Abstract
Introduction
Mesenchymal stem cells (MSCs) are multipotent cells capable of self-renewal and multilineage differentiation. Their multipotential capacity and immunomodulatory properties have led to an increasing interest in their biological properties and therapeutic applications. Currently, the definition of MSCs relies on a combination of phenotypic, morphological and functional characteristics which are typically evaluated upon in vitro expansion, a process that may ultimately lead to modulation of the immunophenotypic, functional and/or genetic features of these cells. Therefore, at present there is great interest in providing markers and phenotypes for direct in vivo and ex vivo identification and isolation of MSCs.
Methods
Multiparameter flow cytometry immunophenotypic studies were performed on 65 bone marrow (BM) samples for characterization of CD13high CD105+ CD45– cells. Isolation and expansion of these cells was performed in a subset of samples in parallel to the expansion of MSCs from mononuclear cells following currently established procedures. The protein expression profile of these cells was further assessed on (paired) primary and in vitro expanded BM MSCs, and their adipogenic, chondrogenic and osteogenic differentiation potential was also determined.
Results
Our results show that the CD13high CD105+ CD45− immunophenotype defines a minor subset of cells that are systematically present ex vivo in normal/reactive BM (n = 65) and that display immunophenotypic features, plastic adherence ability, and osteogenic, adipogenic and chondrogenic differentiation capacities fully compatible with those of MSCs. In addition, we also show that in vitro expansion of these cells modulates their immunophenotypic characteristics, including changes in the expression of markers currently used for the definition of MSCs, such as CD105, CD146 and HLA-DR.
Conclusions
BM MSCs can be identified ex vivo in normal/reactive BM, based on a robust CD13high CD105+ and CD45− immunophenotypic profile. Furthermore, in vitro expansion of these cells is associated with significant changes in the immunophenotypic profile of MSCs.
Similar content being viewed by others
Introduction
Mesenchymal stem cells (MSCs) are nonhematopoietic multipotent stem cells that have the ability for self-renewal and multilineage differentiation [1]. These cells were first described as residing in the bone marrow (BM), which is currently the most extensively studied source for MSCs; however, MSCs have also been successfully isolated from tissues other than BM (e.g., umbilical cord blood [2], the placenta [3], amniotic fluid [4], adipose tissue [5], lung [6], skeletal muscle [7] and the dental pulp [8]). Due to their multipotential capacity [1] and immunomodulatory properties [9, 10], an increasing interest has emerged about the biological properties and the potential clinical application of these cells, as they may represent a potential source for cell-based therapy for tissue repair [11, 12] and for suppressing autoimmunity [13]. In addition, MSCs may also play an important role in the pathogenesis of several diseases, including hematological disorders such as multiple myeloma [14], chronic myeloid leukemia [15] and myelodysplastic syndromes [16, 17].
At present, the identification and definition of MSCs are both based on a combination of phenotypic, morphological and functional characteristics, summarized into three minimal criteria by the International Society for Cellular Therapy (ISCT) [18]. Based on these criteria, MSCs must lack expression of hematopoietic markers (CD19 or cyCD79a, CD11b or CD14, CD45, CD34 and HLA-DR), and show positivity for several other proteins (CD73, CD90 and CD105). Furthermore, MSCs must also be capable of adhering to a plastic surface when maintained in standard culture conditions, and to differentiate to at least three different cell lineages (i.e., osteoblastic, adipocytic and chondrocytic lineages) [18].
Despite the above criteria have contributed to the standardization of MSC studies, the need for in vitro expansion of these cells prior to their characterization has been suggested to potentially modify their immunophenotypic, functional or even genetic features during culture [17, 19–22]; such changes could contribute to explain, at least in part, discrepant results observed in the literature about the characteristics of MSCs [17, 22–27]. Therefore, at present there is a great interest in providing markers for direct in vivo and ex vivo identification and isolation of MSCs [28–31]. In this regard, several studies have identified markers that could be used for positive selection of BM MSCs, such as the nerve growth factor receptor (CD271), the mesenchymal stem cell antigen 1 (MSCA-1), STRO-1, SSEA-4 and the CD13 ectoenzyme [32–34], in addition to CD73, CD90 and CD105. However many of these markers do not provide a clear-cut distinction between MSCs and other BM cells; due to their heterogeneous expression, their overlap with other BM cell populations, or the fact that they have not been systematically used for the identification of MSCs.
In the present study we identified (ex vivo) the CD13high CD105+ CD45– immunophenotype to define a minor population of BM cells that show immunophenotypic, morphologic and functional features which are fully compatible with those of MSCs. Further comparison of the ex vivo immunophenotypic features of such BM MSCs versus those of in vitro expanded MSCs, obtained either through standard isolation procedures or by flow cytometry-based sorting of CD13high CD105+ CD45– cells, showed that the immunophenotypic profile of MSCs undergoes significant changes during in vitro expansion, such changes including downregulation of CD10, CD13 and HLA-DR and upregulation of CD105 and CD146.
