Abstract
Background
Desmoid tumors are extremely rare borderline benign and malignant tumors that do not exhibit accumulation on fluorodeoxyglucose positron emission tomography–computed tomography. In the present study, we report a rare case of a desmoid tumor with fluorodeoxyglucose accumulation at the anastomotic postoperative gastric cancer site.
Case presentation
A 68-year-old Japanese man underwent robot-assisted laparoscopic distal gastrectomy for early-stage gastric cancer in 2019. The pathological diagnosis was stage IA cancer, and no adjuvant chemotherapy was administered. Two years after surgery, a soft mass appeared on the greater curvature side of the anastomosis on computed tomography. Fluorodeoxyglucose positron emission tomography–computed tomography revealed fluorodeoxyglucose accumulation, which suggested a malignancy; therefore, surgery was performed for diagnostic treatment. The histopathological findings led to the diagnosis of a desmoid tumor. The patient has not experienced recurrence to date.
Conclusions
In the present study, we encountered a desmoid tumor arising from the anastomotic site of a postoperative gastric cancer. This case is rare as fluorodeoxyglucose positron emission tomography–computed tomography showed fluorodeoxyglucose accumulation in the desmoid tumor, and a preoperative diagnosis could not be reached. We hope that further studies will improve the accuracy of preoperative diagnosis.
Similar content being viewed by others
Background
Desmoid tumors are extremely rare benign or malignant borderline tumors, and mechanical stimuli such as surgery are considered triggers for their development. Preoperative diagnosis with standard imaging modalities is difficult, and desmoid tumors do not exhibit any accumulation of fluorodeoxyglucose on positron emission tomography–computed tomography (FDG-PET/CT). In the present study, we report the case of a desmoid tumor with fluorodeoxyglucose (FDG) accumulation at the anastomotic site following robot-assisted laparoscopic distal gastrectomy, along with a review of the literature.
Case presentation
A 68-year-old Japanese man was found to have an extensive type 0–IIc lesion in the upper gastric body extending up to the hypogastric lesser curvature on upper gastrointestinal endoscopy in September 2019. Biopsy revealed a poorly differentiated adenocarcinoma; the patient was referred to our department for surgery. He had hypertension and hyperlipidemia. However, he had no family history of malignancy. The patient underwent robot-assisted laparoscopic distal gastrectomy, D1+ lymph node dissection, and Roux-en-Y reconstruction in November 2019. The patient had a good postoperative course and was discharged 6 days postoperatively.
The initial surgical specimen findings were as follows (Fig. 1): a 50 × 35-mm 0–IIc lesion with shallow depression ranging from the upper gastric body to the hypogastric lesser curvature. Histopathological examination revealed a poorly differentiated adenocarcinoma with negative resection margins that were staged IA (pT1aN0M0) according to the Union for International Cancer Control (UICC) TNM Classification of Malignant Tumors, 8th edition.
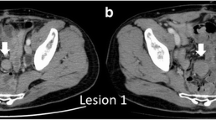
The patient was followed-up without adjuvant chemotherapy; however, a 38 × 36 mm soft tissue mass was noted on the greater curvature side of the residual stomach during computed tomography (CT) 2 years after the procedure. Although partial thickening of the gastric wall was seen retrospectively in a 1-year postoperative CT scan, it was reviewed within normal limits by a radiologist at that time (Fig. 2). No evidence of recurrent metastasis was found at other sites.
The blood test findings were as follows: carcinoembryonic antigen (CEA), 1.8 ng/mL; carbohydrate antigen 19-9 (CA19-9), 8.5 U/mL; and soluble IL-2 receptor, 396 U/mL. Colonoscopy had been performed prior to the first surgery; hence, it was not performed again.
The FDG-PET/CT findings were as follows (Fig. 3): The soft tissue mass on the left side of the residual stomach showed moderate FDG accumulation (SUVmax, 5.25). No FDG accumulation was observed in other areas. Malignant lesions, including recurrent gastric cancer and regional lymph node metastasis, were suspected; however, qualitative diagnosis was difficult. No abnormal accumulation indicating recurrence and metastasis occurred at other sites.
Contrast-enhanced magnetic resonance imaging (MRI) of the abdomen revealed the following findings (Fig. 4): the soft tissue mass on the greater curvature side of the residual stomach exhibited a high, heterogenous signal on T2-weighted images and a low signal on T1-weighted images, and contrast imaging revealed a slow contrast effect. Other malignant lesions such as gastric cancer recurrence, lymph node metastasis, and a gastrointestinal stromal tumor (GIST) were considered as possible differential diagnoses in addition to desmoid tumors; however, none of the findings were typical, and qualitative diagnosis was difficult.
Upper gastrointestinal endoscopic findings were as follows (Fig. 5): no mass lesions were present in the remnant stomach. Transgastric ultrasonography revealed a 43 × 30 mm borderline oligo-hypoechoic mass in the splenic hilum, which was biopsied using endoscopic ultrasound-guided fine needle aspiration (EUS-FNA).
