Abstract
Extracellular vesicles (EVs) are plasma membrane-bound fragments released from several cell types, including mesenchymal stromal cells (MSCs), constitutively or under stimulation. EVs derived from MSCs and other cell types transfer molecules (such as DNA, proteins/peptides, mRNA, microRNA, and lipids) and/or organelles with reparative and anti-inflammatory properties to recipient cells. The paracrine anti-inflammatory effects promoted by MSC-derived EVs have attracted significant interest in the regenerative medicine field, including for potential use in lung injuries. In the present review, we describe the characteristics, biological activities, and mechanisms of action of MSC-derived EVs. We also review the therapeutic potential of EVs as reported in relevant preclinical models of acute and chronic respiratory diseases, such as pneumonia, acute respiratory distress syndrome, asthma, and pulmonary arterial hypertension. Finally, we discuss possible approaches for potentiating the therapeutic effects of MSC-derived EVs so as to enable use of this therapy in clinical practice.
Similar content being viewed by others
Background
In recent decades, the therapeutic potential and safety of mesenchymal stromal cells (MSCs) has been studied in the context of regeneration and immune modulation of injured tissues [1]. Many studies have demonstrated that, when systemically administered, MSCs are recruited to sites of inflammation through still-incompletely understood chemotactic mechanisms [2], stimulate endogenous repair of injured tissues [3], and modulate immune responses [4]. The beneficial effects of MSCs on tissue repair and regeneration are based on their paracrine activity, characterized by the capacity to secrete growth factors, cytokines, and chemokines, which orchestrate interactions within the microenvironment and influence tissue regeneration. These factors can inhibit apoptosis, stimulate proliferation, promote vascularization, and modulate the immune response [5]. Remarkably, conditioned medium collected from MSCs can convey many of these protective effects, suggesting that soluble factors rather than cell–cell contact are the major mechanism of MSC actions [6].
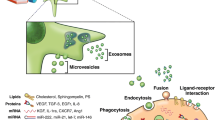
Notably, a growing body of literature suggests that many of these paracrine effects are mediated by extracellular vesicles (EVs) contained in the conditioned medium. EVs are small, spherical membrane fragments including exosomes, microvesicle particles, and apoptotic bodies in accordance with the recommendations of the International Society for Extracellular Vesicles (ISEV) [7]. The EVs are released by cells that are involved in cell-to-cell communication and are capable of altering the fate and phenotype of recipient cells [8]. The exosomes arise from intracellular endosomes, while the microvesicles originate directly from the plasma membrane. These particle types are secreted from a wide range of different cell types, including T and B lymphocytes, dendritic cells (DCs), mast cells, platelets, and MSCs derived from different tissues (bone marrow, placenta, as well as adipose and lung tissues), and can also be isolated in vivo from body fluids such as urine, serum, and bronchoalveolar lavage fluid (BALF) [9, 10]. Nevertheless, the classification of EVs differs depending on their origin, size, and contents (Table 1). Additionally, the number and nature of EVs may be affected by gender, age, circadian rhythms, fasting state, medication exposure, and physical activity [11]. However, whether these different classes of EVs represent distinct biological entities is not evident. Several parameters have been used to characterize the different classes of EVs, including size, ionic composition, sedimentation rate, flotation density on a sucrose gradient, lipid composition, protein cargo, and biogenesis pathway; however, most of these parameters are neither definitive nor exclusive to any specific class of EVs (Fig. 1) [7].
Schematic representation of EVs biogenesis. Vesicles bud directly from the plasma membrane, whereas exosomes originate from ILVs that are generated by inward budding of the limiting membrane of a subgroup of late endosomes called multivesicular bodies (MVBs). MVBs can be directed towards the cell periphery and, after fusion with the plasma membrane, release their content into the extracellular space. miRNA microRNA, MSC mesenchymal stromal cell
Exosomes range in size from 50 to 150 nm, have a homogeneous shape, and are defined as a subtype of EVs derived from specialized intracellular compartments, the multivesicular bodies (MVBs) [12]. Exosomes are constitutively released from cells, but their release is augmented significantly following activation by soluble agonists (cytokines, chemokines, and growth factors), as well as physical, chemical (oxidative stress and hypoxia), and shear stresses [13]. In order to form an exosome, the limiting membrane of the MVBs buds inward, thus forming intraluminal vesicles (ILVs), which then fuse with the plasma membrane to release ILVs as exosomes. This process is mediated by p53-regulated exocytosis, which is dependent on cytoskeletal activation but independent of cell calcium influx [14]. In contrast, microvesicles range from 150 to 1000 nm in size and are more heterogeneous. They are released by budding of small cytoplasmic protrusions, a process dependent on calpain, cytoskeletal reorganization, and intracellular calcium concentration. Calcium ions are responsible for the asymmetric phospholipid distribution of the plasma membrane that yields microvesicle formation [14]. Finally, there is another type of EVs, larger than 1 μm: the apoptotic body, derived from dying cells. DNA, as a residue of the nucleus, is frequently present within these vesicles, as are noncoding RNAs and cell organelles [15].