Materials and methods
BM samples
A total of 65 freshly obtained ethylenediaminetetraacetic acid (EDTA)- or heparin-anticoagulated normal (n = 9) and reactive (n = 56) BM aspirates were obtained from an identical number of individuals (30 males and 35 females; median age 51 years, range 22–76 years) at the University Hospital of Salamanca, Spain, and the Mast Cell Unit of the Hospital Virgen del Valle, Toledo, Spain. Normal BM samples were obtained from healthy donors, while reactive samples corresponded to patients undergoing BM aspiration due to suspected mastocytosis or other hematological disorders, but who did not exhibit any clonal hematological disease. The study was approved by the local Ethics Committee, Comisión de Bioética del Centro de Investigación del Cáncer—IBMCC (USAL-CSIC). In all cases, informed consent was obtained prior to the study, according to the guidelines of the local Ethics Committee, and all experiments were performed following the Declaration of Helsinki.
Ex vivo multiparameter flow cytometry immunophenotypic studies
Multiparameter flow cytometry (MFC) immunophenotypic studies were performed on BM aspirated samples, processed under sterile conditions within the first 24 hours after they were collected. For ex vivo immunophenotypic characterization of BM MSCs, a direct immunofluorescence stain-and-then-lyse technique was used, as previously described in detail [35]. Eight color combinations of monoclonal antibodies (MoAb) were used to identify and characterize BM MSCs (Additional file 1: Table S1). For each sample, a minimum of 1.5 × 106 cells were analyzed per antibody combination using a FACSCanto II flow cytometer (Becton Dickinson Biosciences, San Jose, CA, USA) equipped with the FACSDiva software (Becton Dickinson). Identification of MSCs was performed based on CD13high and CD105+ expression in the absence of CD45 and high but heterogeneous light scatter features (Fig. 1). For data analysis, the INFINICYT software (Cytognos SL, Salamanca, Spain) was used. Expression of individual markers was recorded both as percentage of positive cells and as median fluorescence intensity (MFI; arbitrary fluorescence units scaled from 0 to 262,000) after subtracting the baseline autofluorescence levels observed for MSCs in the corresponding fluorescence detector.
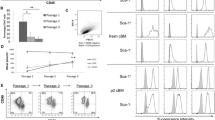
Representative bivariate dot plots and single parameter histograms illustrating the gating strategy and the ex vivo immunophenotypic characteristics of bone marrow CD13high CD105+ CD45– cells. a–h Gating strategy used for the identification of these bone marrow cells based on their unique CD13high CD105+ CD45– and high but heterogeneous light scatter features. i–t Immunophenotypic features of bone marrow CD13high CD105+ CD45– cells from a representative healthy donor. Baseline autofluorescence and expression levels for each protein are indicated in the histogram plot in gray and black, respectively, using the overlay histogram function of the Infinicyt software. SSC sideward light scatter, MFI median fluorescence intensity (arbitrary units scaled from 0 to 262,000)
MSC isolation, expansion and culture
Isolation and expansion of MSCs was performed on 14 heparin-anticoagulated BM samples. Prior to mononuclear cell (MNC) isolation, autologous BM plasma was obtained by centrifugation for (10 minutes at 1200 g). Low density BM MNC were then isolated using a Biocoll density gradient centrifugation step (Biochrom AG, Berlin, Germany). Afterwards, MNC were washed and resuspended in phosphate-buffered saline (PBS) supplemented with 10 % fetal bovine serum (FBS; Gibco, Life Technologies, Paisley, UK). To isolate CD13high CD105+ CD45– cells (i.e., MSC fraction), a four-way fluorescence-activated cell sorter (FACSAria, Becton Dickinson) equipped with the FACSDiva software was used. Before sorting, cells were stained with the CD45 pacific orange (PacO)/CD105 fluorescein isothiocyanate (FITC)/CD73 phycoerythrin (PE)/CD34 peridinin chlorophyll protein (PerCP)–cyanin 5.5 (Cy5.5)/CD117 PE–cyanin 7 (Cy7)/CD13 allophycocyanin (APC) combination of MoAbs for 30 minutes at 4 °C in the dark. Based on this MoAb combination, MSCs were identified and sorted as SSChi CD13high CD73+ CD105+ CD45– CD34– CD117– cells with a purity systematically ≥95 %. In addition, those cells which did not meet the phenotypic criteria for CD13high CD105+ CD45– cells (i.e., non-MSCs) were also isolated; in this later fraction (non-MSC cell fraction) no contamination (<0.01 %) by cells phenotypically compatible with MSCs was detected. The sorted non-MSC and MSC fractions were separately resuspended in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, Steinheim, Germany) supplemented with 20 % autologous plasma, L-glutamine 2 mM and 1 % penicillin-streptomycin (Gibco), and plated in six-well culture plates (Corning Inc., Corning, NY, USA). In parallel, isolation of MSCs using conventional MSC expansion after in vitro culture was also performed, as described elsewhere [1, 36]. Briefly, MNCs were resuspended in DMEM medium supplemented with 15 % FBS, L-glutamine 2 mM and 1 % penicillin-streptomycin, and plated in six-well culture plates at a concentration of 1 × 106 cells/well. Culture plates were maintained in a humidified atmosphere with 5 % CO2 at 37 °C. After 4 days of cell culture (for both purified MSCs and MNCs), nonadherent cells were removed and fresh medium (DMEM plus 15 % FBS, L-glutamine 2 mM and 1 % penicillin-streptomycin) was added; for the sorted non-MSC fraction, cultured cells were washed and the culture medium replaced. These steps were then repeated every 2 days until the culture reached 80 % cell confluence. At this moment, nonadherent cells were discarded while adherent cells were detached with 0.05 % trypsin/EDTA (Gibco) and plated again in two or three culture flasks. This later procedure was repeated every 2 days, three times, in order to ensure that contaminating hematopoietic cells had been washed out of the cell culture.
