Abstract
Introduction
Sporadic inclusion body myositis is the most common adult myopathy in persons aged 50 years and older. The clinical presentation includes a chronic, slowly progressive course with a predilection for weakness of the forearm flexors and quadriceps muscles. Its indolent course makes it a disease frequently missed or misdiagnosed as other neuromuscular conditions by health care professionals. The degenerative processes with amyloid accumulation distinguish sporadic inclusion body myositis from other inflammatory myopathies. Currently, no effective therapy exists. This clinical report highlights the difficulties in diagnosing the disease, examples of misdiagnosis, and inappropriate therapies that can result from misdiagnosis.
Case presentation
We present our clinical experience with 20 patients over a 10-year period and describe in depth two cases, both men, one of Indian ethnicity and the other of Hispanic ethnicity, who were referred to our neuromuscular division for second opinions and diagnosed with sporadic inclusion body myositis years after symptom onset.
Conclusions
Although sporadic inclusion body myositis is rare and without effective therapy, accurate diagnosis is crucial to providing adequate counseling and information about the prognosis and disease course, and to avoiding inappropriate therapy.
Similar content being viewed by others
Introduction
Sporadic inclusion body myositis (s-IBM) is one of several chronic adult inflammatory myopathies. Its prevalence varies, but it may be as high as 35 per 1 million adults over age 50 years, with a slight male predominance [1]. The clinical presentation involves chronic, slowly progressive, distal asymmetric weakness affecting the finger flexors and proximal lower extremity weakness affecting the quadriceps, which later progresses to other proximal and distal muscles. The indolent disease course sometimes lasts many years until patients notice significant deterioration leading to medical care [2–4]. Additional complaints include dysphagia caused by cricothyroid muscle weakness and decreased pharyngeal propulsion [5]. The pathogenesis of s-IBM is not fully understood, and currently there is no known treatment [3, 6]. We present two exemplary cases followed by a summary table that highlights the presentation of 20 patients seen in our clinic over a 10-year period.
Case presentations
The local institutional review board approved this study. The case presentation portions of this report were prepared according to recent standardized guidelines [7–9].
Patient 1
A 58-year-old man of Indian ethnicity with type 2 diabetes mellitus was referred to our hospital for a second-opinion neuromuscular evaluation. Four years prior, he had noted leg weakness, particularly in his thighs. After initial evaluation and diagnostic studies, his neurologist had diagnosed him with deconditioning and prescribed vitamin B12 injections. A subsequent electrodiagnostic study revealed denervation changes in his upper extremity muscles and thoracic paraspinal muscles, leading to a diagnosis of amyotrophic lateral sclerosis (ALS), which the patient carried for nearly 1 year. He was prescribed riluzole and advised to plan for end of life.
When referred to our institution, the patient described weakness while climbing and descending stairs without upper extremity complaints. During his physical examination, he was noted to have atrophy in the quadriceps muscles with moderate weakness. Electromyography (EMG) showed increased membrane instability and early recruitment with fractionation of the motor unit potentials, showing a brief, small, abundant, polyphasic motor unit potential pattern. His creatine kinase (CK) level was elevated at 647IU/L. A muscle biopsy showed myopathic features with rimmed vacuoles characteristic of s-IBM. The patient was informed of his correct diagnosis and counseled on his prognosis. Patient 1 is represented as patient 8 in Table 1.
Patient 2
A 54-year-old Hispanic man developed weakness in his grip, followed 1 year later by lower extremity weakness. His CK level was 2400IU/L, and a muscle biopsy indicated polymyositis (PM). His initial therapy included prednisone followed by other immunosuppressive medications, including azathioprine, methotrexate, and mycophenolate mofetil. Although the patient’s CK level decreased to 450IU/L, his condition continued to progress. Nine years later, he was evaluated at our neuromuscular clinic. At that time, he was unable to get up from a seated position, locked his knees while ambulating to avoid falls, and had severe weakness in his left hand and moderate weakness in his right hand. During his physical examination, he was noted to have asymmetric atrophy in the forearm flexor and the quadriceps muscles, with severe asymmetric weakness of knee extension and slight weakness in ankle dorsiflexion and plantarflexion. EMG showed active denervation with significant brief, small, abundant, polyphasic motor unit potentials. A repeat muscle biopsy showed variation in muscle fiber size, endomysial fibrosis, chronic inflammatory cells with macrophages, and rimmed vacuoles with basophilic stippling, all characteristic of s-IBM. The patient was counseled on his correct s-IBM diagnosis, and immunosuppressive therapy was discontinued. Patient 2 is represented as patient 9 in Table 1.