The different EVs can be isolated from body fluids or in vitro cultured cells by specific standardized protocols, and characterized by differential ultracentrifugation, ultrafiltration, and immunoprecipitation with the use of antibody-loaded magnetic cell beads [16]. These procedures are critical because all types of vesicles, as well as membrane fragments, are normally present in the starting material and can contaminate specific EVs preparations. One major challenge in EVs research is therefore to standardize methods for isolation and analysis. Additionally, it is difficult to distinguish between exosomes and microvesicles because of their overlapping characteristics and the lack of discriminating markers [17]. Nevertheless, among the many subtypes of EVs, exosomes have emerged as physiologically relevant and powerful components of the MSC secretome [18].
The content of EVs consists of proteins, lipids, and nucleic acids; microvesicles and apoptotic bodies also have organellar contents. Since the effects of EVs usually depend on their cell of origin and may be influenced by physiological stress or pathological conditions, they could be used as biomarkers to diagnose, prognosticate, or predict diseases and their natural history [14]. Many reports have shown that the functions of EVs reflect, at least in part, those of their originating cells; differences between them occur because the EVs composition may be modified, suggesting that preferential packaging or exclusion of material occurs [19]. Information on the protein, lipid, and RNA expressions of EVs is collected in VESICLEPEDIA (http://www.microvesicles.org) [20], while the exosomes of different cell types and organisms are described in the ExoCarta database [21]. EVs play an important role in intercellular communication and are capable of modifying the activity of target cells through direct surface receptor interactions, receptor transfer between cells, protein delivery to target cells, or horizontal transfer of genetic information [22]. They are involved in cellular processes such as angiogenesis modulation, cell proliferation, and immune regulation [23]. EVs are therefore particularly attractive for their therapeutic potential, especially MSC-derived EVs, which appear to be an important tool to harness the clinical benefits of MSC therapy while using cell-free strategies based on the MSC secretome. These strategies may reduce the risks associated with engraftment of MSCs, such as possible immune reactions against MSCs and development of ectopic tissue. Since EVs carry a wide array of signals, several studies have been performed evaluating their implication in animal models of organ injury, including lung diseases. Nonetheless, comprehensive insight regarding the full scope of molecules packaged in MSC-derived EVs and their role in tissue regeneration has yet to be gained, and additional studies are needed to provide greater detail [9, 23].
Characteristics of MSC-derived EVs
MSC-derived EVs express surface molecules, such as CD29, CD73, CD44, and CD105, which are characteristic of their cells of origin. Among the MSC-derived EVs, the exosomes are those best characterized. Exosomes are known to conserve a set of proteins, including tetraspanins, involved in cell targeting (CD63, CD81, and CD9); heat-shock proteins Hsp60, Hsp70, and Hsp90 [24]; ALG-2-interacting protein X (Alix) and tumor susceptibility gene 101 (Tsg101), which are involved in their biogenesis from MVBs; integrins and annexins, which are important for transport and fusion [20]; and major histocompatibility complex classes I and II [25]. Microvesicles lack proteins of the endocytic pathway, but are rich in cholesterol and lipid raft-associated proteins, such as integrins and flotillins. Although tetraspanins are commonly used as unique markers for exosomes, they can be detected in microvesicles in some cases [26]. Several studies have been conducted evaluating the potential role of MSC-derived EVs in physiological and pathological conditions and their possible applications in the therapy of different diseases [12, 15]; however, few studies have evaluated the RNA and protein content of these vesicles.
MSC-derived EVs are enriched by distinct classes of RNAs that could be transferred to target cells and translated into proteins, resulting in an alteration of target cell behavior [27]. In particular, MSC-derived EVs contain transcripts involved in control of transcription (transcription factor CP2, clock homolog), cell proliferation (retinoblastoma-like 1, small ubiquitin-related modifier 1), and immune regulation (interleukin 1 receptor antagonist) [27]. Additionally, MSC-derived EVs contain noncoding RNA, microRNAs (miRNAs) that mediate posttranscriptional control of gene expression and, as such, modulate survival and metabolic activities of recipient cells [28]. These miRNAs can be present both in EVs and/or in their cells of origin [9]. The miRNAs detected in MSC-derived EVs are usually related to development, cell survival, and differentiation, while some MSC-derived EVs-enriched miRNAs are more closely associated with regulation of the immune system [9]. Comprehensive information on the complete RNA content of MSC-derived EVs is not currently available, however, and whether adult MSCs from different sources share similar RNA repertoires remains unknown. A recent study compared the RNA profile of exosomes released by adult MSCs from two different sources: adipose-derived MSCs (ASCs) and bone marrow-derived MSCs (BM-MSCs). Despite substantial similarity between the most represented RNAs in the ASC and BM-MSC exosomes, their relative proportions are different [29].
