Abstract
Introduction
Tuberculosis is a major health problem worldwide. Sudan has high burden of tuberculosis (TB) with a prevalence of 209 cases per 100,000 of the population and it is commonly presented with pulmonary disease but involvement of the gastrointestinal tract is not uncommon. Abdominal tuberculosis comprises about 1–3 % of all cases of tuberculosis and about 12% of extrapulmonary tuberculosis. It involves the ileocecal region, but involvement of stomach and duodenum are rare sites. Here we present an unusual case of gastric outlet obstruction due to gastric tuberculosis.
Case presentation
A 54-year-old Sudanese man presented with a non-bile stain persistent projectile vomiting, and epigastric pain for two years associated with marked loss of weight. There is no fever or cough. He was on antacid, physical examination showed BMI 18 and stable vital signs. He was not pale or jaundiced, there was no cervical lymphadenopathy and chest was clear. Abdominal examination was normal apart of positive succussion splash. The results of haematological tests were normal, ESR was 30 mm/hr, hepatitis B, C and HIV were negative. Upper gastrointestinal endoscopy showed that the stomach was full of fluid and food particles and ulcerated mass in the pylorus extended to the proximal part of the duodenum with severe narrowing of the pylorus. The lesion biopsied and the result revealed active inflammatory cells, cryptitis and multiple lymphoid follicles, no malignancy seen. Sonographic test showed hypodense pyloric mass, enlarged para-aortic and mesenteric lymph nodes and mild pelvic ascites. A computed tomography scan of the abdomen and pelvis showed antral hypodense lesions multiple mesenteric lymphadenopathies peritoneal thickening and ascites. Chest X-ray was normal. Intra-operative findings were dilated stomach and pylorus mass with multiple mesenteric lymph nodes, peritoneal and omental seedlings all over with small nodules on the surface of the liver, gastro-jejunostomy was done. Histopathology confirmed the diagnosis of abdominal tuberculosis. Postoperative event was uneventful. Patient received anti-tuberculous.
Conclusions
Here we presented an unusual case of gastric outlet obstruction due to primary gastric tuberculosis, patient underwent surgery to relief his symptoms and received anti-tuberculous.
Similar content being viewed by others
Introduction
Tuberculosis (TB) is a major health problem worldwide and during 2008 there were 8.9 to 9.9 million reported cases of TB all over the world, most of them in Africa and Asia [1]. Sudan has a high burden of TB with a prevalence of 209 cases per 100,000 of the population and 50,000 incident cases during 2009 [2]. Pulmonary TB accounted for 73.4 % of all patients with TB in Sudan while extrapulmonary TB accounted for 26.6 %. In Sudan, patients with TB usually had less education than average, and more male patients than female patients were infected [3]. Involvement of the gastrointestinal (GI) tract is not uncommon and it often involves the ileocecal region [4]. Abdominal TB comprises approximately 1 to 3 % of all cases of TB and approximately 12 % of extrapulmonary TB [5]. The stomach and duodenum are rare sites for TB and are usually a result of secondary spread from a primary pulmonary disease. A series by Rao et al. reported an incidence of gastroduodenal TB of only 0.5 % [4] and isolated gastric TB without evidence of a lesion elsewhere is even rarer [6]. Duodenal and gastric TB were found in only 1 % of patients with pulmonary TB with associated human immunodeficiency virus (HIV) infection in non-endemic areas; duodenal obstruction due to TB is very rare and needs a high index of suspicions for diagnosis [7]. The pyloric stenosis resulting from TB is even rarer than gastroduodenal TB. This, however, should be considered in patients who come from areas where the disease is endemic [8]. On clinical examination gastric TB resembles peptic ulcer disease or malignancy and it may be difficult to distinguish; the possible routes of infection include direct infection of the mucosa, hematogenous spread, extension from neighboring tuberculous lesion [9] or secondary to pulmonary TB [10]. A few cases of primary gastroduodenal TB were reported in the literature [11]. Primary gastric TB is usually a diagnostic challenge and may mimic peptic ulcer disease and even a neoplasia [12] or other conditions, including inflammatory bowel disease, malignancy and other infectious diseases [13]. The reason for this rarity is attributed to the bactericidal property of gastric acid, scarcity of lymphoid tissue in the gastric wall and intact gastric mucosa of the stomach [14, 15]. Gastroduodenal TB has three forms of presentation: obstruction, upper GI bleeding, and gastric or periampullary mass suggestive of malignancy [4].
