Abstract
Background and aims
Stem cell therapy (SCT) for the treatment of Crohn’s disease (CD) is still in its infancy, and whether SCT is associated with improved outcomes is unclear. We performed a meta-analysis to evaluate the efficacy and safety of patients receiving SCT.
Methods
Electronic databases were searched for studies that reported the use of stem cells for the treatment of patients with CD. Raw data from included studies were pooled for effect estimates. Subgroup analyses were performed for exploration of heterogeneity regarding all outcomes.
Results
We analyzed 21 studies comprising 514 patients with active CD. A random-effects meta-analysis of studies of SCT as systemic infusion showed 56% (95% confidence interval (CI) 33–76, n = 150) of patients achieved clinical response. Similarly, random-effects pooled rates of clinical or endoscopic remission were 46% (95% CI 25–69, n = 116) and 15% (95% CI 0–50, n = 48), respectively. A random-effects meta-analysis of all perianal CD studies showed that 57% (95% CI 44–69%, n = 251) of patients had healed fistula with SCT, with an odds ratio of 3.83 (95% CI 1.06–13.86, n = 121, P = 0.04) versus control. The pooled rate of clinical recurrence was high at 16% (95% CI 4–34, n = 101) with follow-up >12 months. The pooled rates of severe adverse events (SAEs) and SAEs related to SCT were 12% (95% CI 6–23, n = 378) and 8% (95% CI 3–18, n = 378), respectively. The Egger test suggests no publication bias existed for fistula healing (P = 0.36), but did for clinical response (P = 0.003).
Conclusions
SCT seems potentially effective and may serve as an alternative treatment for refractory active CD. Toxicity will remain the most significant barrier to systemic SCT in patients with CD.
Similar content being viewed by others
Background
Crohn's disease (CD) is a chronic relapsing inflammatory condition of the gastrointestinal tract that can result in lifelong ill health [1]. Immunosuppressive drugs, including biologicals, are the standard of care for CD, but some patients do not respond or lose response to treatment [2, 3]. Therefore, alternative treatments for refractory CD remain an unmet need.
Hematopoietic stem cell transplantation (HSCT) might play a role in some of these treatment-resistant cases [4]. Previous studies have indicated that allogeneic HSCT may reset the immune system at a genetic level [5, 6], and autologous HSCT eliminates aberrant clones by immunoablation and replacement with uncommitted stem cells (SCs), leading to de novo generation of an altered T-cell repertoire [7]. Case reports and series describe long-term treatment-free disease regression with autologous [8,9,10] and allogeneic [5, 6] HSCT in some [6, 11, 12] but not all patients [8,9,10] with CD. However, the recent Autologous Stem Cell Transplantation International Crohn Disease (ASTIC) trial [13] failed to demonstrate a statistically significant improvement in sustained disease remission at 1-year of autologous HSCT compared with conventional therapy. These findings raise doubt about HSCT for patients with refractory CD.
Perianal fistulas are very common complications, appearing in 25% of CD patients. Of these, approximately half are complex. The only approved drug that has shown efficacy in a randomized clinical trial is infliximab [14, 15]. Few treatment options exist for drug treatment-refractory patients, and repeated surgical options are associated with a significant risk of permanent stoma. These findings emphasize the need for novel treatment options for treatment-refractory complex perianal fistulas in patients with CD. Initial clinical results [16, 17] suggested mesenchymal stem cells (MSCs) might have therapeutic potential in this setting.
Given the immunoregulatory potential of SCs, multiple studies have been conducted to assess the safety and efficacy of stem cell therapy (SCT) in CD. In this study, we perform a meta-analysis of feasibility and toxicity of SCT for the treatment of CD.
Methods
The study was performed following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [18].