Adipogenic, chondrogenic and osteogenic in vitro differentiation of MSCs
In order to fulfill the ISCT criteria for the definition of MSCs [18], adipogenic, osteogenic and chondrogenic differentiation was performed on both sorted MSCs and MNC-derived MSCs, as previously described [17, 37]. Briefly, MSCs were resuspended and plated in a 24-well culture plate at a concentration of 1 × 104 cells/well with either conditioned (differentiation) medium or with standard growth medium (DMEM plus 15 % FBS, L-glutamine 2 mM and 1 % penicillin-streptomycin); fresh medium was added every 2 days. To induce adipogenic differentiation, cells were incubated for 8 days with the STEMPRO® Adipogenesis Differentiation Kit medium (Invitrogen Life Science, Grand Island, NY, USA), according to the manufacturer’s instructions. The presence of neutral lipids was visualized by standard staining with Oil Red O (Sigma-Aldrich, St. Louis, MO, USA) [38]. In parallel, cells were also grown in osteogenic induction medium (STEMPRO® Osteogenesis Differentiation Kit medium (Invitrogen Life Sciences)) to induce osteogenic differentiation. Detection of osteoblasts was performed both after 8 days of culture using alkaline phosphatase staining [39], and after 11 days of culture through demonstration of the presence of calcium deposits using the alizarin red S (Sigma-Aldrich) staining [40]. To induce chondrogenesis, 2.5 × 104 cells were placed in a 15-ml polypropylene tube and centrifuged (150 g for 10 minutes) in order to form a pelleted cellular micromass at the bottom of the tube. The cell pellet was cultured in 500 μl chondrogenic induction medium (STEMPRO® Chondrogenesis Differentiation Kit medium) following the recommendations of the manufacturer. Fresh chondrogenic differentiation medium was added every 2 days; after 27 days, the micromass was fixed, embedded in paraffin, cut in a microtome and stained with toluidine blue (Sigma-Aldrich) [41].
Multiparameter flow cytometry immunophenotypic studies of in vitro expanded MSCs
For the assessment of the phenotype of in vitro expanded MSCs (i.e., the expression of those proteins previously evaluated on ex vivo MSCs) a direct immunofluorescence technique was used as previously described in detail [42]. Briefly, 2 × 105 cells were stained (30 minutes at 4 °C in the dark) with eight-color combinations of MoAbs (Additional file 1: Table S1); stained cells were subsequently measured in a FACSCanto II flow cytometer equipped with the FACSDiva software.
Statistical analyses
For all continuous variables, their median, mean, standard deviation, range and both the 25th and 75th, and the 10th and 90th percentiles, were calculated; for categorical variables, frequencies were reported. Statistical significance (P value ≤0.05) was determined by the non-parametric Kruskal-Wallis and Mann-Whitney U tests (for continuous variables in case of independent samples) or the Wilcoxon's and Friedman nonparametric tests for paired samples. For all statistical analyses the SPSS 18.0 software (SPSS, Chicago, IL, USA) was used.
Results
Frequency and ex vivo immunophenotypic features of CD13high CD105+ CD45– cells in normal and reactive BM samples
Overall, a CD13high CD105+ CD45– cell population was identified in every BM sample analyzed. Such cells were present at relatively low frequencies (median 0.028 %; range 0.0004–0.61 %) in all BM samples studied. Overall, they showed high but heterogeneous light scatter features, associated with lack of expression of early hematopoietic cell markers (CD34–, CD45–, CD117–), monocyte–macrophage related proteins (CD11b–, CD14–) and B cell lineage markers (CD19–) (Table 1 and Fig. 1). Conversely, expression of classical MSC-related markers (CD73, CD90, CD105 and CD146) was systematically observed in this cell compartment for all BM samples analyzed. Despite the above markers being detected in the whole CD13high CD105+ CD45– cell population (100 % of the cells; Table 1), different expression levels for individual antigens were observed for distinct markers; thus, CD146 was typically dimly expressed, whereas the pattern of expression of CD90 was heterogeneous among different samples (normalized MFI ranging from 1870 to 260,738 arbitrary fluorescence units), as well as among cells from individual samples (Fig. 1). Expression of CD105 and CD73 was also detected in 100 % of the cells with homogeneously positive-to-moderate positive expression levels (Fig. 1). Reactivity for CD10 and HLA-DR were heterogeneous and typically restricted to only a subset of the whole CD13high CD105+ CD45– cell population (62 % to 97 % and 50 % to 100 % of the CD13high CD105+ CD45– cells, respectively). Expression of other, more recently described, MSC-associated markers (nerve growth factor receptor (CD271), MSCA-1, SSEA-4 and STRO-1) was also assessed in a smaller subset of samples (n = 5). In these samples, co-expression of CD271, MSCA-1, SSEA-4 and STRO-1 was also systematically observed on CD13high CD105+ CD45– cells (Additional file 2: Figure S1). Nevertheless, whereas CD271 and MSCA-1 were strongly expressed on these cells, expression of STRO-1 was heterogeneous and SSEA-4 was only dimly expressed (Additional file 2: Figure S1).