Case presentation
A 54-year-old Sudanese man presented with persistent non-bile-stained projectile vomiting and epigastric pain for 2 years associated with marked loss of weight. He had no jaundice, fever or change in bowel habits. He did not have a cough or hemoptysis and his other systems were unremarkable. He had no significant past medical history, no history of TB, HIV infection or diabetes, he was not hypertensive, and there was no family history of a similar condition or TB. He was on antacid medicine; he was not a tobacco smoker and neither was he an alcoholic. A physical examination showed a body mass index (BMI) of 18, normal vital signs, he was not pale or jaundiced, there was no cervical lymphadenopathy and his chest examination was clear. His abdomen was flat, moved with respiration, with no dilated veins, surgical scars or cautery marks and hernia orifices were intact. There was no tenderness, masses, organomegaly or ascites; his succussion splash was positive. The results of hematological tests were normal, his erythrocyte sedimentation rate (ESR) was 30 mm/hour, and hepatitis B, C and HIV were negative.
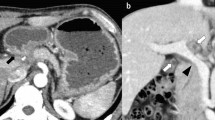
An upper GI endoscopy showed that his stomach was full of fluid and food particles and an ulcerated pyloric mass extended to the proximal part of his duodenum with severe narrowing. Multiple biopsies were taken and histopathology revealed gastric mucosa heavily infiltrated by florid active inflammatory cells disrupting the glands, which consisted of neutrophils, lymphocytes and plasma cells. The glands exhibited cryptitis and regenerative changes with the presence of multiple lymphoid follicles. No Helicobacter pylori, dysplasia or evidence of malignancy was seen. A sonographic test showed a 4.4×2.5 cm hypodense focal soft tissue mass in his pyloric region with enlarged para-aortic and mesenteric lymph nodes, there was minimal pelvic ascites, normal liver and other organs. A computed tomography scan of his abdomen and pelvis showed nodular hypodense lesions measuring 30 mm surrounding the antrum of his stomach with gastric dilatation and multiple mesenteric lymphadenopathies measuring 40 mm. Peritoneal thickening and ascites were also noted, otherwise, he had a normal liver, spleen, pancreas, kidneys, pelvic organs as well as aorta and inferior vena cava (IVC; Figs. 1 and 2), and a normal chest X-ray. A decision was made to relieve the obstruction. Intraoperative findings were: dilated stomach and 8×7 cm mass at the gastric pylorus with multiple mesenteric lymph nodes, and peritoneal and omental seedlings all over with small nodules on the surface of the liver; a gastrojejunostomy was done with multiple biopsies from the mass and the lymph nodes which showed caseating material during dissection (Figs. 3, 4, 5, 6 and 7). The result of histopathology confirmed the diagnosis of abdominal TB (Fig. 8). The patient’s postoperative course was uneventful and he started feeding on day four; he was discharged in good condition. Moreover, he was on serial follow up which showed that he gained weight of more than 1 kg over 20 days. He was referred to the TB eradication program for antituberculous therapy and screening for pulmonary TB which was negative (acid-fast bacilli, AAFB).
Discussion
Although abdominal TB can develop at any age, it is most common in patients between 25 and 45 years of age and females slightly predominate [16]. Patients with gastroduodenal TB can present with obstruction or mass and an endoscopic biopsy has a poor yield [4]. Gastric lesions typically cause dyspeptic complaints, and generally, peptic ulcer is suspected. If the patient has lost weight, in addition to these complaints, gastric cancer should be considered first [17]. Gleason et al. reviewed 49 patients with duodenal TB; they found that the most common presenting symptoms were pain (73 %) and vomiting (55 %), whereas GI bleeding was rare (16 %) [18]. A report by Chetri et al. described a case of gastric TB presenting as non-healing gastric ulcer and out of five cases, three presented with gastric outlet obstruction, which is the most common presentation of gastric TB [19]. It may present as multiple shallow ulcers, especially on the lesser curvature of the stomach [20], or as a nondescript hypertrophic submucosal mass [21]. Another study showed that long-term therapy with H2 blockers increases the incidence of gastroduodenal TB [22]. In investigations of patients, a chest X-ray may show evidence of pulmonary