Perin EC, Borow KM, Silva GV, Demaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circul Res. 2015;117:576-84.
Golpanian S, Elkhorazaty J, Mendizabal A, Difede DL, Suncion V, Karantalis V, Fishman JE, Ghersin E, Balkan W, Hare JM. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol. 2015;65:125.
Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, Cernelc P, Torreamione G, Haddad F, Wu JC. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013;128:42–9.
Lezaic L, Socan A, Poglajen G, Peitl PK, Sever M, Cukjati M, Cernelc P, Wu JC, Haddad F, Vrtovec B. Intracoronary transplantation of CD34(+) cells is associated with improved myocardial perfusion in patients with nonischemic dilated cardiomyopathy. J Card Fail. 2015;21:145–52.
Mushtaq M, DL DF. Rationale and design of the percutaneous stem cell injection delivery effects on neomyogenesis in dilated cardiomyopathy (the POSEIDON-DCM study). J Cardiovasc Transl Res. 2014;7:769–80.
Martino HF, Oliveira PS, Souza FC, Costa PC, Assunção E Silva E, Villela R, Gaze M, Weitzel LH, Oliveira A and Muccillo FB. A safety and feasibility study of cell therapy in dilated cardiomyopathy. Braz J Med Biol Res. 2010;43:989.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Chichester: Wiley; 2011.
Chang KL, Jun YU, Zhang SX. Intracoronary autologous myeloid stem cells transplantation in the treatment of dilated cardiomyopathy. Med J Natl Defending Forces Northwest China. 2010;29:6-8.
Chen Y, Gao EM, Gao CY, Xu Y, Huang KJ, Niu ZM, Zhu ZY, Yang HH, Mw LI. Effects of intracoronary autologous bone marrow mononuclear cells transplantation in patients with dilated cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi. 2008;36:1087.
Chen S, Liu Z, Tian N, Zhang J, Yei F, Duan B, Zhu Z, Lin S, Kwan TW. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552–6.
Hamshere S, Arnous S, Choudhury T, Choudry F, Mozid A, Yeo C, Barrett C, Saunders N, Gulati A, Knight C. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. Eur Heart J. 2015;36:3061–9.
Heldman AW, Difede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, Mcniece IK. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62.
Henry TD, Traverse JH, Hammon BL, East CA, Bruckner B, Remmers AE, Recker D, Bull DA, Patel AN. Safety and efficacy of ixmyelocel-T: an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circul. Res. 2014;115:730–7.
Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, East C, Remmers AE, Goodrich J, Desai AS. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016;387:2412.
Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, Mesquita CT, Belém L, Vaughn WK, Rangel FO. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004;110:213–8.
Sant'Anna RT, James F, Valle FH, Iran C, Nardi NB, Sant'Anna JRM, Abrahão NI, Kalil RAK. Direct intramyocardial transthoracic transplantation of bone marrow mononuclear cells for non-ischemic dilated cardiomyopathy: INTRACELL, a prospective randomized controlled trial. Rev Bras Cir Cardiovasc. 2014;29:437–47.
Seth S, Bhargava B, Narang R, Ray R, Mohanty S, Gulati G, Kumar L, Airan B, Venugopal P, Group ASCS. The ABCD (autologous bone marrow cells in dilated cardiomyopathy) trial a long-term follow-up study. J Am Coll Cardiol. 2010;55:1643.
Song YB. Effect and security of intracoronary transplantation of autologous bone marrow stem cells on patients with dilated cardiomyopathy. Chinese J Cardiovasc Rehabil Med. 2008;17:348-50.
Souza ASBD, Souza WKSB, Costa SA, Freitas EMDM, Carvalho G, Sá LAB, Rassi S. Incidence of ventricular arrhythmias after stem cell therapy in patients with Chagas cardiomyopathy. Arq Bras Cardiol. 2014;102:489–94.
Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torreamione G, Haddad F. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients 5-year follow-up. Circul. Res. 2013;112:165–73.
Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, Haddad F, Torre-Amione G. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J Card Fail. 2011;17:272–81.
Wang JA, Xie XJ, He H, Sun Y, Jiang J, Luo RH, Fan YQ, Dong L. A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy. Chinese J Cardiol. 2006;34:107.
Xiao W, Guo S, Gao C, Dai G, Gao Y, Li M, Wang X, Hu D. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017;58:238-44.
Xiao WT, Gao LJ, Gao CY, Gao YJ, Dai GY, Li MW, Wang XP. Comparative study on the efficacy of intracoronary infusion with various types of autologous bone marrow stem cells for patients with dilated cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi. 2012;40:575–8.
Yan JJ, Gao CY, Gao YJ, Dai GY, Xiao WT, Liu J. Autologous bone marrow mesenchymal stem cells transplantation for dilated cardiomyopathy. J Chinese Pract Diagnosis Therapy. 2012;26:544-46.