Literature search
We identified relevant literature (published articles and abstracts) by performing a systematic search of three databases: PubMed, Cochrane Library CENTRAL, and Embase (initial search February 5, 2015; updated October 15, 2016). Keywords used were (all fields): (“inflammatory bowel disease” or “crohn’s disease” or “crohn disease” or “enteritis”) and (“stem cell*” OR “precursor cell*” OR “progenitor cell*” OR “Stromal cell*”), and any appropriate abbreviations. For PubMed, all relevant MeSH terms were used. The final queries were validated by manual review and matching results.
The conference proceeding abstracts for annual meetings of European (European Crohn’s and Colitis Organisation congress) and American (Digestive Disease Week) Congresses were searched between 2002 and 2016.
Study selection
We finally performed a manual selection of studies which satisfied the following criteria: (a) observational (prospective or post hoc analysis of prospectively obtained cohort) or interventional design (randomized or non-randomized); (b) established diagnosis of CD by accepted criteria not in clinical or endoscopic remission at study outset; and (c) clear definition of efficacy and adverse events (AEs). Studies with exclusively pediatric patients (<15 years), a diagnosis of ulcerative colitis, indeterminate colitis, or an unclear diagnosis of CD and case reports were also excluded.
Data extraction and quality assessment
Eligible articles were reviewed in a blind manner by two different investigators (YQ and MYL), and the results of the primary research studies were abstracted onto specially designed data extraction forms. Disagreement in data extraction was resolved by consensus with MHC.
Assessment of the quality of randomized controlled trials (RCTs) and observational studies was performed using the Cochrane risk of bias tool [19] and Newcastle Ottawa Quality Assessment Scale (NOS) [20], respectively. For the NOS, studies scoring >7 (of 9) were considered high quality.
Outcomes assessed
The primary endpoint was clinical efficacy (e.g., clinical response or remission, fistula healing as defined by the primary study) of SCs for the treatment of patients with CD. Secondary outcomes were safety (e.g., AEs, severe AEs (SAEs)) and other measures of disease activity (endoscopic remission, clinical recurrence) of SCT for CD.
Data synthesis and analysis
We calculated incidence estimates with the variance-stabilizing double arcsine transformation [21] because the inverse variance weight in fixed-effects meta-analyses is suboptimum when dealing with binary data with low incidences. Additionally, the transformed incidences are weighted very slightly towards 50%, and studies with incidences of zero can thus be included in the analysis. We used the Wilson method [22] to calculate 95% confidence intervals (CIs) around these estimates because the asymptotic method produces intervals which can extend below zero. Heterogeneity due to study variation was assessed using the χ2 test [23, 24], with a P value of <0.10 being considered as statistically significant.
Publication bias
Funnel-plot asymmetry as proposed by Egger et al. [25] was used to investigate the possibility of publication bias.
The meta-analysis was performed using the metaprop command of the meta package in R (version 3.2.0) [26] and Stata (version 12.1) with the commands metareg (for metaregression). All statistical tests were two-sided, and statistical significance was defined as a P value <0.05.
Outcomes
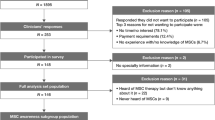
The initial search strategy yielded 4828 abstracts for review, of which 108 were selected for detailed review. Seventy-one studies were excluded for being non-human or safety studies or studies with exclusively pediatric patients, or for having a diagnosis of UC or indeterminate colitis or mixed irritable bowel syndrome for which the CD data could not be separated. Sixteen studies were further excluded because they were case studies or duplicate studies (Fig. 1; Additional file 1: Table S1).
The main characteristics of the included 20 studies [9, 10, 13, 16, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] are detailed in Tables 1 and 2. The included two Park et al. studies [32, 37] and two Cho et al. studies [33, 38] were not duplicated. Among the included 20 studies, three [9, 10, 13] were about the systemic infusion of hematopoietic stem cells, seven [27, 29,30,31, 36, 39, 40] the systemic infusion of bone marrow-derived MSCs (BMSCs), and ten [16, 28, 32,33,34,35, 37, 38, 41, 42] the local injection of MSCs; the numbers of transplanted SCs ranged from 107 to 1.5 × 1010. All studies defined clinical or endoscopic response with the Crohn’s Disease Activity Index (CDAI) and CD Endoscopic Index of Severity (CDEIS). All 15 observational studies were considered as high quality (scoring >7) using the NOS and the 2/6 RCTs [13, 27, 28, 37, 39, 42] had low risk of bias. Detailed quality assessments are provided in Additional file 2: Figure S1, Additional file 1: Table S1 and Additional file 3: Table S2.