Proteome analysis may be equally important. Characterization of the content of BM-MSC-derived EVs identified several proteins, among which are mediators controlling self-renewal and differentiation. Interestingly, this analysis revealed a number of surface markers, such as platelet-derived growth factor receptor, epidermal growth factor receptor, and plasminogen activator, urokinase receptor; signaling molecules of the RAS-mitogen-activated protein kinase, Rho GTPase, and Cell division control protein 42 pathways; cell adhesion molecules; and additional MSC antigens [30], supporting a possible role for such vesicles in tissue repair. Treatment of cell-derived EVs with specific growth factors can change the phenotype and protein content of these vesicles; for example, ASCs treated with platelet-derived growth factor have been shown to produce EVs with enhanced angiogenic activity [31]. This wide distribution of biological activities gives MSC-derived EVs the potential to elicit diverse cellular responses and interact with many cell types.
Mechanisms of action and biological activities of EVs
EVs may interact with recipient cells by different mechanisms: interactions at the cell surface, internalization into endocytic compartments, and fusion with plasma membranes (Fig. 1) [32]. The efficiency of EVs uptake has been observed to correlate directly with intracellular and microenvironmental acidity [33]. Following ligand interaction, EVs may deliver their contents to the recipient cell that reprogrammed them. Recently, EVs from stem cells were demonstrated to shuttle a cysteine-selective transport channel (cystinosin) that restores function in mutant target cells [34]. EVs may also mediate the horizontal transfer of genetic information, such as subsets of mRNA and miRNA, from the cell of origin, thereby inducing alterations in the phenotype and behavior of recipient cells by different pathways [35]. In this line, EVs produced by murine embryonic stem cells may reprogram hematopoietic progenitors by delivering not only proteins but also mRNA for several pluripotent transcription factors [36], whereas pretreatment of these EVs with RNase inhibited the observed biological effects, thus suggesting the contribution of EVs-derived mRNA [36]. Stem cells may therefore modulate their biological effects by delivering genetic information and altering the gene expression of target cells. Interestingly, the exchange of genetic information may be bidirectional: from injured cells to bone marrow-derived or resident stem cells; or from stem cells to injured cells. In this context, Dooner et al. [37] reported that bone marrow stem cells cocultured with injured lung cells expressed genes for lung-specific proteins, such as surfactant B and C, and Clara cell-specific proteins, which may be attributed to the transfer of lung-specific mRNAs to bone marrow cells via EVs released from the injured lung cells.
Additionally, EVs derived from injured and immune cells may induce stem cell recruitment and differentiation of resident stem cells present in several organs during adulthood, thus contributing to physiologic tissue repair [13]. Nevertheless, depending on their cells of origin, EVs can exert immunostimulatory or immunosuppressive effects [38]. Alveolar macrophages infected with mycobacteria release EVs containing pathogen-derived proinflammatory molecules and secrete Hsp70, which activates the nuclear factor-κB pathway by stimulating toll-like receptors (TLRs) [15], leading to the secretion of proinflammatory cytokines [14, 24]. On the other hand, EVs secreted by DCs are able to induce humoral responses against antigens processed by DCs before EVs purification, yielding strong protection against infection [39]. EVs may also modulate the function of target cells. For instance, EVs derived from lipopolysaccharide-activated monocytes induce apoptosis in target cells through transfer of caspase-1 [40]. Furthermore, proteomic analysis of damaged tissues usually reveals they are depleted of many rate-limiting ATP-generating enzymes, and are thus unable to utilize the restored oxygen supply to produce ATP. This depletion could be supplemented by the proteome of MSC-derived exosomes, which has a cargo rich in enzymatically active glycolytic enzymes and other ATP-generating enzymes, such as adenylate kinase and nucleoside-diphosphate kinase [41].
However, MSC-derived EVs have received more emphasis in the literature and have been most widely studied. In this line, EVs released from human MSCs have been shown to contain ribonucleoproteins involved in the intracellular trafficking of RNA and selected patterns of miRNA, suggesting dynamic regulation and compartmentalization of RNA involved in the development, regulation, regeneration, and cell differentiation, which contribute to recovery processes after injury of adult tissues (Fig. 2) [42]. Indeed, MSC-derived EVs exert an important inhibition in the differentiation and activation of T cells and their interferon-gamma (IFN-γ) release in vitro, as well as stimulating the secretion of anti-inflammatory cytokines (interleukin (IL)-10 and transforming growth factor beta (TGF-β)) and generation of regulatory T cells [43], suggesting that MSC-derived exosomes are relevant immunomodulatory therapeutic agents (Fig. 2). Additionally, treatment with MSC-derived EVs activates an M2 macrophage-like phenotype in the lung parenchyma, which is known to promote tissue repair and limit injury [44].