Of note, with the exception of the expression of CD90, which was significantly decreased in reactive (versus normal) samples, no differences were observed between normal and reactive BM MSCs for all other markers analyzed (Table 2).
In vitro expansion, and osteogenic, adipogenic, and chondrogenic differentiation of CD13high CD105+ CD45– cells
Since the CD13high CD105+ CD45– BM cell population showed immunophenotypic features which were compatible with those of MSCs [18], we aimed to determine whether these cells also displayed the minimal functional criteria for MSCs (adherence to plastic and multipotent differentiation potential to the osteogenic, adipogenic and chodrogenic cell lineages), compared with donor-matched MSCs isolated from the same BM by the conventional method of density gradient purification followed by plastic adherence. In order to demonstrate that the CD13high CD105+ CD45– BM cell fraction contained virtually all BM MSCs, the negative BM cell fraction corresponding to all BM cells depleted on the CD13high CD105+ CD45– cell population was also cultured in parallel. Overall, both the FACS-sorted CD13high CD105+ CD45− cells and conventional in vitro cultured MSCs showed a similar ability to adhere to plastic; in addition, no significant differences were found regarding the growth rate of the two cells fractions (Fig. 2). In contrast, no adherent cells were observed in the BM cell fraction depleted from CD13high CD105+ CD45− cells at the time when the former two cell populations reached an 80 % confluent layer (passage 1).
Growth kinetics (a) and immunophenotypic features (b–h) of mesenchymal stem cells (MSCs) obtained from BM mononuclear cells (MNCs) using a standard protocol for in vitro MSC expansion compared to the growth kinetics of FACS-sorted and in vitro cultured CD13high CD105+ CD45– BM cells (identified as CD13high cells). a Bars and vertical lines correspond to median ± 95 % confidence interval values. b–h Notched boxes extend from the 25th to 75th percentile values; the lines in the middle and vertical lines correspond to median values and both the 10th and 90th percentiles, respectively. Normalized median fluorescence intensity (MFI) values are displayed after subtracting the background autofluorescence levels observed for each individual marker-associated fluorochrome channel (arbitrary units scaled from 0 to 262,000). NS not significant (P > 0.05)
The multipotent differentiation potential of both FACS-sorted CD13high CD105+ CD45– cells and MNC-cultured MSCs was evaluated in parallel in five different samples. Both fractions showed a normal osteogenic, adipogenic and chondrogenic differentiation after three passages, as assessed via: 1) determination of the alkaline phosphatase activity and the detection of extracellular calcium deposits using Alizarin Red staining (Fig. 3, panels II–III) after 8 and 11 days of culture with conditioned medium, respectively; 2) the Oil Red O staining for detection of lipid droplets after 8 days of culture with conditioned medium (Fig. 3, panel IV) and; 3) staining for cartilaginous extracellular matrix performed with toluidine blue after 27 days of culture with conditioned medium (Fig. 3, panel V).
The functional features of mesenchymal stem cells (MSCs) observed in vitro after culture of Biocoll isolated mononuclear cells (MNCs) with conventional standard plastic adherence methods (left panels) versus FACS-purified and cultured CD13high CD105+ CD45– BM cells (right panels). Panels I a and I b show undifferentiated and unstained MSCs cultured for 24 days (passage 3). Panels II through V display cells which have been induced to differentiate in vitro towards the osteogenic (Panels II and III), adipogenic (Panels IV) and chondrogenic (Panels V) differentiation pathways. Osteogenic differentiation was assessed using alkaline phosphatase (Panel II) and alizarin red S (Panel III) stainings at days +8 and +11, respectively; adipogenesis was determined using the oil red O staining at day +8 (Panel IV), and chondrogenesis was evaluated by toluidine blue staining at day +27 (Panel V). For each staining technique in Panels II–IV, undifferentiated controls counterstained with hematoxylin are also displayed (Panels a and c)
Immunophenotypic features of ex vivo and in vitro expanded FACS-sorted CD13high CD105+ CD45− cells versus MSCs obtained using conventional cell culture methods
Once compared to donor-matched MSCs isolated from BM MNCs by conventional plastic adherence methods, in vitro expanded FACS-sorted CD13high CD105+ CD45– cells showed similar immunophenotypic features. Thus, they displayed similar levels of CD10, CD13, CD73, CD90, CD105 and CD146, and at the same time both cultured cell populations lacked CD11b, CD14, CD19, CD34, CD45, CD117 and HLA-DR expression. In contrast, when FACS-purified and in vitro cultured CD13high CD105+ CD45– cells were compared with donor-matched (recently obtained, noncultured) ex vivo CD13high CD105+ CD45– BM cells, the former showed significantly higher levels of CD105 (P = 0.03) and CD146 (P ≤ 0.04) expression (Fig. 2f and g). In contrast, expression of both CD10 and CD13, despite remaining positive, was significantly decreased (P ≤ 0.04) after in vitro culture of CD13high CD105+ CD45– cells (Fig. 2b and c). Interestingly, expression of HLA-DR, which was detected on ex vivo BM CD13high CD105+ CD45– cells, was not detected on either of the two in vitro expanded donor-matched MSC populations (P = 0.01) (Fig. 2h).