Efficacy of SCT as a systemic infusion for CD
Clinical response
Eleven studies [16, 27, 29,30,31, 33,34,35,36, 40, 41] reported raw data on clinical response of patients with SCT. Rates of clinical response ranged from 10 to 100%. The random-effects pooled rate of clinical response was 56% (95% CI 33–76, n = 150); heterogeneity was pronounced (χ2 = 1.88, P < 0.001; I 2 = 78.6%; Fig. 2a). Subgroup analyses were performed to assess whether the source of the stem cells, i.e., HSCT or MSCT, and the method of SC infusion had substantial effects on the efficacy of SCs as well as decreased the high heterogeneity.
Six studies [27, 29,30,31, 36, 40] reported raw data on the outcome of patients with bone marrow-derived MSC (BMSC) therapy. The pooled rate of clinical response was 59% (95% CI 34–80, n = 79) compared to 51% (95% CI 13–88, five studies [16, 33,34,35, 41], n = 71) for patients who had ASC infusion (Table 3).
Seven studies [16, 29, 31, 33, 35, 40, 41] reported results in patients with autologous SCT. The pooled rate of clinical response was 31% (95% CI 17–49, n = 89) compared to 86% (95% CI 71–94, four studies [27, 30, 34, 36], n = 61) for patients who underwent allogeneic SCT (Table 3).
The pooled rate of clinical response was 66% (95% CI 39–86, five studies [27, 29, 30, 36, 40], n = 59) in patients who received systemic SCT compared to 45% (95% CI 16–79, six studies [16, 31, 33,34,35, 41], n = 91) for patients with local SCT (Table 3).
Clinical remission
Eight studies [9, 10, 13, 27, 29, 30, 36, 43] reported raw data on clinical remission (mainly defined as a CDAI <150; Tables 1 and 2) of patients with SCT. A random-effects meta-analysis of all studies of SCT as systemic infusion showed that 46% (95% CI 25–69, n = 116) of patients achieved clinical remission after infusion of SCs (Fig. 2b). Heterogeneity was modest (χ2 = 1.11, P = 0.002; I 2 = 68.5%).
Four studies [9, 10, 13, 43] reported raw data on the outcome of patients with HSCT. The pooled rate of clinical remission was 73% (95% CI 36–93, n = 69) in patients who received HSCT compared to 23% (95% CI 7–54, four studies [27, 29, 30, 36], n = 47) of patients who received MSCT (Table 3).
Endoscopic remission
Four studies [10, 29, 36, 43] reported raw data on the endoscopic remission of patients with SCT. Rates of endoscopic remission ranged from 0–50% (Fig. 2c). The random-effects pooled rate of endoscopic remission was 15% (95% CI 0–50, n = 48); heterogeneity was pronounced (χ2 = 0.42, P < 0.001; I 2 = 83.9%).
Efficacy of SCT for perianal CD (local therapy)
Thirteen studies [8, 10, 16, 28, 31,32,33,34,35, 37, 39, 41, 42] reported raw data on the efficacy of SCT for perianal CD of patients with SC injection. A random-effects meta-analysis of all perianal CD studies showed that 57% (95% CI 44%–69%, n = 251) of patients had healed fistula after local MSC injection (Fig. 3a) with mild heterogeneity (χ2 = 0.47, P = 0.003; I 2 = 59.9%).
Nine studies [16, 28, 32,33,34,35, 37, 41, 42] reported raw data on the outcome of patients who received ASC injection. The random-effects pooled rate of fistula healing was 60% (95% CI 44–75, n = 203) compared to 29% (95% CI 3–85, two studies [8, 10], n = 24) for patients who received HSCT and 62% (95% CI 42–79, two studies [31, 39], n = 24) for patients who received BMSC (Table 3).