Scheme illustrating extracellular vesicle (EV) function related to tissue repair. The exchange of proteins and genetic information (mRNA and miRNA) from MSCs or resident stem cells contributes to tissue repair. IFN interferon, IL interleukin, miRNA microRNA, TGFβ transforming growth factor beta, Treg regulatory T cell
The immunomodulatory effects of BM-MSCs and derived EVs have been analyzed in vitro. BM-MSCs and their EVs exhibit similar inhibitory activity against B-cell proliferation, but EVs display less inhibitory activity on differentiation and antibody release of B cells compared with BM-MSCs. Moreover, BM-MSCs are more efficient than EVs at inhibiting T-cell proliferation. In one study, incubation of both T cells and B cells with EVs led to a decrease in granulocyte–macrophage colony-stimulating factor and IFN-γ and an increase in IL-10 and TGF-β compared with BM-MSCs [45].
Therapeutic potential of MSC-derived EVs in lung diseases
MSC-derived EVs have shown to be a promising therapy enabling tissue repair and wound healing. The effects of MSC-derived EVs can be potentiated under some conditions, such as exposure to hypoxia and coculture with animal or human serum obtained in pathologic conditions. These methods may induce the release and potentiate the effects of these EVs due to stimulation and the presence of cytokines and chemotactic and growth factors, which not only increase EVs release but may also modify their content, leading to enhancement of beneficial effects.
EVs are also important vehicles for drug delivery because of their lipid bilayer and aqueous core, since they can carry both lipophilic and hydrophilic drugs [46]. Furthermore, EVs present several advantages for this purpose, such as: presence of protein and genetic materials, which enables active loading of biological material; high tolerability in the body due to the presence of inhibitors of complement and phagocytosis [30]; protection against degradative enzymes or chemicals; and ability to cross the plasma membrane to deliver their cargo to target cells [9, 47] and home to target tissues [9, 46]. Electroporation [48] and viral packaging strategies [49] have been used to load therapeutically active cargo molecules (e.g., small-molecule drugs or small interfering RNA (siRNA)) into EVs [48, 49].
While a predominant mechanism of MSCs in tissue repair through paracrine activity has already been suggested, some studies are being carried out to better understand the mechanisms associated with the beneficial effects of MSC-derived EVs in lung diseases, such as asthma, pulmonary arterial hypertension (PAH), acute respiratory distress syndrome (ARDS), and pneumonia (Table 2), and how they can be potentiated for translation to clinical practice.
Asthma
Asthma is a chronic inflammatory disease characterized by airway constriction and inflammation, which may lead to structural changes in the airways, often in response to allergens, infections, and air pollutants [50]. Even though several therapeutic strategies are currently available to reduce airway inflammation, no treatment has so far been able to hasten repair of the damaged lung [51]. In this line, some studies reported that MSCs reduced lung inflammation and remodeling in experimental allergic asthma [52–54].
EVs are released from several cells that are involved in allergies, including mast cells, DCs, T cells, and bronchial epithelial cells (BECs) in the lungs. For example, mast cell-derived EVs induce DC maturation, and DC-derived EVs can transport allergens and activate allergen-specific T-helper (Th) type 2 cells [55]. Among several potential mechanisms, BECs exposed to compressive stress – thus simulating the bronchoconstriction seen in asthma – produce EVs bearing tissue factor which may participate in promotion of subepithelial fibrosis and angiogenesis [56]. In short, available data indicate the potential contributions of BEC-derived EVs to the pathogenesis of asthma. Additionally, these findings may lead to the development of future treatments for asthma patients that target the inhibition of EVs secretion by these cells.
Several phenotypic and functional alterations have been observed in BALF EVs from asthmatics compared with healthy patients. These include higher expression of CD36, which has been implicated in bacterial recognition and may play a role in asthma exacerbations in response to bacterial infections [57], and that the EVs contain miRNAs, critical regulators of specific pathogenic events [58] which may act as biomarkers of lung diseases, such as the let-7 (let-7a–let-7e) and miRNA-200 (miR-200b and miR-141) families [59]. Further, incubation of BECs with BALF EVs from asthmatic patients resulted in increased leukotriene and IL-8 release [60].
Additionally, administration of BALF-derived EVs from mice sensitized and challenged with ovalbumin has been shown to inhibit IgE response, Th2 cytokine production, and airway inflammation in experimental asthma [61]. Similar behavior was observed with asthmatic serum-derived EVs, protecting against allergic airway inflammation and reducing BALF eosinophil counts, IgE levels, and Th2 response. EVs from different sources may therefore play a role in the development of asthma and allergy, either as a failure to induce effective tolerance or as enhancers of an already established response. In short, EVs may be therapeutic targets in anti-allergy treatment.
Recently, the therapeutic effects of EVs derived from human MSCs (hMSCs) and mouse MSCs (mMSCs) were investigated in experimental asthma. The authors observed that systemic administration of EVs from either hMSCs or mMSCs were each effective – in some cases, more effective than the administration of hMSCs or mMSCs themselves – in mitigating allergic airway hyperresponsiveness and lung inflammation, and altered the phenotype of antigen-specific CD4 T cells in a model of severe, acute, mixed Th2/Th17-mediated eosinophilic and neutrophilic airway allergic inflammation in immunocompetent mice. Additionally, blocking EVs release led to an absence of protective effects associated with both hMSCs and mMSCs [62].