Retrospective chart review
Table 1 shows 20 patients (16 men, 4 women) seen at our neuromuscular division between 2004 and 2014 (including patients 1 and 2 in the present report listed as patients 8 and 9, respectively). The patients’ average age at initial evaluation at our clinic was 67.8 years (standard deviation, 11.5; range, 43–85). The average number of months from symptom onset to diagnosis was 70.0 (standard deviation, 54.8; range, 6–240). One patient did not follow up to confirm the diagnosis but was clinically diagnosed with s-IBM. Our clinic population had characteristics of s-IBM similar to those described in previous reports. The topography was proximal leg weakness presenting as difficulty with ambulation and rising from chairs, in addition to finger flexor weakness presenting as difficulty handling objects and opening jars and bottles. Four patients had dysphagia.
Several patients were misdiagnosed before evaluation at our institute: two patients (patients 9 and 17) diagnosed with PM who were given immunosuppressive medications; three (patients 8, 18, and 19) who were diagnosed with ALS; one (patient 5) diagnosed with entrapment neuropathy who underwent cubital tunnel release with no improvement; one (patient 14) who was diagnosed with extrapyramidal symptoms of Parkinson’s disease versus radiculopathy; and one (patient 16) who was diagnosed with s-IBM and given etanercept. The remaining 12 patients were referred without diagnosis or for second opinions for myopathy.
(Please note the standard deviations given above for time until the final correct diagnosis, and see below for discussion of trends and how our findings coincide with the existing published literature.)
Discussion
s-IBM is both a myodegenerative and neurodegenerative disease with β-amyloid-related deposits and an inflammatory disease with endomysial lymphocytic infiltration. Accumulation of misfolded proteins and inadequate intracellular repair mechanisms characterize the disease [10]. There is also expression of major histocompatibility complex (MHC) class I molecules [3]. A 43 kDa muscle protein, recently identified as cytosolic 5′-nucleotidase 1A, was discovered in 2013 as an autoantigen for s-IBM autoantibodies [11–13]. This finding indicates the potential role of humoral immunity in the pathogenesis of the disease in addition to a cytotoxic T-cell-mediated component. Furthermore, identification of this target of s-IBM autoantibodies could lead to the development of a diagnostic blood test, potentially eliminating the need for invasive testing such as muscle biopsy.
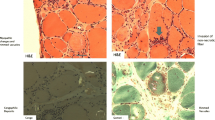
Although muscle biopsy can confirm the diagnosis of s-IBM, the results may be negative owing to sampling error or to end-stage muscle wasting. Endomysial inflammation and degeneration with misfolded protein aggregates and/or inclusions [10] and atrophic fibers [14] are often present. Congo red staining or immunofluorescence shows amyloid β-pleated sheets in and around vacuoles [3]. There are rimmed vacuoles within the myofibril. In cases where the inclusion bodies are not found, the presence of inflammation alone can lead to an erroneous diagnosis of PM [4, 15]. If there is no definite diagnosis, then biopsy should be repeated.
Currently, there is no cure for s-IBM. Some patients may initially show a limited response to corticosteroids. Other treatments available for inflammatory myopathies have efficacy in s-IBM in specific cases [3, 16]. Patients with dysphagia may benefit from intravenous immunoglobulin (IVIG) therapy, along with balloon dilation or botulinum toxin injection [16–20]. Although this disease is rare and incurable, making the correct diagnosis is crucial to directing the patient to physical therapy for weakness, gait training, and education to prevent falls. Occupational therapy may improve a patient’s ability to engage in activities of daily living. Appropriate patients with swallowing complaints should be referred to a speech therapist for proper education regarding diet consistency and aspiration precautions. Patients may be assured that s-IBM is not a motor neuron disease or a rapidly deteriorating myopathic condition that is life-threatening [6].