Discussion
In recent years, growing interest has emerged about MSCs due to their unique biological properties (e.g., their low immunogenicity, their immunomodulatory properties, availability, self-renewal and differentiation capacities) [1, 9, 43, 44] which make them a good candidate for cell therapy, as well as their involvement in the pathogenesis of several diseases [14, 15, 17]. However, the need for in vitro expansion of MNC fractions of BM cells for the definition of MSCs (which may affect the natural properties of these cells) together with the lack of (universally accepted) specific antigens and phenotypes for their ex vivo identification and positive isolation, has led to a growing interest in the identification of potential markers/phenotypes and the establishment of specific strategies for accurate in vivo and ex vivo identification of MSCs [28, 30]. In recent years, important advances have been achieved in this regard and several markers such as CD271 and MSCA-1 have been identified which can be used for purification of MSCs [32, 45–47]. However, some of these markers are dimly expressed and do not provide a sufficient definition of MSCs versus other cells in the sample (e.g., SSEA-4 or CD146) and/or their expression is not restricted to MSCs (e.g., STRO-1 is also expressed by nucleated erythroid cells) [32–34]. In addition, other markers have been found to be associated to, but not specific of, MSCs such as CD13, CD105 and CD90 [33, 34]. Here we show that the CD13high CD105+ CD45– immunophenotype defines a unique minor subset of BM cells that display MSC features which can be detected ex vivo on fresh BM aspirated samples prior to cell culture. Furthermore, we show that in vitro expansion of these cells modulates their immunophenotypic characteristics including the expression of markers currently used in the definition of MSCs; these results reinforce the need for a more detailed ex vivo characterization of MSCs.
In this study, CD13high CD105+ CD45– cells were systematically present in all normal/reactive BM samples analyzed. In the BM samples studied, these cells represented a minor cell compartment which displayed a unique and clearly different phenotype, distinct from that of all BM cell populations described so far. Therefore, these cells showed features distinct from those of B cells (CD19–), monocyte/macrophage lineage cells (CD11b–, CD14–), erythroid precursor cells (CD13hi, CD73+/++, CD90+/+++), hematopoietic precursor cells (CD34–, CD117–) and endothelial cells (CD34–), also lacking the expression of pan-leukocyte markers (CD45–) [18]. Further characterization of these cells revealed immunophenotypic features previously described to be associated with MSCs (e.g., CD10, CD73, CD90, CD146, CD271, MSCA-1, SSEA-4 and STRO-1 expression) [1, 18]. In fact, the immunophenotypic pattern of these CD13high CD105+ CD45– BM cells is fully compatible with the widely described MSC profile, except for the expression of HLA-DR which was found to be systematically expressed in all or a substantial fraction of all ex vivo studied CD13high CD105+ CD45– BM cells. Of note, HLA-DR has been previously reported to be absent on MSCs unless they had been stimulated (e.g., by interferon-γ) [18, 48]. CD13high CD105+ CD45– cells were isolated using a large set of markers, defined upon their ex vivo characterization in a large number of BM samples, in order to ensure a high cell purity. Based on this, sorted CD13high CD105+ CD45– BM cells showed similar growth kinetics, plastic adherence ability and osteogenic, adipogenic and chondrogenic differentiation potential as compared to those exhibited by donor-matched MSCs isolated using conventional in vitro culture methods. These results suggest that, in fact, this minor ex vivo BM CD13high CD105+ CD45– cell population corresponded to multipotent MSCs. In contrast, no adherent cells with MSC functions were observed in the BM cell fraction depleted on this specific minor cell population. These findings suggest that the CD13high CD105+ CD45– phenotype would identify the overall MSC population. Alternatively, MSCs could consist of a heterogeneous cell population which includes a pool of different cells with distinct proliferation and differentiation capacities [32], the MSCs not fulfilling the CD13high CD105+ CD45– criteria potentially displaying a lower proliferation and/or plastic adherence potential or being highly diluted and, consequently, requiring more time and/or distinct culture conditions to expand.