Pulmonary arterial hypertension
PAH is a disease characterized by hyperplasia and hypertrophy of smooth muscle cells in small pulmonary arteries, associated with an increase in endothelial cell proliferation that leads to remodeling of pulmonary vessels and, consequently, an increase in mean pulmonary arterial pressure and right ventricular overload. Data obtained from patients with PAH show that the severity of PAH is related to an increase in circulating EVs released from stimulated or endothelial cells undergoing apoptosis, probably due to release of soluble vascular cellular adhesion molecule VCAM-1, and that proinflammatory markers, such as monocyte chemoattractant protein MCP-1 and highly specific C-reactive protein, were elevated in PAH patients. In addition, a further increase in endothelium-derived CD105 microparticles was observed in pulmonary arterial blood compared with venous blood in patients with PAH [63]. Inflammation plays an important role in the development of human PAH and there are several animal models of this condition, such as monocrotaline-induced and hypoxia-induced PAH in rodents.
Despite significant progress in elucidating PAH pathophysiology and treatment, few PAH therapies are available and all have limited effectiveness. Many studies have therefore investigated the effects of MSC therapy in PAH, and demonstrated benefit. In a recent investigation, lung-derived and plasma-derived EVs generated from monocrotaline-induced PAH led to increased right ventricular mass and pulmonary vascular wall thickness, resulting in PAH-like changes in healthy mice This effect may be promoted directly by EVs on the pulmonary vasculature or by differentiation of bone marrow cells to endothelial progenitor cells that induce pulmonary vascular remodeling [64]. This suggests that EVs presented altered expressions of miRNAs involved in pulmonary vascular remodeling. Conversely, in hypoxia-induced PAH, MSC-derived EVs protected against elevation of right ventricular systolic pressure and development of right ventricular hypertrophy, whereas EVs-depleted medium and fibroblast-derived EVs had no effect. These beneficial effects of MSC-derived EVs can be related to suppression of hypoxic pulmonary macrophage influx and hypoxic activation of signal transducer and activator of transcription STAT3, combined with induction of proinflammatory and proproliferative mediators – including MCP-1 and hypoxia-inducible mitogenic factor HIMF – and increased pulmonary levels of the key miRNAs miR-17 and miR-204, the expressions of which are reduced in human pulmonary hypertension [65]. However, the animal models in which these effects were tested are not considered good representations of preclinical models of PAH. The beneficial effects observed with EVs treatment of PAH therefore require more in-depth investigation before they can be considered practice changing.
Acute respiratory distress syndrome
ARDS is a severe clinical condition characterized by alveolar-capillary damage, accumulation of protein-rich debris in the alveolar airspace, and progressive respiratory failure [66]. Although major improvements in treatment and supportive care of ARDS have been achieved, its mortality rate remains around 40 % [67].
Recently, some studies reported that MSCs can be a promising therapeutic approach for ARDS through paracrine effects [68–70]. Additionally, MSC-derived EVs have been shown to produce beneficial effects in experimental endotoxin-induced ARDS, reducing lung inflammation [71]. hMSC-derived EVs were therapeutically effective following Escherichia coli endotoxin-induced ARDS, thus reducing extravascular lung water, total protein levels in BALF, edema, and neutrophil infiltration. These beneficial effects were associated with an increase in keratinocyte growth factor (KGF) expression, as they were partially eliminated after delivery of EVs derived from KGF siRNA-pretreated MSCs [72]. Moreover, ischemic preconditioning can potentiate the protective effect of MSCs in endotoxin-induced ARDS through the secretion of exosomes since it confers strong protection against cell death and promotes their differentiation potential by activating multiple signaling pathways which open new avenues for therapeutic approaches [73].
Pneumonia
Bacterial pneumonia is among the main causes of respiratory failure in critically ill patients. Despite improvements in supportive care and appropriate antibiotic use, morbidity and mortality remain high [74]. Several studies have reported efficacy of MSCs in preclinical models of pneumonia due to their ability to secrete paracrine factors such as growth factors, anti-inflammatory cytokines, and antimicrobial peptides [75]. Outer-membrane vesicle release is a conserved phenomenon among pathogenic and nonpathogenic Gram-negative bacteria [76]. Nevertheless, little is known regarding Gram-positive EVs, especially their biogenesis and role in host–pathogen interactions. EVs from Streptococcus pneumonia, one of the leading causes of bacterial pneumonia worldwide, have only recently been characterized [77] and found to exhibit high immunogenicity due to the presence of the toxin pneumolysin.