Nine studies [8, 10, 16, 31,32,33, 35, 39, 41] reported results in patients with autologous SCT with a pooled rate of fistula healing of 62% (95% CI 44–77, n = 113) compared to 47% (95% CI 33–61, four studies [28, 34, 37, 42], n = 138) for patients who underwent allogeneic SCT (Table 3). No heterogeneity existed between the studies.
The pooled rate of fistula healing was 29% (95% CI 3–85, two studies [8, 10], n = 24) for patients who received systemic SCT compared to 60% (95% CI 47–72, 11 studies [16, 28, 31,32,33,34,35, 37, 39, 41, 42], n = 227) for patients who received local SCT (Table 3).
Comparison of SC versus control
Among the 13 studies, five [27, 28, 37, 39, 42] were double-armed; both Onken et al. [27] and Park et al. [37] compared two different doses of SCs. Thus, three studies [28, 39, 42] were pooled, yielding an odds ratio (OR) of 3.83 (95% CI 1.06–13.86, n = 121, P = 0.04) for achieving fistula healing in patients who received SCT versus control (Fig. 3b). Heterogeneity was modest (χ2 = 6.37, P = 0.04; I 2 = 69%).
Recurrence
Six studies [9, 10, 31, 33, 35, 43] reported raw data on recurrence for patients with SCT. Rates of recurrence ranged from 0 to 58% with a follow-up of >12 months (Fig. 4). The random-effects pooled rate of recurrence was 16% (95% CI 4–34, n = 101); heterogeneity was pronounced (χ2 = 0.15, P = 0.004; I 2 = 71.3%).
Three studies [9, 10, 43] reported raw data on the outcome of patients with HSCT. The pooled rate of clinical remission was 25% (95% CI 6–64, n = 46) for patients who received HSCT compared to 17% (95% CI 5–45, four studies [33, 35], n = 43) for patients who received ASCs and 4% (95% CI 0–40, four studies [31], n = 12) for patients who received BMSCs.
Safety profile
Overall, the included studies demonstrate that administration of SCs could lead to minor adverse reactions like perianal sepsis; however, serious adverse reactions leading to hospitalization are less common and perhaps related to underlying CD activity (Table 2). Eighteen [9, 10, 13, 16, 17, 27, 29, 31, 33,34,35,36,37,38,39,40,41,42] of 20 studies reported raw data on severe adverse events (SAEs) of patients with SCT. Rates of SAE ranged from 0–83%. The random-effects pooled rate of SAEs was 12% (95% CI 6–23, n = 378); heterogeneity was modest (χ2 = 1.68, P < 0.001; I 2 = 73.8%; Fig. 5). The random-effects pooled rate of SAEs related to SCT was 8% (95% CI 3–18, n = 378); heterogeneity was modest (P < 0.001; I 2 = 74.2.6%).
Publication bias and heterogeneity
A funnel plot generated for the primary outcome suggested no publication bias (Fig. 6), as did an Egger test for fistula healing (t = −0.97, P = 0.36), but not for clinical response (t = −4.91, P = 0.003).
Discussion
Our meta-analysis suggests that SCT has good therapeutic potential with relatively low risk of AEs for patients with CD, particularly for those who have perianal disease and are treated with local therapy.
MSCs have regenerative and immunomodulatory properties which lead to reduction of inflammation and healing of affected intestinal tissue. A previous meta-analysis showed that 40.5% (95% CI 7.5–78.5) of patients achieved remission after systemic infusion of MSCs [44] compared to 23% (95% CI 7–54) in our study. Autologous and allogeneic MSC administration are both being evaluated; their unique cell surface HLA characteristics allow for therapy with unrelated donor cells without increasing the risk of rejection by the host. According to our study, the pooled rate of clinical remission was 32% (95% CI 11–62) for patients who underwent allogeneic MSCT compared to 61% (95% CI 25–88) for patients who underwent autologous SCT (Table 3). However, patients who underwent allogeneic MSCT had a relatively higher pooled rate of clinical response compared with patients who underwent autologous SCT (86 versus 31%). Whether allogeneic MSCT has a superior therapeutic efficacy for CD warrants further study.
