Abstract
Purpose
In recent decades, diverse nomograms have been proposed to predict extraprostatic extension (EPE) in prostate cancer (PCa). We aimed to systematically evaluate the accuracy of MRI-inclusive nomograms and traditional clinical nomograms in predicting EPE in PCa. The purpose of this meta-analysis is to provide baseline summative and comparative estimates for future study designs.
Materials and methods
The PubMed, Embase, and Cochrane databases were searched up to May 17, 2023, to identify studies on prediction nomograms for EPE of PCa. The risk of bias in studies was assessed by using the Prediction model Risk Of Bias ASsessment Tool (PROBAST). Summary estimates of sensitivity and specificity were obtained with bivariate random-effects model. Heterogeneity was investigated through meta-regression and subgroup analysis.
Results
Forty-eight studies with a total of 57 contingency tables and 20,395 patients were included. No significant publication bias was observed for either the MRI-inclusive nomograms or clinical nomograms. For MRI-inclusive nomograms predicting EPE, the pooled AUC of validation cohorts was 0.80 (95% CI: 0.76, 0.83). For traditional clinical nomograms predicting EPE, the pooled AUCs of the Partin table and Memorial Sloan Kettering Cancer Center (MSKCC) nomogram were 0.72 (95% CI: 0.68, 0.76) and 0.79 (95% CI: 0.75, 0.82), respectively.
Conclusion
Preoperative risk stratification is essential for PCa patients; both MRI-inclusive nomograms and traditional clinical nomograms had moderate diagnostic performance for predicting EPE in PCa. This study provides baseline comparative values for EPE prediction for future studies which is useful for evaluating preoperative risk stratification in PCa patients.
Critical relevance statement
This meta-analysis firstly evaluated the diagnostic performance of preoperative MRI-inclusive nomograms and clinical nomograms for predicting extraprostatic extension (EPE) in prostate cancer (PCa) (moderate AUCs: 0.72–0.80). We provide baseline estimates for EPE prediction, these findings will be useful in assessing preoperative risk stratification of PCa patients.
Key points
• MRI-inclusive nomograms and traditional clinical nomograms had moderate AUCs (0.72–0.80) for predicting EPE.
• MRI combined clinical nomogram may improve diagnostic accuracy of MRI alone for EPE prediction.
• MSKCC nomogram had a higher specificity than Partin table for predicting EPE.
• This meta-analysis provided baseline and comparative estimates of nomograms for EPE prediction for future studies.
Graphical Abstract

Similar content being viewed by others
Introduction
Oncologic radicality and good functional outcomes are two clinical priorities for the surgical treatment of prostate cancer patients. Nerve-sparing surgery could be chosen to preserve urinary continence and erectile function for low-risk patients. However, nerve-sparing surgery in high- or moderate-risk patients is associated with an increased risk of positive surgical margins (PSMs) in postoperative specimens. Therefore, planning the therapeutic schedule for PCa patients must be based on comprehensive risk assessment and staging. Making these clinical decisions depends on the predicted probability of EPE of prostate cancer. EPE is associated with a high risk of PSMs and early biochemical recurrence and can cause a worse prognosis than organ-confined tumors [1, 2]. Consequently, accurate prediction of EPE is of high priority in clinical, radiotherapy, and surgical decision-making.
There are several widely used clinical prediction tools for evaluating EPE, such as the Partin table [3], the Cancer of the Prostate Risk Assessment (CAPRA) score [4], and the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram [5]. These tools rely on conventional clinical and histopathological parameters, such as clinical T stage, prostate-specific antigen (PSA) level and biopsy Gleason score (GS). However, the diagnostic performance of these nomograms was varied, with reported areas under the curve (AUCs) ranging from 0.60 to 0.86. MRI has good tissue resolution, and some multiparametric MRI findings like capsular irregularity and bulge, curvilinear contact length (CCL) > 15 mm and invasion of periprostatic fat are associated with pathological EPE [6]. Although MRI was reported to be insensitive for diagnosing EPE (57%) by de Rooij et al., it does offer high specificity (91%) [7]. Particularly, several MRI-EPE grading systems were proposed in recent years, such as the ESUR score [8] and the EPE grade [9], which are promising to promote structure of MRI-EPE report and improve diagnostic performance. In addition, many studies have suggested that integrating MRI information with clinical characteristics might result in more precise clinical staging for PCa [10]. However, some scholars reported that MRI did not significantly increase the precision of the clinical nomogram [11, 12]. There has no well-defined MRI-inclusive nomogram to predict EPE in PCa; thus far, it is still inconclusive how to use MRI characteristics to improve the diagnostic accuracy of traditional clinical nomograms.