Recently, in an in vivo model of E. coli pneumonia in mice, hMSC-derived EVs were as effective as their parent stem cells in improving survival and mitigating lung inflammation, protein permeability, and bacterial growth. The antimicrobial effect of hMSC-derived EVs was exerted in part through enhancement of monocyte phagocytosis of bacteria, which could be further increased by prestimulation of hMSCs with a TLR-3 agonist before EVs release. Uptake of hMSC-derived EVs through the CD44 receptor into injured human monocytes and alveolar epithelial cells was critical for their therapeutic effects. Another factor that should be stressed is that hMSC-derived EVs decreased tumor necrosis factor alpha secretion by lipopolysaccharide-primed human monocytes and restored intracellular ATP levels in injured human alveolar epithelial type II cells, suggesting immunomodulatory and metabolomic effects of EVs. Additionally, administration of a KGF neutralizing antibody abrogated the survival advantage mediated by hMSC-derived EVs, suggesting a possible mechanism for their therapeutic effect [78].
Conclusions
Several studies have reported that MSCs may repair damaged tissue by modifying target cell function through paracrine mechanisms without directly replacing injured cells. The role of EVs in this mechanism would be to exchange genetic material, which could explain the observed phenotypic and functional changes of MSCs [79]. This genetic material transfer may lead to the production of soluble factors, thus regulating cell proliferation, apoptosis, and/or inflammation and immune response.
EVs present many advantages over stem cells, such as homing ability to target tissue, preventing undesired accumulation in other organs, and absence of any innate toxicity or association with long-term maldifferentiated engrafted cells, tumor generation, or immune rejection after stem cell injection. However, the mechanisms associated with the beneficial effects induced by MSC-derived EVs require further investigation. In this line, the following points in particular warrant better evaluation: which signaling regulates the transfer of biologically active molecules within EVs, which surface receptors may yield selective specificity, and which stimuli are responsible for triggering EVs release. Understanding these EVs mechanisms may allow their use as diagnostic markers, for the delivery of drugs and genes, and as new therapeutic strategies. Although some studies have reported beneficial effects of MSC-derived EVs in asthma, ARDS, PAH, and pneumonia, many issues must be addressed before their use in clinical settings, including: the need for large-scale EVs production from MSCs; the need for criteria defining the potency of EVs, due to different preparations and MSC sources; the long-term effects of EVs; and the biodistribution of EVs in each respiratory disease.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ASC:
-
Adipose-derived mesenchymal stromal cell
- BALF:
-
Bronchoalveolar lavage fluid
- BEC:
-
Bronchial epithelial cell
- BM-MSC:
-
Bone marrow-derived mesenchymal stromal cell
- DC:
-
Dendritic cell
- EV:
-
Extracellular vesicle
- hMSC:
-
Human mesenchymal stromal cell
- Hsp:
-
Heat-shock proteins
- IFNγ:
-
Interferon gamma
- IL:
-
Interleukin
- ILV:
-
Intraluminal vesicle
- ISEV:
-
International Society for Extracellular Vesicles
- KGF:
-
Keratinocyte growth factor
- miRNA:
-
MicroRNA
- mMSC:
-
Mouse mesenchymal stromal cell
- MSC:
-
Mesenchymal stromal cell
- MVB:
-
Multivesicular body
- PAH:
-
Pulmonary arterial hypertension
- siRNA:
-
Small interfering RNA
- TGF-β:
-
Transforming growth factor beta
- Th:
-
T-helper
- TLR:
-
Toll-like receptor
References
Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559.
Hofmann NA, Ortner A, Jacamo RO, Reinisch A, Schallmoser K, Rohban R, Etchart N, Fruehwirth M, Beham-Schmid C, Andreeff M, Strunk D. Oxygen sensing mesenchymal progenitors promote neo-vasculogenesis in a humanized mouse model in vivo. PLoS One. 2012;7(9):e44468.
Bell GI, Meschino MT, Hughes-Large JM, Broughton HC, Xenocostas A, Hess DA. Combinatorial human progenitor cell transplantation optimizes islet regeneration through secretion of paracrine factors. Stem Cells Dev. 2012;21(11):1863–76.
Zhao S, Wehner R, Bornhauser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19(5):607–14.
Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thebaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012;21(15):2789–97.
Goolaerts A, Pellan-Randrianarison N, Larghero J, Vanneaux V, Uzunhan Y, Gille T, Dard N, Planes C, Matthay MA, Clerici C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol. 2014;306(11):L975–85.
Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913.
Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–48.
Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359.
Almqvist N, Lonnqvist A, Hultkrantz S, Rask C, Telemo E. Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology. 2008;125(1):21–7.
Quesenberry PJ, Goldberg LR, Aliotta JM, Dooner MS, Pereira MG, Wen S, Camussi G. Cellular phenotype and extracellular vesicles: basic and clinical considerations. Stem Cells Dev. 2014;23(13):1429–36.
Chen J, Li C, Chen L. The role of microvesicles derived from mesenchymal stem cells in lung diseases. Biomed Res Int. 2015;2015:985814.