When considering HSCT, the question remains whether its risk to benefit ratio justifies it. Observations of case reports published to date suggest that sustained clinical remission with HSCT is initially likely to result from lymph ablation by drugs used in the conditioning regimen; altered immune reconstitution may be a later effect. Temporarily disrupting the immunological memory and ceasing the chronic inflammatory burden using non-ablative HSCT has been shown to be effective in non-controlled study designs [9]. However, the recent ASTIC trial [13] failed to demonstrate a statistically significant improvement in sustained disease remission at 1-year of HSCT. According to our study, the pooled rate of clinical remission was 73% (95% CI 36–93) for patients who underwent autologous HSCT, which was higher than 23% (95% CI 7–54) for patients who received ASC. Based on these trial findings, further study of HSCT in patients with refractory CD may be warranted.
When considering fistulizing CD, the existing pharmacological treatments for complex perianal fistulas have low efficacy in inducing fistula healing (antibiotics 21–48%; thiopurines 20–40%; anti-TNFs 23% complete responders) [45]. In our study, the random-effects meta-analysis of all perianal CD studies showed that 57% (95% CI 44–69) of patients had healed fistula after local MSC injection, which is superior to all the above-mentioned treatments. The pooled OR for achieving fistula healing in patients who received SC injection versus control was 3.83 (95% CI 1.06–13.86, P = 0.04).
Whether SCT is associated with improved long-term outcomes for CD patients is unclear. Burt et al. [9] reported that the percentage of clinical relapse-free survival after autologous nonmyeloablative HSCT was 91% at 1 year, 57% at 3 years, and 19% at 5 years. The percentage of patients in remission, steroid-free, or medication-free at any post-transplantation evaluation interval more than 5 years after HSCT remained at or greater than 70, 80, and 60%, respectively. Ciccocioppo et al. [31] reported the efficacy of MSCs for refractory complex CD fistulae declined over time and fistula relapse-free survival was 50% at 2 years and 37% at 4 years. Cho et al. [38] reported that 83.3% (20/24) of patients who received MSCs maintained complete fistula closure at 2 years. In the present meta-analysis, the pooled rate of clinical recurrence was 16% (95% CI 4–34) with a follow-up >12 months. Studies assessing the long-term safety and efficacy of systemic MSC therapy for luminal CD are lacking. Further studies that investigate the impact of periodic MSC administration on long-term outcomes are necessary to establish SCT as maintenance therapy.
The safety of SCT remains a major concern that must be addressed before it can be officially approved for use for CD. During the first 12-month follow-up, viral infections were the most commonly observed complications of autologous HSCT [46]. This is further confirmed by the ASTIC trial [13], with most importantly proven or presumed infections associated with the pancytopenia induced by the conditioning regimen. In contrast, Kniazev et al. [47] revealed no differences in the development of acute post-transfusion reactions, infectious complications, exacerbations of chronic inflammatory diseases, severe infectious complications, malignant transformation, and fatal cases in patients who received MSC therapy during 5-year follow-up. In our analysis, the pooled rate of SAEs and SAEs related to SCT were 12% (95% CI 6–23) and 8% (95% CI 3–18), respectively. When subgrouped by source of SCs, patients who received HSCs had a pooled rate of SAEs of 35% (95% CI 5–86), which is higher than with either BMSC (10%, 95% CI 2–42) or ASC (12%, 95% CI 7–18) infusion. MSCT does not require preparatory regimens involving high-dose chemotherapy and/or radiation; HSCT is thus associated with less procedure-related mortality. Overall, autologous HSCT for patients with refractory CD is feasible, but extraordinary supportive measures need to be implemented.