Bartolucci J, Verdugo FJ, Carrion F, Abarzúa E, Goset C, Lamich R, Sanhueza P, Pedreros P, Nazzal C, Khoury M. Long-term results of intracoronary transplantation of autologous bone marrow cells in dilated cardiomyopathy. Rev Med Chile. 2015;143:415–23.
Bartolucci JG, Verdugo FJ, Gonzà lez PL, Larrea RE, Abarzua E, Goset C, Rojo PG, Palma I, Lamich R, Pedreros PA. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial). Circul. Res. 2017;121:CIRCRESAHA.117.310712.
Nesteruk J, Voronina N, Kundt G, Donndorf P, Klopsch C, Kaminski A, Duckers HJ, Steinhoff G. Stem cell registry programme for patients with ischemic cardiomyopathy undergoing coronary artery bypass grafting: what benefits does it derive? Esc Heart Failure. 2017;4:105–11.
Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229–37.
Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–35.
Ishida M, Tomita S, Nakatani T, Fukuhara S, Hamamoto M, Nagaya N, Ohtsu Y, Suga M, Yutani C, Yagihara T, Yamada K, Kitamura S. Bone marrow mononuclear cell transplantation had beneficial effects on doxorubicin-induced cardiomyopathy. J Heart Lung Transplant. 2004;23:436–45.
Jezierska-Woźniak K, Mystkowska D, Tutas A, Jurkowski MK. Stem cells as therapy for cardiac disease - a review. Folia Histochem Cytobiol. 2011;49:13.
Dongming H, Eyas Al-Shaykh Y, Brinton TJ, Ping Z, Pamela R, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:150–6.
Mäkelä J, Anttila V, Ylitalo K, Takalo R, Lehtonen S, Mäkikallio T, Niemelä E, Dahlbacka S, Tikkanen J, Kiviluoma K, Juvonen T, Lehenkari P. Acute homing of bone marrow-derived mononuclear cells in intramyocardial vs. intracoronary transplantation. Scand Cardiovasc J. 2009;43:366–73.
Zhang J, Lin L, Zong W. Bone marrow mononuclear cells transfer for patients after ST-elevated myocardial infarction: a meta-analysis of randomized control trials. Yonsei Med J. 2018;59:611–23.
Murry CE, Field LJ, Philippe M. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112:3174–83.
Ledford KJ, Zeigler F, Bartel RL. Ixmyelocel-T, an expanded multicellular therapy, contains a unique population of M2-like macrophages. Stem Cell Res Ther. 2013;4:134.
Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, Stern TP, Watling S, Bartel RL. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther. 2012;20:1280.
Ledford KJ, Murphy N, Zeigler F, Bartel RL. Potential beneficial effects of ixmyelocel-T in the treatment of atherosclerotic diseases. Stem Cell Res Ther. 2013;4:135.
Tongers J, Losordo DW, Ulf L. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197.
Martin P, Petr W, Petr K, Tomas K, Otto L. Images in cardiovascular medicine. Early tissue distribution of bone marrow mononuclear cells after transcoronary transplantation in a patient with acute myocardial infarction. Circulation. 2005;112:63–5.
Geng Y. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann N Y Acad Sci. 2003;10:687–97.
Florea V, Rieger AC, Difede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, Tompkins BA, Khan A, Schulman IH, Landin AM. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the TRIDENT study). Circul Res. 2017;121:CIRCRESAHA.117.311827.
Quyyumia AA, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E, Willerson J. CD34 cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98–105.
Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, TD H. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117:576–84.
Wen Y, Ding J, Zhang B, Gao Q. Bone marrow-derived mononuclear cell therapy for nonischaemic dilated cardiomyopathy—a meta-analysis. Eur J Clin Investig. 2018;48:e12894.
Oh H, Ito H, Sano S. Challenges to success in heart failure: cardiac cell therapies in patients with heart diseases. J Cardiol. 2016;68:361–7.
Hare JM, Fishman JE, Gary G, Velazquez DF, Zambrano JP, Suncion VY, Melissa T, Eduard G, Johnston PV, Brinker JA. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79.
Hare JM, Difede DL, Castellanos AM, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ. Randomized comparison of allogeneic vs. autologous mesenchymal stem cells for non–ischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiol. 2017;68:526-37.
Acknowledgements
Not applicable
Funding
Authors did not receive any funding.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article (and its additional files).
Author information
Authors and Affiliations
Contributions
WC and ZJL contributed to the conception and design of the study. BYZ and YJL performed the database search and study selection. BYZ and YJL reviewed and extracted data from the publication. In cases of disagreements, WC and ZJL were reached to solve them. WC and BYZ performed the statistical analysis. WC, ZJL, and YJL analyzed and interpreted the results. WC and ZJL contributed to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, C., Li, J., Zhang, B. et al. Safety and efficacy of bone marrow-derived cells therapy on cardiomyopathy: a meta-analysis. Stem Cell Res Ther 10, 137 (2019). https://doi.org/10.1186/s13287-019-1238-5
Published:
DOI: https://doi.org/10.1186/s13287-019-1238-5