Bruno S, Camussi G. Role of mesenchymal stem cell-derived microvesicles in tissue repair. Pediatr Nephrol. 2013;28(12):2249–54.
Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27(8):3037–42.
Fujita Y, Kosaka N, Araya J, Kuwano K, Ochiya T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol Med. 2015;21(9):533–42.
Fierabracci A, Del Fattore A, Luciano R, Muraca M, Teti A. Recent advances in mesenchymal stem cell immunomodulation: the role of microvesicles. Cell Transplant. 2015;24(2):133–49.
Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2: 10.3402/jev.v2i0.20389.
Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–3.
Katsuda T, Kosaka N, Takeshita F, Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13(10–11):1637–53.
Chen J, Liu Z, Hong MM, Zhang H, Chen C, Xiao M, Wang J, Yao F, Ba M, Liu J, Guo ZK, Zhong J. Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS One. 2014;9(12):e115316.
Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9(21):4997–5000.
Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2011;7(2):105–15.
Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 2015;11(1):150–60.
Bruno S, Deregibus MC, Camussi G. The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol Lett. 2015;168(2):154–8.
Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol Lung Cell Mol Physiol. 2002;283(3):L510–7.
Dale GL, Remenyi G, Friese P. Tetraspanin CD9 is required for microparticle release from coated-platelets. Platelets. 2009;20(6):361–6.
Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22(5):772–80.
Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64.
Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127.
Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11(2):839–49.
Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014;12:26.
Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472.
Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–22.
Iglesias DM, El-Kares R, Taranta A, Bellomo F, Emma F, Besouw M, Levtchenko E, Toelen J, van den Heuvel L, Chu L, Zhao J, Young YK, Eliopoulos N, Goodyer P. Stem cell microvesicles transfer cystinosin to human cystinotic cells and reduce cystine accumulation in vitro. PLoS One. 2012;7(8):e42840.
Camussi G, Deregibus MC, Cantaluppi V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans. 2013;41(1):283–7.
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–56.
Dooner MS, Aliotta JM, Pimentel J, Dooner GJ, Abedi M, Colvin G, Liu Q, Weier HU, Johnson KW, Quesenberry PJ. Conversion potential of marrow cells into lung cells fluctuates with cytokine-induced cell cycle. Stem Cells Dev. 2008;17(2):207–19.
Bourdonnay E, Zaslona Z, Penke LR, Speth JM, Schneider DJ, Przybranowski S, Swanson JA, Mancuso P, Freeman CM, Curtis JL, Peters-Golden M. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med. 2015;212(5):729–42.
Qazi KR, Torregrosa Paredes P, Dahlberg B, Grunewald J, Eklund A, Gabrielsson S. Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax. 2010;65(11):1016–24.
Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009;4(9):e7140.
Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–8.
Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7):e11803.
Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1–2):47–54.
Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–44.
Conforti A, Scarsella M, Starc N, Giorda E, Biagini S, Proia A, Carsetti R, Locatelli F, Bernardo ME. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014;23(21):2591–9.
Lai Y, Long Y, Lei Y, Deng X, He B, Sheng M, Li M, Gu Z. A novel micelle of coumarin derivative monoend-functionalized PEG for anti-tumor drug delivery: in vitro and in vivo study. J Drug Target. 2012;20(3):246–54.
Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6(4):481–92.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–90.
Gyorgy B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–64.
Martinez FD, Vercelli D. Asthma. Lancet. 2013;382(9901):1360–72.
Papierniak ES, Lowenthal DT, Harman E. Novel therapies in asthma: leukotriene antagonists, biologic agents, and beyond. Am J Ther. 2013;20(1):79–103.
Abreu SC, Antunes MA, de Castro JC, de Oliveira MV, Bandeira E, Ornellas DS, et al. Bone marrow-derived mononuclear cells vs. mesenchymal stromal cells in experimental allergic asthma. Respir Physiol Neurobiol. 2013;187(2):190–8.
Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011;29(7):1137–48.
Lathrop MJ, Brooks EM, Bonenfant NR, Sokocevic D, Borg ZD, Goodwin M, Loi R, Cruz F, Dunaway CW, Steele C, Weiss DJ. Mesenchymal stromal cells mediate Aspergillus hyphal extract-induced allergic airway inflammation by inhibition of the th17 signaling pathway. Stem Cells Transl Med. 2014;3(2):194–205.
Admyre C, Telemo E, Almqvist N, Lotvall J, Lahesmaa R, Scheynius A, Gabrielsson S. Exosomes—nanovesicles with possible roles in allergic inflammation. Allergy. 2008;63(4):404–8.
Park JA, Sharif AS, Tschumperlin DJ, Lau L, Limbrey R, Howarth P, Drazen JM. Tissue factor-bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J Allergy Clin Immunol. 2012;130(6):1375–83.
Baranova INKR, Bocharov AV, Vishnyakova TG, Chen Z, Remaley AT, Csako G, et al. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J Immunol. 2008;181(10):7147–56.