TB in up to 20 % of cases [23] and upper GI endoscopy may reveal duodenal bulb deformity [24]. Endoscopic biopsy has a poor yield even in ulcerated lesions and endoscopic biopsy rarely reveals granulomas because of the predominantly submucosal location of these lesions and the failure of routine endoscopic biopsies to include the submucosa [17]. The diagnosis of duodenal TB is usually made after surgical intervention (exploratory laparotomy) and it is very rarely made preoperatively [25]; however, Sharma et al. reported that endoscopic ultrasonography (EUS) is an excellent modality for characterizing the lesion, as well as obtaining a sample for cytological confirmation of the diagnosis [26]. Multiple intraoperative fine-needle aspiration cytology (FNAC) may be taken from the diseased portion of the duodenum to establish the histopathological diagnosis if not established by any other means [7]. When the diagnoses of TB are established before surgery, most lesions regress with appropriate antitubercular treatment and do not require excision [27, 28]. Minimally invasive procedures such as laparoscopic, endoscopic and percutaneous biopsy should be used for diagnosis of intraperitoneal TB as a first step in diagnosis, and laparotomy should be performed only when complications develop or diagnosis remains unclear in spite of these diagnostic modalities [16]. Surgery is usually required for diagnosis or therapy, after which patients respond well to antituberculous treatment. In areas endemic for TB, a good biopsy from the site of gastroduodenal bleeding or mass lesion and the surrounding lymph nodes should always be obtained [4]. In patients with gastric outlet obstruction, gastrojejunostomy is preferred over pyloroplasty, as intense fibrosis around the pyloroduodenal junction precludes safe pyloroplasty [29]. Puri et al. showed that endoscopic therapy in combination with antituberculous therapy is recommended as the first-line therapy for gastroduodenal TB and surgical intervention is reserved for the minority in whom endoscopic therapy fails [30].
Conclusions
Primary gastric TB is rare, it is usually a diagnostic challenge and may present with gastric outlet obstruction. It should be suspected in TB-endemic areas. Surgery is often required for diagnosis and therapy.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- GI:
-
Gastrointestinal
- HIV:
-
Human immunodeficiency virus
- TB:
-
Tuberculosis
References
World Health Organization Global tuberculosis control: a short update to the 2009 report. 3rd WHO annual report on global TB control. 1999
World Health Organization. Global tuberculosis control: WHO Report 2010. Geneva: WHO; 2010.
Abdallah TM, Ali AAA. Epidemiology of tuberculosis in Eastern Sudan. Asian Pac J Trop Biomed. 2012;2(12):999–1001.
Rao YG, Pande GK, Sahni P, Chattopadhyay TK. Gastroduodenal tuberculosis management guidelines, based on a large experience and a review of the literature. Can J Surg. 2004;47:364–8.
Sheer TA, Coyle WJ. Gastrointestinal tuberculosis. Curr Gastroenterol Rep. 2003;5:273–8.
Subei I, Attar B, Schmitt G, Levendoglu H. Primary gastric tuberculosis: a case report and literature review. Am J Gastroenterol. 1987;82:769–72.
Upadhyaya VD, Kumar B, Lal R, Sharma MS, Singh M, Rudramani. Primary duodenal tuberculosis presenting as gastric-outlet obstruction: Its diagnosis. Afr J Paediatr Surg. 2013;10(2):83–6.
Haydee Buluran F, Felix Z, Ena Lyn A, Norberto E. Duodenal tuberculosis presenting as gastric outlet obstruction: A case report. World J Gastrointest Endosc. 2011;3(1):16–9.
Gupta B, Mathew S, Bhalla S. Pyloric obstruction due to gastric tuberculosis: An endoscopic diagnosis. Postgrad Med J. 1990;66:62–5.
Brody JM, Miller DK, Zeman RK, Klappenbach RS, Jaffe MH, Clark LR, et al. Gastric tuberculosis: A manifestation of acquired immunodeficiency syndrome. Radiology. 1986;159:347–8.
Gheorghe L, Băncilă I, Gheorghe C, Herlea V, Vasilescu C, Aposteanu G. Antro-duodenal tuberculosis causing gastric outlet obstruction – a rare presentation of a protean disease. Rom J Gastroenterol. 2002;11:149–52.
Zengin K, Taskin M, Cicek Y, et al. Primary gastric tuberculosis mimicking gastric tumor that results in pyloric stenosis. European Surgery. 2003;35(4):220–1.
Jadvar H, Mindelzun RE, Olcott EW, Levitt DB. Still the great mimicker: abdominal tuberculosis. Am J Roentgenol. 1997;168:1455–60.
Amarapurkar DN, Patel ND, Amarapurkar AD. Primary gastric tuberculosis – a report of 5 cases. BMC Gastroenterol. 2003;3:6.