Of note, when immunophenotypic studies were repeated on expanded MSC fractions, no differences were observed between donor-matched in vitro cultured MSCs which had been isolated from the BM by the conventional density gradient (e.g. Biocoll) purification followed by plastic adherence method and in vitro expanded CD13high CD105+ CD45– (FACS-purified) cells. Interestingly, both in vitro expanded cell populations lacked expression of HLA-DR (previously detected on the CD13high CD105+ CD45– cells from all samples studied ex vivo), suggesting the ex vivo identified CD13high CD105+ CD45– cells in fact fulfill all minimal criteria for the definition of MSCs, since such immunophenotypic criteria were established on cultured cells [18]. In addition, since HLA-DR is known to be expressed on in vitro expanded MSCs upon activation [48], it could be argued that, for example, the BM extraction process could lead to activation of these cells, which could “return” to a resting state upon stabilization in culture. However, when both in vitro expanded fractions of MSCs were compared with their donor-matched ex vivo CD13high CD105+ CD45– cell population, for the remaining MSC markers, significantly higher expression levels were detected for CD105 and CD146 among the cultured cells, in association with lower CD10 and CD13 expression. These immunophenotypic differences may contribute to explain the lack of consensus regarding the antigenic profile (and also morphological characteristics) of BM MSCs in vivo, and at the same time they would support previous results suggesting in vitro modulation of the expression of several MSC-related markers (e.g., HLA-DR or CD146) after long-term cell culture [20, 21], particularly when different culture conditions are used for MSC expansion (e.g., CD10, CD73, CD90, CD105, CD146) [44, 49], or the processes associated with cell adherence and growth on plastic (e.g., CD44, CD34) varies [28, 50]. Such in vitro antigen expression-associated changes may potentially contribute to explain also some of the discrepancies reported in the literature as regards the genetic, phenotypic and functional properties of in vitro cultured MSCs [17, 22–27]. Consequently, the here described CD13high CD105+ CD45– MSC-specific phenotype may contribute to a more accurate and standardized ex vivo characterization of MSCs, and a better understanding of the in vivo biological and functional features of this cell population, therefore contributing also to establish more accurately the normal versus altered MSC immunophenotypic transcriptional and functional profiles in different disease conditions. Further studies, in larger series of BM samples from patients with distinct disease conditions, are necessary to fully characterize these cells and to determine whether the expression of CD13, CD105 and CD45 is stable enough to be used as a backbone combination for the identification and characterization of BM MSCs in patients with distinct hematological malignancies and other hematological and nonhematological disorders.
Conclusions
In summary, here we demonstrate for the first time that BM MSCs can be identified ex vivo in normal/reactive BM, based on a robust CD13high CD105+ and CD45– inmunophenotypic profile. In addition, we also show that in vitro expansion of these cells is associated with significant changes in the immunophenotypic profile of MSCs. Further studies are needed for more detailed ex vivo characterization of these cells as a basis for the identification of altered genotypes and phenotypes in BM MSCs from patients diagnosed with different disease conditions, such as myeloid and lymphoid malignancies.
Abbreviations
- APC:
-
Allophycocyanin
- BM:
-
Bone marrow
- Cy5.5:
-
Cyanin 5.5
- Cy7:
-
Cyanin 7
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- EDTA:
-
Ethylenediaminetetraacetic acid
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- ISCT:
-
International Society for Cellular Therapy
- MFC:
-
Multiparameter flow cytometry
- MFI:
-
Median fluorescence intensity
- MNC:
-
Mononuclear cell
- MoAb:
-
Monoclonal antibodies
- MSC:
-
Mesenchymal stem cell
- PacO:
-
Pacific orange
- PBS:
-
Phosphate-buffered saline
- PE:
-
Phycoerythrin
- PerCP:
-
Peridinin chlorophyll protein
References
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7.
Kim HS, Shin TH, Yang SR, Seo MS, Kim DJ, Kang SK, et al. Implication of NOD1 and NOD2 for the differentiation of multipotent mesenchymal stem cells derived from human umbilical cord blood. PLoS One. 2010;5:e15369.
Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy. 2004;6:543–53.
Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450–6.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28.
Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest. 2007;117:989–96.
Gao X, Usas A, Tang Y, Lu A, Tan J, Schneppendahl J, et al. A comparison of bone regeneration with human mesenchymal stem cells and muscle-derived stem cells and the critical role of BMP. Biomaterials. 2014;35:6859–70.
Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836–42.
Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–32.
Sempere JM, Martinez-Peinado P, Arribas MI, Reig JA, De La Sen ML, Zubcoff JJ, et al. Single cell-derived clones from human adipose stem cells present different immunomodulatory properties. Clin Exp Immunol. 2014;176:255–65.
Psaltis PJ, Carbone A, Nelson AJ, Lau DH, Jantzen T, Manavis J, et al. Reparative effects of allogeneic mesenchymal precursor cells delivered transendocardially in experimental nonischemic cardiomyopathy. JACC Cardiovasc Interv. 2010;3:974–83.
Lee JW, Gupta N, Serikov V, Matthay MA. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther. 2009;9:1259–70.
Uccelli A, Prockop DJ. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr Opin Immunol. 2010;22:768–74.
Garcia-Gomez A, Sanchez-Guijo F, Del Canizo MC, San Miguel JF, Garayoa M. Multiple myeloma mesenchymal stromal cells: contribution to myeloma bone disease and therapeutics. World J Stem Cells. 2014;6:322–43.