The biopsy results were as follows: the tumor contained multiple spindle-shaped cells, and immunostaining for CD34, c-kit, desmin, S-100, and β-catenin was performed to distinguish spindle-shaped tumors; however, no significant positive findings were obtained. The biopsy specimen did not allow us to estimate the histological type of the tumor as it did not conclusively suggest a GIST, smooth myxoid, schwannoma, or desmoid tumor.
Based on the above-mentioned examination results, local recurrence of gastric cancer, lymph node metastasis, a GIST, a smooth myxoid, schwannoma, and a desmoid tumor were regarded as possible differential diagnoses. Although the possibility of a benign tumor remained, a definitive diagnosis could not be made histopathologically, and moderate FDG accumulation could not rule out a malignant lesion; thus, we decided to operate for diagnostic treatment. Preoperative CT revealed a suspicious finding of splenic arteriovenous invasion; therefore, total residual gastrectomy and splenectomy were planned.
The surgical findings were as follows: the abdomen was opened via a median upper abdominal incision. A 50 × 40 mm soft tissue mass originating from the gastrojejunal anastomosis involving the spleen and splenic arteriovenous was observed on the greater curvature side of the anastomosis. Additionally, a partial invasion of the pancreas was observed. The remaining stomach, spleen, splenic artery and vein, and part of the pancreas, including the gastrojejunal anastomosis, along with the soft tissue mass, were removed as a single lump. Regarding reconstruction, only esophago-jejunal anastomosis was performed, sparing previous jejuno-jejunal anastomosis.
The operation time was 279 minutes, and blood loss was 1100 mL; however, no blood transfusion was required. Pathological findings of the resected specimen were as follows (Fig. 6): a 45 × 40 × 40-mm soft tissue mass extending from the muscularis propria to the serosa at the gastrojejunal anastomosis. The tumor was a white, substantial nodule, and its growth involved the gastrojejunal anastomosis. Although fibroblasts and spindle-shaped cells were observed inside the tumor, the Ki-67 index was about 2%, and atypia was not observed. Immunostaining was positive for vimentin, desmin, and β-catenin and negative for α-SMA, S-100, CD34, c-kit, STAT6, estrogen receptor (ER), and progesterone receptor (PgR). Based on the above, a desmoid tumor was diagnosed.
Pathological findings. The arrows show the tumor. Tumor growth involving anastomosis is observed (a). Hematoxylin–eosin staining showing spindle-shaped cell proliferation without atypia (b). Immunostaining is positive for vimentin (c) and β-catenin (d) expression and negative for Ki-67 (e) expression
Postoperative course
The patient developed a postoperative pancreatic fistula that resolved after drainage. He was discharged from the hospital on postoperative day 16 and has not experienced any recurrence for 6 months.
Discussion and conclusions
Desmoid tumors are a type of fibromatosis that are on the borderline between being benign and malignant, with an annual incidence of 2–4 per million people [1]. Pathologically, desmoid tumors are characterized by the proliferation of differentiated fibroblasts, presence of collagen fibers between cells, invasive growth patterns, absence of malignant features, and local recurrence without metastasis [2]. The three types of desmoid lesions are as follows: extra-abdominal, abdominal wall, and intra-abdominal desmoid, and the frequency of their occurrence is 43%, 49%, and 8%, respectively [1]. The etiology of desmoid tumors is unknown; however, genetic familial adenomatous polyposis (FAP), trauma, history of abdominal surgery, and female hormones may be involved [3].
MRI has excellent tissue resolution and is the most useful imaging technique for diagnosing desmoid tumors, with T1-weighted images showing equal signals and T2-weighted images showing high or heterogeneous signals [4]. However, desmoid tumors have no characteristic imaging findings, and making a definitive preoperative diagnosis is considered extremely difficult. FDG-PET/CT has recently been performed in some cases. As a desmoid tumor is a benign tumor, FDG accumulation is generally low. Suzumura et al. have reported 12 cases of intra-abdominal desmoid tumors with FDG uptake on PET/CT. They have reported that only one case showed high accumulation; however, the remaining 11 cases showed only mild accumulation with the maximum standardized uptake value (SUVmax) ranging between 1.95 and 3.9 [5]. The cause of hyperaccumulation of FDG is not clear; however, some reports indicate that SUVmax increased with tumor growth, whereas other reports suggest that SUVmax reflects the efficacy of drug therapy [6, 7]. Therefore, hyperaccumulation of FDG in the present case may suggest that the desmoid tumor tended to increase in size.