Lin OS, Wu SS, Yeh KT, Soon MS. Isolated gastric tuberculosis of the cardia. J Gastroenterol Hepatol. 1999;14:258–61.
Akgun Y. Intestinal and peritoneal tuberculosis: changing trends over 10 years and a review of 80 patients. Can J Surg. 2005;48(2):131–6.
Cem Eray İ, Rencüzoğulları A, Yalav O, et al. Primary gastric tuberculosis mimicking gastric cancer. Ulus Cerrahi Derg. 2015;31(3):177–179.
Gleason T, Prinz RA, Kirsch EP, Jablokow V, Greenlee HB. Tuberculosis of the duodenum. Am J Gastroenterol. 1979;72:36–40.
Chetri K, Prasad KK, Jain M, Choudhuri G. Gastric tuberculosis presenting as non-healing ulcer: case report. Trop Gastroenterol. 2000;21:180–1.
Quantrill SJ, Archer GJ, Hale RJ. Gastric tuberculosis presenting with massive hematemesis in association with acute myeloid leukemia. Am J Gastroenterol. 1996;91:1259–60.
Rohwedder J. Abdominal tuberculosis: a disease poised for reappearance. N Y State J Med. 1989;89:252–4.
Placido RD, Pietroletti R, Leardi S, Simi M. Primary gastroduodenal tuberculosis infection presenting as pyloric outlet obstruction. Am J Gastroenterol. 1996;91:807–8.
Mukherji B, Singhal AK. Intestinal tuberculosis. Proc Assoc Surg East Afr. 1968;2:71–5.
Tandon RK, Pastakia B. Duodenal tuberculosis as seen by duodenoscopy. Am J Gastroenterol. 1976;66:483–6.
Eti S, Vijai Datta U, Basant K, Moniak Sen S. Primary Duodenal tuberculosis presenting as gastric-outlet obstruction - is pathological diagnosis is always possible. World J Surg Res. 2012;1:12–16.
Sharma V, Rana SS, Gunjan D, Chhabra P, Sharma R, Bhasin DK. Primary gastric tuberculosis mimicking a submucosal tumor. J Dig Endosc. 2015;6:130–2.
Gilinsky NH, Marks IN, Kottler NE, Price SK. Abdominal tuberculosis: a 10-year review. S Afr Med J. 1983;64(22):849–57.
Anand BS, Nanda R, Sachdev GK. Response of tuberculous stricture to antituberculous treatment. Gut. 1988;29:62–9.
Thompson JN, Keshavarzian A, Rees HC. Duodenal tuberculosis. J R Coll Surg Edinb. 1984;29:292–5.
Puri AS, Sachdeva S, Mittal VV, Gupta N, Banka A, Sakhuja P, et al. Endoscopic diagnosis, management and outcome of gastroduodenal tuberculosis. Indian J Gastroenterol. 2012;31(3):125–9.
Acknowledgement
We would like to thank the Head of Medical Records for her help and all the medical team in Ibnsina Specialized Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NA and MMAI admitted the patient and requested the relative investigations and the postoperative follow up. NA wrote the manuscript. NA, MMAI, AMM, EEA and MSEA participated in its design and coordination and helped to draft the manuscript and reviewed the paper for English editing. All authors read and approved the final manuscript.
Authors’ information
Nassir Alhaboob Arabi: MD (surgery) MRCSEd, assistant professor of surgery, OIU University, Department of GI Surgery, Ibnsina Specialized Hospital, Khartoum, Sudan.
Abdulmagid M. Musaad: FRCSI, professor of surgery, OIU University, Department of GI Surgery, Ibnsina Specialized Hospital, Khartoum, Sudan.
Elsaggad Eltayeb Ahmed: associate professor of surgery, Alnilin University, Department of GI Surgery, Ibnsina Specialized Hospital, Khartoum, Sudan.
Mohammed M. A. M. Ibnouf: registrar of surgery, Department of GI Surgery, Ibnsina Specialized Hospital, Khartoum, Sudan.
Muataz Salah Eldin Abdelaziz: associate professor of surgery, OIU University, Department of GI Surgery, Ibnsina Specialized Hospital, Khartoum, Sudan.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Arabi, N.A., Musaad, A.M., Ahmed, E.E. et al. Primary gastric tuberculosis presenting as gastric outlet obstruction: a case report and review of the literature. J Med Case Reports 9, 265 (2015). https://doi.org/10.1186/s13256-015-0748-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-015-0748-8