Our university neuromuscular clinic sees approximately six new patients with s-IBM each year. The median age of the predominantly male population in our present report was 69 years, and patients’ ages ranged from 43 to 85 years. The median time from symptom onset to final diagnosis was 54 months and ranged from 6 months to 20 years, reflective of the slow disease course that helps to differentiate s-IBM from other disease mimickers. Experienced neurologists and neuromuscular specialists can diagnose s-IBM based on its topography of weakness of quadriceps and forearm flexors, but the diagnosis is often missed, delayed, or incorrect. Common misdiagnoses are PM, immune-mediated neuromuscular disease, entrapment neuropathies, and motor neuron disease. Erroneous diagnosis can lead to inappropriate therapy, as illustrated by some of the cases described here and in a previous report of patients who received years of corticosteroids and immunosuppressive medication and experienced serious side effects [6]. IVIG was previously used for treatment of s-IBM until a controlled clinical trial showed lack of efficacy [2]. Needless surgical treatments for radiculopathy or entrapment neuropathy have occurred, as illustrated by patient 5 in our series.
Although s-IBM is a well-recognized neuromuscular diagnosis that is seen in neurology and neuromuscular specialty clinics, it is well known that it can masquerade as other disorders [6]. The cases described here confirm and highlight the fact that s-IBM is still difficult to diagnose and remains frequently misdiagnosed. Our data show that the time to diagnosis averaged 5.83 years, a delay similar to that described by Lotz and coworkers in 1989 [21]. The fact that this has remained unchanged for 25 years is disappointing but not entirely surprising, given the symptoms and neurological topography, including asymmetric weakness, finger flexor weakness, loss of grip strength, gait difficulty, and dysphagia, that overlap with those of non-s-IBM conditions. The electrodiagnostic findings in our clinical neurophysiology laboratory in this case series also coincide with previous reports of mixed patterns, which may confuse non-neuromuscular clinicians.
We find several pitfalls in the diagnosis of s-IBM. First is an overreliance on electrophysiology. Second, muscle biopsies sometimes do not have all the cardinal histological features, including endomysial inflammation, newer findings of MHC class II upregulation and invasion of non-necrotic muscle fibers by lymphocytes, and mitochondrial changes [22–24]. Also, clinicians may be misled by an incomplete biopsy appearance, with patchy inflammatory changes being more florid early and patchy degenerative changes more florid later in the disease course. A third pitfall arises when patients present early with either atypical symptoms, such as camptocormia or foot drop, or an incomplete clinical picture. Clues to look for in a clinical examination include long finger flexor and quadriceps weakness, as confirmed in our present study. It has been suggested that s-IBM can be made by clinical diagnosis alone [25]. To the general practice clinician, however, we recommend that all cases of suspected s-IBM be referred to a center specializing in neuromuscular disease so that an appropriate diagnosis can be made and inappropriate treatments are avoided.
Limitations of our study include the relatively small number of patients. Our present study of 20 patients is only half the number of the series previously reported by Lotz and colleagues (40 patients) [21]. Also, our study may have university and/or tertiary geographic referral bias, along with limitations of our single-center experience. We do hope that our aforementioned clinical points are clear, however, regarding potential misdiagnosis and the importance of correct diagnosis.
Conclusions
s-IBM is still difficult to diagnose and unfortunately remains frequently misdiagnosed, with a delay in accurate diagnosis of just over 5½ years, which has not changed over the last 25 years. Although s-IBM is rare and without effective therapy, accurate diagnosis is of crucial importance to providing adequate patient counseling and information about the prognosis and course of the disease. Cases of suspected s-IBM should be referred to a center specializing in neuromuscular disease so that an appropriate diagnosis can be made and inappropriate treatments are avoided.