This meta-analysis also has limitations. First, assessment of the methodological quality determined that there were deficiencies in all the studies evaluated. Although only six RCTs [13, 17, 27, 37, 39, 42] met the inclusion criteria, the majority of studies included are phase I/IIa clinical trials. Second, clinical response exhibited statistical heterogeneity, which likely reflects the variability of definitions for clinical response, study design, sources of SCs, and time to clinical or endoscopic assessment. We used a random effects model to conservatively account for the clinical and statistical heterogeneity in the pooled studies. We also performed subgroup analyses to examine differences in the overall effect estimate.
Conclusions
So far, not enough studies have so far been performed to have a clear view about the use of SCT for CD; up to now the findings are encouraging but not conclusive. SCT seems potentially effective for refractory CD and has high efficacy in inducing fistula healing. Based on subgroup analysis, systemic allogeneic BMSC transfusion had relatively higher rates of inducing clinical response, while autologous ASC local injection had relatively higher rates of inducing fistula closure. Studies are needed to standardize MSC injection in often complex fistulas with multiple tracts to determine optimal dosing and source. Systemic infusion of SCs is not yet ready for the clinic and faces multiple challenges. Toxicity will remain the most significant barrier to HSCT in patients with CD.
Abbreviations
- AE:
-
adverse event
- ASC:
-
Adipose mesenchymal stem cell
- ASTIC:
-
Autologous Stem Cell Transplantation International Crohn Disease
- BMSC:
-
Bone marrow-derived MSC
- CD:
-
Crohn’s disease
- CDAI:
-
Crohn’s Disease Activity Index
- CI:
-
Confidence interval
- HSCT:
-
Hematopoietic stem cell transplantation
- MSC:
-
Mesenchymal stem cell
- NOS:
-
Newcastle Ottawa Quality Assessment Scale
- OR:
-
Odds ratio
- RCT:
-
Randomized controlled trial
- SAE:
-
Severe adverse event
- SC:
-
Stem cell
- SCT:
-
Stem cell therapy
- TNF:
-
Tumor necrosis factor-α
References
Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605.
Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–7.
Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106:674–84.
Duijvestein M, van den Brink GR, Hommes DW. Stem cells as potential novel therapeutic strategy for inflammatory bowel disease. Journal of Crohn's and Colitis 2008;2:99–106.
Engelhardt KR, Shah N, Faizura-Yeop I, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;131:825–30.
Lopez-Cubero SO, Sullivan KM, McDonald GB. Course of Crohn’s disease after allogeneic marrow transplantation. Gastroenterology. 1998;114:433–40.
Muraro PA, Douek DC, Packer A, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201:805–16.
Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128:552–63.
Burt RK, Craig RM, Milanetti F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood. 2010;116:6123–32.
Hasselblatt P, Drognitz K, Potthoff K, et al. Remission of refractory Crohn’s disease by high-dose cyclophosphamide and autologous peripheral blood stem cell transplantation. Aliment Pharmacol Ther. 2012;36:725–35.
Kreisel W, Potthoff K, Bertz H, et al. Complete remission of Crohn’s disease after high-dose cyclophosphamide and autologous stem cell transplantation. Bone Marrow Transplant. 2003;32:337–40.
Hawkey CJ. Stem cell transplantation for Crohn’s disease. Best Pract Res Clin Haematol. 2004;17:317–25.
Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA. 2015;314:2524–34.
Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–405.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–85.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23.
Garcia-Olmo D, Herreros D, Pascual M, et al. Treatment of enterocutaneous fistula in Crohn’s Disease with adipose-derived stem cells: a comparison of protocols with and without cell expansion. Int J Colorectal Dis. 2009;24:27–30.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–41.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–11.
Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–12.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557.
Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Schwarzer G. Meta: An R package for meta-analysis[J]. R news. 2007;7(3):40–5.
Onken J, Jaffe T, Custer L. W1237 long-term safety of prochymal adult mesenchymal stem cells in Crohn’s disease. Gastroenterology. 2008;134:A-661.
Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86.
Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–9.
Lazebnik L, Knyazev O, Konoplyannikov A, et al. T1751 Transplantation of allogenic mesenchimal stem cells is a new method of biological therapy of Crohn’s disease. Gastroenterology. 2010;138:S-571.
Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788–98.
Park KJ, Oh H-K, Ha H-K, et al. Adipose-derived stem cell treatment for persistent perineal wound in complex Crohn’s perianal abscess/fistula. Gastroenterology. 2012;142:S-76.
Cho YB, Lee WY, Park KJ, et al. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22:279–85.
de la Portilla F, Alba F, Garcia-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–23.
Lee WY, Park KJ, Cho YB, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells. 2013;31:2575–81.
Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71.
Park KJ, Kim JS, Kim WH, et al. 507 Allogeneic adipose-derived stem cells for the treatment of Crohn’s perianal fistula: a phase I/IIa clinical study. Gastroenterology. 2014;146:S-1016.
Cho YB, Park KJ, Yoon SN, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015;4:532–7.
Molendijk I, Bonsing BA, Roelofs H, et al. Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2015;149:918–27. e6.
Dhere T, Copland I, Garcia M, et al. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn’s disease--a phase 1 trial with three doses. Aliment Pharmacol Ther. 2016;44:471–81.
Lightner AL, Dozois E, Fletcher JG, et al. Early results using an adipose derived mesenchymal stem cells coated fistula plug for the treatment of refractory perianal fistulizing Crohns disease. Gastroenterology. 2016;150:S483–4.
Panés J, García-Olmo D, Van Assche G, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–90.
Cassinotti A, Onida F, Annaloro C, et al. Autologous haematopoietic stem cell transplantation without CD34+ cell selection for refractory Crohn’s disease: the milan experience after 5 years. Dig Liver Dis. 2012;44(Supplement 2):S62–3.
Dave M, Mehta K, Luther J, et al. Mesenchymal stem cell therapy for inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2696–707.
Gecse KB, Bemelman W, Kamm MA, et al. A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–92.
Jauregui-Amezaga A, Rovira M, Marin P, et al. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn’s disease. Gut. 2016;65:1456–62.
Kniazev OV, Parfenov AI, KOnopliannikov AG, et al. Safety of mesenchymal stromal cell therapy for inflammatory bowel diseases: results of a 5-year follow-up. Ter Arkh. 2015;87:39–44.
Acknowledgements
None.
Funding
This project was supported by grants from the National Natural Science Foundation of China (#81270473, #81470821, #81670498, #81630018), the Pearl River S&T Nova Program of Guangzhou (#201610010126), Guangdong Science and Technology (#2014A020212128, #2016A020214006), and the Fundamental Research Funds for Sun Yat-sen University (#15ykpy12).
Availability of data and materials
Availability of data and materials can be assessed both in the Method part and the Results part.
Authors’ contributions
YQ and M-yL: study design, data collection, statistical analysis, interpretation, and manuscript drafting/revision. M-hC and S-hZ: study conception and design and critical revision of the manuscript for important intellectual content. RF, TF, B-lC, RM, YH, and Z-rZ: study conception and design and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of the paper. All the tables and figures are original for this article.
Consent for publication
All the authors have looked through the manuscript and approved the submission.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
Characteristics of excluded studies. (DOCX 35 kb)
Additional file 2: Figure S1.
Risk of bias within studies assessed by Cochrane risk of bias assessment tool. (TIF 1067 kb)
Additional file 3: Table S2.
Assessment of quality of observational studies using Newcastle Ottawa Quality Assessment Scale (NOS) (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Qiu, Y., Li, My., Feng, T. et al. Systematic review with meta-analysis: the efficacy and safety of stem cell therapy for Crohn’s disease. Stem Cell Res Ther 8, 136 (2017). https://doi.org/10.1186/s13287-017-0570-x
Published:
DOI: https://doi.org/10.1186/s13287-017-0570-x