Chandia M, Sayagues JM, Gutierrez ML, Chillon ML, Aristizabal JA, Corrales A, et al. Involvement of primary mesenchymal precursors and hematopoietic bone marrow cells from chronic myeloid leukemia patients by BCR-ABL1 fusion gene. Am J Hematol. 2014;89:288–94.
Santamaria C, Muntion S, Roson B, Blanco B, Lopez-Villar O, Carrancio S, et al. Impaired expression of DICER, DROSHA, SBDS and some microRNAs in mesenchymal stromal cells from myelodysplastic syndrome patients. Haematologica. 2012;97:1218–24.
Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, Robledo C, Villaron EM, Hernandez-Campo P, et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009;23:664–72.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7.
Bogdanova A, Berzins U, Nikulshin S, Skrastina D, Ezerta A, Legzdina D, et al. Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J Stem Cells. 2014;9:135–48.
Dighe PA, Viswanathan P, Mruthunjaya AK, Seetharam RN. Effect of bFGF on HLA-DR expression of human bone marrow-derived mesenchymal stem cells. J Stem Cells. 2013;8:43–57.
Yu Y, Park YS, Kim HS, Kim HY, Jin YM, Jung SC, et al. Characterization of long-term in vitro culture-related alterations of human tonsil-derived mesenchymal stem cells: role for CCN1 in replicative senescence-associated increase in osteogenic differentiation. J Anat. 2014;225:510–8.
Zhao ZG, Xu W, Yu HP, Fang BL, Wu SH, Li F, et al. Functional characteristics of mesenchymal stem cells derived from bone marrow of patients with myelodysplastic syndromes. Cancer Lett. 2012;317:136–43.
Blau O, Baldus CD, Hofmann WK, Thiel G, Nolte F, Burmeister T, et al. Mesenchymal stromal cells of myelodysplastic syndrome and acute myeloid leukemia patients have distinct genetic abnormalities compared with leukemic blasts. Blood. 2011;118:5583–92.
Flores-Figueroa E, Arana-Trejo RM, Gutierrez-Espindola G, Perez-Cabrera A, Mayani H. Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk Res. 2005;29:215–24.
Flores-Figueroa E, Montesinos JJ, Flores-Guzman P, Gutierrez-Espindola G, Arana-Trejo RM, Castillo-Medina S, et al. Functional analysis of myelodysplastic syndromes-derived mesenchymal stem cells. Leuk Res. 2008;32:1407–16.
Mafi P, Hindocha S, Mafi R, Griffin M, Khan WS. Adult mesenchymal stem cells and cell surface characterization - a systematic review of the literature. Open Orthop J. 2011;5:253–60.
Soenen-Cornu V, Tourino C, Bonnet ML, Guillier M, Flamant S, Kotb R, et al. Mesenchymal cells generated from patients with myelodysplastic syndromes are devoid of chromosomal clonal markers and support short- and long-term hematopoiesis in vitro. Oncogene. 2005;24:2441–8.
Hall SR, Jiang Y, Leary E, Yavanian G, Eminli S, O'Neill DW, et al. Identification and isolation of small CD44-negative mesenchymal stem/progenitor cells from human bone marrow using elutriation and polychromatic flow cytometry. Stem Cells Transl Med. 2013;2:567–78.
Jones EA, English A, Kinsey SE, Straszynski L, Emery P, Ponchel F, et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70:391–9.
Martins AA, Paiva A, Morgado JM, Gomes A, Pais ML. Quantification and immunophenotypic characterization of bone marrow and umbilical cord blood mesenchymal stem cells by multicolor flow cytometry. Transplant Proc. 2009;41:943–6.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34.
Battula VL, Treml S, Bareiss PM, Gieseke F, Roelofs H, de Zwart P, et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94:173–84.
Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–19.
Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells International. 2012;2012:975871.
Sanchez ML, Almeida J, Vidriales B, Lopez-Berges MC, Garcia-Marcos MA, Moro MJ, et al. Incidence of phenotypic aberrations in a series of 467 patients with B chronic lymphoproliferative disorders: basis for the design of specific four-color stainings to be used for minimal residual disease investigation. Leukemia. 2002;16:1460–9.
Amaral AT, Manara MC, Berghuis D, Ordonez JL, Biscuola M, Lopez-Garcia MA, et al. Characterization of human mesenchymal stem cells from ewing sarcoma patients. Pathogenetic implications. PLoS One. 2014;9:e85814.
Garayoa M, Garcia JL, Santamaria C, Garcia-Gomez A, Blanco JF, Pandiella A, et al. Mesenchymal stem cells from multiple myeloma patients display distinct genomic profile as compared with those from normal donors. Leukemia. 2009;23:1515–27.
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301.
Sanchez-Guijo FM, Blanco JF, Cruz G, Muntion S, Gomez M, Carrancio S, et al. Multiparametric comparison of mesenchymal stromal cells obtained from trabecular bone by using a novel isolation method with those obtained by iliac crest aspiration from the same subjects. Cell Tissue Res. 2009;336:501–7.
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13.