Consent
Written informed consent was obtained from both of the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- b:
-
Biceps
- b/l:
-
Bilateral
- COX:
-
Cytochrome oxidase
- CK:
-
Creatine kinase
- DF:
-
Dorsiflexors
- EE:
-
Elbow extension
- EF:
-
Elbow flexion
- EMG:
-
Electromyography
- FE:
-
Finger extension
- FF:
-
Finger flexion
- HA:
-
Hip abduction
- HE:
-
Hip extension
- HF:
-
Hip flexion
- IBM:
-
Inclusion body myositis
- IVIG:
-
Intravenous immunoglobulin
- KE:
-
Knee extension
- KF:
-
Knee flexion
- LE:
-
Lower extremity
- MHC:
-
Major histocompatibility complex
- PF:
-
Plantarflexors
- PM:
-
Polymyositis
- pt:
-
Patient
- PT:
-
Physical therapy
- R:
-
Right
- RA:
-
Rheumatoid arthritis
- SA:
-
Shoulder abduction
- s-IBM:
-
Sporadic inclusion body myositis
- UE:
-
Upper extremity
- WE:
-
Wrist extension
- WF:
-
Wrist flexion
References
Dalakas MC. Sporadic inclusion body myositis–diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol. 2006;2(8):437–47.
Dalakas MC, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K. Treatment of inclusion-body myositis with IVIg: a double-bind, placebo-controlled study. Neurology. 1997;48:712–16.
Dimachkie MM, Barohn RJ. Inclusion body myositis. Curr Neurol Neurosci Rep. 2013;13:321.
Engel WK, Askanas V. Inclusion-body myositis: clinical, diagnostic, and pathologic aspects. Neurology. 2006;66(2 Suppl 1):S20–9.
Murata KY, Kouda K, Tajima F, Kondo T. A dysphagia study in patients with sporadic inclusion body myositis (s-IBM). Neurol Sci. 2012;33(4):765–70.
Schellenberg KL, Johnston WS, Kalra S, Resch L, Johnson ES. Inclusion body myositis masquerading as amyotrophic lateral sclerosis. Can J Neurol Sci. 2010;37(5):687–91.
Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, et al. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol. 2014;67(1):46–51.
Rison RA, Kidd MR, Koch CA. The CARE (CAse REport) guidelines and the standardization of case reports. J Med Case Rep. 2013;7:261.
Rison RA. A guide to writing case reports for the Journal of Medical Case Reports and BioMed Central Research Notes. J Med Case Rep. 2013;7:239.
Askanas V, Engel WK, Nogalska A. Inclusion body myositis: a degenerative muscle disease associated with intra-muscle fiber multi-protein aggregates, proteasome inhibition, endoplasmic reticulum stress and decreased lysosomal degradation. Brain Pathol. 2009;19(3):493–506.
Salajegheh M, Lam T, Greenberg SA. Autoantibodies against a 43 kDa muscle protein in inclusion body myositis. PLoS One. 2011;6(5):e20266.
Larman HB, Salajegheh M, Nazareno R, Lam T, Sauld J, Steen H, et al. Cytosolic 5′-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol. 2013;73(3):408–18.
Pluk H, van Hoeve BJ, van Dooren SH, Stammen-Vogelzangs J, van der Heijden A, Schelhaas HJ, et al. Autoantibodies to cytosolic 5′-nucleotidase 1A in inclusion body myositis. Ann Neurol. 2013;73(3):397–407.
Hilton-Jones D, Miller A, Parton M, Holton J, Sewry C, Hanna MG. Inclusion body myositis: MRC Centre for Neuromuscular Diseases, IBM workshop, London, 13 June 2008. Neuromuscul Disord. 2010;20(2):142–7.
Chahin N, Engel AG. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology. 2008;70(6):418–24.
Tawil R, Griggs RC. Inclusion body myositis. Curr Opin Rheumatol. 2002;14(6):653–7.
Breithaupt M, Schmidt J. Update on treatment of inclusion body myositis. Curr Rheumatol Rep. 2013;15:329.
Cherin P, Pelletier S, Teixeira A, Laforet P, Simon A, Herson S, et al. Intravenous immunoglobulin for dysphagia of inclusion body myositis. Neurology. 2002;58:326.
Murata KY, Kouda K, Tajima F, Kondo T. Balloon dilation in sporadic inclusion body myositis patients with dysphagia. Clin Med Insights Case Rep. 2013;6:1–7.