Levanen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, Skold CM, Svartengren M, Grunewald J, Gabrielsson S, Eklund A, Larsson BM, Woodruff PG, Erle DJ, Wheelock AM. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131(3):894–903.
Pinkerton M, Chinchilli V, Banta E, Craig T, August A, Bascom R, Cantorna M, Harvill E, Ishmael FT. Differential expression of microRNAs in exhaled breath condensates of patients with asthma, patients with chronic obstructive pulmonary disease, and healthy adults. J Allergy Clin Immunol. 2013;132(1):217–9.
Torregrosa Paredes P, Esser J, Admyre C, Nord M, Rahman QK, Lukic A, Radmark O, Gronneberg R, Grunewald J, Eklund A, Scheynius A, Gabrielsson S. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy. 2012;67(7):911–9.
Prado NME, Segura E, Fernández-García H, Villalba M, Théry C, Rodríguez R, Batanero E. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J Immunol. 2008;181(2):519–25.
Cruz FFBZ, Goodwin M, Sokocevic D, Wagner DE, Coffey A, Antunes M, Robinson KL, Mitsials SA, Kourembanas S, Thane K, Hoffman AM, McKenna DH, Rocco PRM, Weiss DJ. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cell Transl Med. 2015;4(11):1302–16.
Bakouboula B, Morel O, Faure A, Zobairi F, Jesel L, Trinh A, Zupan M, Canuet M, Grunebaum L, Brunette A, Desprez D, Chabot F, Weitzenblum E, Freyssinet JM, Chaouat A, Toti F. Procoagulant membrane microparticles correlate with the severity of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177(5):536–43.
Aliotta JM, Pereira M, Amaral A, Sorokina A, Igbinoba Z, Hasslinger A, El-Bizri R, Rounds SI, Quesenberry PJ, Klinger JR. Induction of pulmonary hypertensive changes by extracellular vesicles from monocrotaline-treated mice. Cardiovasc Res. 2013;100(3):354–62.
Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601–11.
Matthay MA, Howard JP. Progress in modelling acute lung injury in a pre-clinical mouse model. Eur Respir J. 2012;39(5):1062–3.
Li L, Jin S, Zhang Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int J Clin Exp Med. 2015;8(3):3825–32.
Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929–39.
Maron-Gutierrez T, Silva JD, Asensi KD, Bakker-Abreu I, Shan Y, Diaz BL, Goldenberg RC, Mei SH, Stewart DJ, Morales MM, Rocco PR, Dos Santos CC. Effects of mesenchymal stem cell therapy on the time course of pulmonary remodeling depend on the etiology of lung injury in mice. Crit Care Med. 2013;41(11):e319–33.
Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303(11):L967–77.
Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913–9.
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32(1):116–25.
Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77(1):134–42.
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–93.
Hackstein H, Lippitsch A, Krug P, Schevtschenko I, Kranz S, Hecker M, Dietert K, Gruber AD, Bein G, Brendel C, Baal N. Prospectively defined murine mesenchymal stem cells inhibit Klebsiella pneumoniae-induced acute lung injury and improve pneumonia survival. Respir Res. 2015;16:123.
MacDonald IA, Kuehn MJ. Offense and defense: microbial membrane vesicles play both ways. Res Microbiol. 2012;163(9–10):607–18.
Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martin-Pena R, Gonzalez-Reyes JA, Jimenez-Munguia I, Gomez-Gascon L, Fernandez J, Luque-Garcia JL, Garcia-Lidon C, Estevez H, Pachon J, Obando I, Casadevall A, Pirofski LA, Rodriguez-Ortega MJ. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J Proteomics. 2014;106:46–60.
Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324–36.
Quesenberry PJ, Aliotta JM. The paradoxical dynamism of marrow stem cells: considerations of stem cells, niches, and microvesicles. Stem Cell Rev. 2008;4(3):137–47.
Acknowledgements
The authors would like to express their gratitude to Mrs Moira Elizabeth Schottler, Mr Filippe Vasconcellos, and Mrs Martha McMahan for their assistance in editing the article.
This study was supported by the Brazilian Council for Scientific and Technological Development (CNPq), the Rio de Janeiro State Research Foundation (FAPERJ), the Coordination for the Improvement of Higher Education Personnel (CAPES), and the Department of Science and Technology—Brazilian Ministry of Health (DECIT/MS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no potential competing interests.
Authors’ contributions
SCA, DJW, and PRMR are responsible for the concept of the review. SCA was responsible for writing the first draft of the manuscript. DJW and PRMR were responsible for assisting with study selection. DJW and PRMR were responsible for critical review of the manuscript. All authors read and approved the manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Abreu, S.C., Weiss, D.J. & Rocco, P.R.M. Extracellular vesicles derived from mesenchymal stromal cells: a therapeutic option in respiratory diseases?. Stem Cell Res Ther 7, 53 (2016). https://doi.org/10.1186/s13287-016-0317-0
Published:
DOI: https://doi.org/10.1186/s13287-016-0317-0