Peran M, Ruiz S, Kwiatkowski W, Marchal JA, Yang SL, Aranega A, et al. Activin/BMP2 chimeric ligands direct adipose-derived stem cells to chondrogenic differentiation. Stem Cell Res. 2013;10:464–76.
Domingues PH, Teodosio C, Ortiz J, Sousa P, Otero A, Maillo A, et al. Immunophenotypic identification and characterization of tumor cells and infiltrating cell populations in meningiomas. Am J Pathol. 2012;181:1749–61.
Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon SJ, et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446:983–9.
Carrancio S, Lopez-Holgado N, Sanchez-Guijo FM, Villaron E, Barbado V, Tabera S, et al. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp Hematol. 2008;36:1014–21.
Attar A, Ghalyanchi Langeroudi A, Vassaghi A, Ahrari I, Maharlooei MK, Monabati A. Role of CD271 enrichment in the isolation of mesenchymal stromal cells from umbilical cord blood. Cell Biol Int. 2013;37:1010–5.
Kuci Z, Seiberth J, Latifi-Pupovci H, Wehner S, Stein S, Grez M, et al. Clonal analysis of multipotent stromal cells derived from CD271+ bone marrow mononuclear cells: functional heterogeneity and different mechanisms of allosuppression. Haematologica. 2013;98:1609–16.
Kim YH, Yoon DS, Kim HO, Lee JW. Characterization of different subpopulations from bone marrow-derived mesenchymal stromal cells by alkaline phosphatase expression. Stem Cells Dev. 2012;21:2958–68.
Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36:350–9.
Hagmann S, Moradi B, Frank S, Dreher T, Kammerer PW, Richter W, et al. Different culture media affect growth characteristics, surface marker distribution and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. BMC Musculoskelet Disord. 2013;14:223.
Lin CS, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14:1159–63.
Acknowledgments
This work was supported by grants from the Instituto de Salud Carlos III, FEDER, Ministry of Economy and Competitivity, Madrid, Spain (grant PI11/02399; RETICEF RD12/0043/0021; RTICC RD12/0036/0048); Fundación Ramon Areces, Madrid, Spain (grant CIVP16A1806); Fundación Científica de la Asociación Española Contra el Cáncer (AECC); Consejería de Sanidad, Gerencia Regional de Salud de Castilla y León (SACYL) (grant BIO/SA24/13) and Fundación Samuel Solórzano Barruso (University of Salamanca, Spain); CT was supported by a grant co-financed by the European Social Fund and the Junta de Castilla y León (Spain). The authors would like to thank the Compared Molecular Pathology Unit from the Cancer Research Center (CSIC-USAL) for their technical assistance regarding the staining procedures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
CT and CM performed experiments, analyzed/interpreted results, designed the figures and wrote the paper. AM and ATA performed experiments, analyzed/interpreted results and critically reviewed the paper. PB and MLS performed the cell sorting of the distinct cellular subpopulations and critically reviewed the paper. SM and ACG-M analyzed/interpreted results and critically reviewed the paper. IA-T, MD-C and JFB performed the clinical follow-up of the individuals analyzed in this study and critically reviewed the paper. AO, MCC and JdPM designed the research, supervised the study and wrote the paper. All authors read and approved the final manuscript.
Carmen Muñiz and Cristina Teodosio are both considered as first authors.
Javier del Pino Montes and Alberto Orfao are both considered as last authors.
Carmen Muñiz Cristina Teodosio Javier del Pino Montes and Alberto Orfao contributed equally to this work.
Additional files
Additional file 1: Table S1.
List of antibodies used for the identification, characterization and isolation of bone marrow mesenchymal stem cells, including CD13high CD105+ and CD45– cells. (DOC 41 kb)
Additional file 2: Figure S1.
Representative bivariate dot plots and single parameter histograms showing the ex vivo expression profile of widely-accepted markers (CD271, MSCA-1, SSEA-4 and STRO-1) on bone marrow CD13high CD105+ CD45– cells. Panels A through C show the gating strategy used for the identification of these bone marrow cells based on their unique CD13high CD105+ CD45– features. Panels D through G illustrate the immunophenotypic features of bone marrow CD13high CD105+ CD45– cells from a representative healthy donor. Panel H summarizes the expression of CD271, MSCA-1, SSEA-4 and STRO-1 on CD13high CD105+ CD45– cells from five healthy donors. Baseline autofluorescence and expression levels for each protein are indicated in the histogram plot in gray and black, respectively, using the overlay histogram function of the Infinicyt software. On panel H the open circles represent individual samples, short horizontal and vertical lines display median values and the 95 % confidence interval of the levels of antigen expression (MFI values), respectively. SSC sideward light scatter, MFI median fluorescence intensity (arbitrary units scaled from 0 to 262,000). (TIFF 1440 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Muñiz, C., Teodosio, C., Mayado, A. et al. Ex vivo identification and characterization of a population of CD13high CD105+ CD45− mesenchymal stem cells in human bone marrow. Stem Cell Res Ther 6, 169 (2015). https://doi.org/10.1186/s13287-015-0152-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-015-0152-8