Schneider I, Thumfart WF, Pototschnig C, Eckel HE. Treatment of dysfunction of the cricopharyngeal muscle with botulinum A toxin: introduction of a new, noninvasive method. Ann Otol Rhinol Laryngol. 1994;103:31–5.
Lotz BP, Engel AG, Nishino H, Stevens JC, Litchy WJ. Inclusion body myositis: observations in 40 patients. Brain. 1989;112(Pt 3):727–47.
Needham M, Corbett A, Day T, Christiansen F, Fabian V, Mastaglia FL. Prevalence of sporadic inclusion body myositis and factors contributing to delayed diagnosis. J Clin Neurosci. 2008;15(12):1350–3.
Brady S, Squier W, Sewry C, Hanna M, Hilton-Jones D, Holton JL. A retrospective cohort study identifying the principal pathological features useful in the diagnosis of inclusion body myositis. BMJ Open. 2014;4:e004552.
Rose MR, Working Group ENMCIBM. 188th ENMC International Workshop: inclusion body myositis, 2–4 December 2011, Naarden, the Netherlands. Neuromuscul Disord. 2013;23:1044–55.
Brady S, Squier W, Hilton-Jones D. Clinical assessment determines the diagnosis of inclusion body myositis independently of pathological features. J Neurol Neurosurg Psychiatry. 2013;84:1240–6.
Acknowledgments
We gratefully acknowledge the patients described in this report. We also acknowledge and are grateful to Jennifer Kelly Shepphird, PhD, of JKS Science & Medical Writing, LLC (www.jkswriting.com) for her assistance in production and revision of Table 1 as well as calculation of some statistics and general manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AC and SRB examined patients 1 and 2. SRB examined all cases presented in Table 1. AC wrote the initial manuscript. SRB supervised the coordination of clinical care, rewrote and edited the manuscript, did an additional literature search and performed and interpreted all of the electrodiagnostic studies. RAR reviewed the manuscript and revised and further edited the manuscript using an additional literature search. SRB and RAR were responsible for the intellectual content of the report, along with critical appraisals, suggestions and revisions. All authors participated in and provided significant contributions to the writing of the manuscript. All authors read and approved the final manuscript.
Authors’ information
AC is a recent former clinical neurophysiology fellow at the University of Southern California Keck School of Medicine and Los Angeles County Medical Center (currently in private practice). SRB is a professor of neurology at the University of Southern California Keck School of Medicine and Los Angeles County Medical Center. SRB is director of the University of Southern California Neuromuscular Program, a fellow of the American Academy of Neurology and the American Association of Neuromuscular and Electrodiagnostic Medicine, and board-certified by the American Board of Psychiatry and Neurology in neurology, clinical neurophysiology, pain medicine, and neuromuscular medicine. SRB is also board-certified by the American Board of Electrodiagnostic Medicine in electrodiagnostic medicine. SRB is a member of the advisory board and the scientific committee of the Myasthenia Gravis Foundation of California. RAR is a deputy editor of the Journal of Medical Case Reports; an associate neurology editor of BMC Neurology, Grand Rounds and WebmedCentral; and a section editor of BMC Research Notes. RAR practices general neurology at Neurology Consultants Medical Group, serves as medical director of the PIH Health Hospital-Whittier Stroke Program and the PIH Health Hospital-Whittier Non-Invasive Vascular Laboratory, is a clinical assistant professor of neurology at the University of Southern California Keck School of Medicine and Los Angeles County Medical Center, and is a fellow of the American Association of Neuromuscular and Electrodiagnostic Medicine. RAR is board-certified by the American Board of Psychiatry and Neurology in neurology and vascular neurology, and neurocritical care and neuroimaging by the United Council of Neurologic Subspecialties. RAR is also board-certified by the American Board of Electrodiagnostic Medicine in electrodiagnostic medicine. RAR is a former president of the Los Angeles Neurological Society and is a fellow of the American Academy of Neurology and the American Neurological Association.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chilingaryan, A., Rison, R.A. & Beydoun, S.R. Misdiagnosis of inclusion body myositis: two case reports and a retrospective chart review. J Med Case Reports 9, 169 (2015). https://doi.org/10.1186/s13256-015-0647-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-015-0647-z