As our patient was a postoperative gastric cancer patient, the moderate accumulation on FDG-PET/CT could not be ruled out as a recurrence of gastric cancer or lymph node metastasis. We decided to perform EUS-FNA in this case because some studies have mentioned that preoperative EUS-FNA should have been performed and some have reported that the diagnosis was made using needle biopsy [8, 9]. In Japan, EUS-FNA is not recommended in cystic masses because it can cause peritoneal dissemination; however, in this case, we performed EUS-FNA because of the suspicion of a substantial mass [10]. Although we could only identify spindle-shaped tumors, we could not reach a definitive diagnosis. If the gastric cancer stage had been higher, chemotherapy may have been considered an option due to the possibility of recurrence of gastric cancer. However, in the present case, the stage was so early that recurrence was unlikely and surgery was the only treatment option for diagnostic purposes.
The local recurrence rate of desmoid tumors is extremely high, ranging between 56% and 86% [11]. Therefore, the possibility of recurrence should be considered, and the patient should be carefully monitored.
In the present study, we encountered a desmoid tumor arising from the anastomotic site of a postoperative gastric cancer. The present case is rare in that FDG-PET/CT showed desmoid tumor accumulation, and a preoperative diagnosis could not be made. We hope that further studies will improve the accuracy of preoperative diagnosis.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FDG-PET/CT:
-
Fluorodeoxyglucose positron emission tomography–computed tomography
- CEA:
-
Carcinoembryonic antigen
- CA19-9:
-
Carbohydrate antigen 19-9
- MRI:
-
Magnetic resonance imaging
- GIST:
-
Gastrointestinal stromal tumor
- EUS-FNA:
-
Endoscopic ultrasound-guided fine-needle aspiration
- ER:
-
Estrogen receptor
- PgR:
-
Progesterone receptor
- FAP:
-
Familial adenomatous polyposis
- SUVmax:
-
Maximum standardized uptake value
References
Reitamo JJ, Häyry P, Nykyri E, Saxën E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77:665–73.
Stout AP, Latters R. Tumors of the soft tissues. Atlas of tumor pathology, 2nd series. Fascicle 1. Washington DC: Armed Forces Institute of Pathology; 1967. p. 17–22.
Häyry P, Reitamo JJ, Tötterman S, Hopfner-Hallikainen D, Sivula A. The desmoid tumor. II. Analysis of factors possibly contributing to the etiology and growth behavior. Am J Clin Pathol. 1982;77:674–80.
Taniguchi T, Iwama Y, Sugimori C, Imagunbai T, Fushimi Y, Kawasaki R, et al. Radiologic features of mesenteric desmoid tumor. Jpn J Clin Radiol. 2002;47:1836–42.
Suzumura K, Kondo Y, Okada T, Iimuro Y, Kuroda N, Torii I, et al. A case of an intra-abdominal desmoid tumor with FDG uptake on PET. J Jpn Surg Assoc. 2014;75:573–8.
Basu S, Nair N, Banavali S. Uptake characteristics of fluorodeoxyglucose (FDG) in deep fibromatosis and abdominal desmoids: potential clinical role of FDG-PET in the management. Br J Radiol. 2007;80:750–6.
Mitsuyoshi T, Masataka I, Tsunekazu M, Hirofumi Y, Mitsugu S. A case report of omental desmoid tumor diagnosed preoperatively as local rectal cancer recurrence. JJCS. 2009;34:640–50.
Kohira Y, Yajima K, Iwasaki Y, Yuu K, Oohinata R, Takahashi K, et al. Laparoscopic resection for desmoid tumor arising from the anastomotic site following laparoscopic distal gastrectomy. Jpn J Gastroenterol Surg. 2017;50:630–8.
Masashi I, Masayuki A, Yoshiaki Y, Kenichi S. Two cases of intraabdominal desmoid tumor in which PET/CT contributed to diagnosis. J Jpn Surg Assoc. 2010;71:2722–7.
Kazuhiko S, Yuki H, Yusuke N, Yoshifumi T, Michitaka I, Masaaki K. Current status of endoscopic ultrasound guided fine needle aspiration (EUS-FNA) in Niigata Cancer Center Hospital. J Niigata Cancer Cant Hosp. 2022;60:55–60.
Clark SK, Phillips RK. Desmoids in familial adenomatous polyposis. Br J Surg. 1996;83:1494–504.
Acknowledgements
We thank Yoshifumi Arai and Matsuyoshi Maeda, who are affiliated with the Department of Pathology, Toyohashi Municipal Hospital. Moreover, we would like to thank Editage (www.editage.com) for English language editing.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
KK wrote the initial draft of the manuscript. KH assisted in the preparation of the manuscript. TK, MF, TA, AA, and TY contributed to reviewing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This report did not include experiments on animals or humans. The patient consented to the use of his personal data for the purpose of this case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kawashima, K., Hiramatsu, K., Kato, T. et al. A desmoid tumor with fluorodeoxyglucose accumulation arising from the anastomotic site of postoperative gastric cancer: a case report. J Med Case Reports 16, 423 (2022). https://doi.org/10.1186/s13256-022-03635-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-022-03635-w