The previous study has summatively determined the accuracy of MRI for diagnosing EPE [7]. To our knowledge, a systematic review and meta-analysis evaluating preoperative MRI-inclusive or traditional clinical nomograms for predicting EPE has not been performed. A comprehensive systematic review is valuable for assessing the vast amount of currently available information. The purpose of this systematic review and meta-analysis is to provide baseline summative estimates for EPE prediction of PCa as well as evaluate the influence of prediction variables, to provide comparative estimates for future trial designs. We assessed study methods, adherence to reporting guidelines, and risk of bias of studies.
Materials and methods
Study design and search strategy
This systematic review and meta-analysis was registered with PROSPERO (CRD42022361098). This study was conducted following the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies [13].
A systematic literature search was performed on the PubMed, Embase, and Cochrane databases up to May 17, 2023. The search terms included prostate cancer, prostate neoplasm, prostate carcinoma, extranodal extension, extraprostatic extension, extracapsular extension, nomogram, risk model, Partin table, and prediction. No language restriction was applied. We also manually reviewed the reference lists of the included studies.
Study selection and data extraction
Two investigators (both with 6 years of research experience) independently assessed all citations according to the predefined inclusion and exclusion criteria. Disagreements were resolved through discussion and consensus. The following inclusion criteria were used: (1) primary studies for developing, validating, or updating preoperative nomograms/models (combining multiple clinical and MRI characteristics or clinical characteristics alone) to predict pathological EPE (pT3 stage) of PCa patients; and (2) studies published in English.
One investigator (with > 6 years of research experience) extracted the characteristics of all included studies independently, including study type; country; reference standards; patient age; sample size; clinical and MRI predictors; and TP, FN, TN, and FP values. To reduce the risk of data duplication and overlapping cohorts, for studies that reported multiple readers, we only extracted the results of reader 1 as a representative and incorporated them into the meta-analysis. For studies that reported multiple sensitivities/specificities of the same cohort based on different algorithms, we only extracted the highest sensitivity/specificity. For studies that reported multiple sensitivities/specificities based on different cohorts or different nomograms, we extracted all of them as independent contingency table results.
Quality and risk of bias assessment
We assessed eligible studies for adherence to reporting guidelines according to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) checklist, which consists of 37 items in 22 criteria to aid transparent reporting of studies that develop and/or validate prediction models [14]. Five items (4b: “Specify the key study dates, including start of accrual; end of accrual; and, if applicable, end of follow-up”, 5c: “Give details of treatments received, if relevant”, 11: “Provide details on how risk groups were created, if done”, 14b: “If done, report the unadjusted association between each candidate predictor and outcome”, and 22: “Give the source of funding and the role of the funders for the present study”) were omitted since they were irrelevant to the quality assessment in this review. We deleted the element “when” in Item 6a.
The Prediction model Risk Of Bias ASsessment Tool (PROBAST) [15] was used to assess the risk of bias and applicability of each study by two investigators independently. The tool includes four domains (participants, predictors, outcome, and analysis) for risk of bias assessment and three domains (participants, predictors, and outcomes) for applicability assessment, consisting of 23 signal questions in total. Any disagreement between the two investigators was resolved by a third investigator (with > 25 years of research experience), and consensus was finally reached in all domains.
Statistical analysis
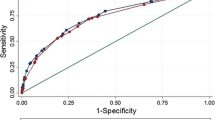
Meta-analysis was performed with the recommended bivariate random-effects meta-analysis model [16]. Statistical heterogeneity was assessed with an I2 estimate [17]. Contingency tables were used to construct hierarchical summary receiver operating characteristic (HSROC) curves to calculate pooled sensitivities and specificities [18]. Funnel plots and Egger’s test were used to identify publication bias. A statistical significance of p < 0.05 indicated the presence of bias. Univariate meta-regression analysis was performed to investigate potential heterogeneity from predictors of nomograms, as well as other baseline parameters, including side-specific or whole-gland pathological EPE-based, pathological EPE rate, MRI predictor and slice thickness. Meta-analysis was conducted with Stata version 15.0.
Results
The search strategy yielded a total of 671 results (299 from Embase, 362 from PubMed, and 10 from Cochrane Library). After duplicates were removed (101), 570 studies were screened. After screening abstracts and full texts, 522 articles were excluded; the exclusion criteria are shown in Fig. 1. Finally, forty-eight full-text studies were assessed as eligible, with a total of 57 contingency tables. Twenty-two studies [9, 11, 12, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] head-to-head compared the diagnostic performance of the MRI-inclusive nomogram with that of the traditional clinical nomogram but lacked data to calculate TP, FN, TN and FP; they therefore were included in the qualitative analysis only. The remaining 26 studies (13 for MRI-inclusive nomograms [29, 38,39,40,41,42,43,44,45,46,47,48,49], eight for clinical nomograms [50,51,52,53,54,55,56,57] and 5 for both MRI-inclusive nomograms and clinical nomograms [58,59,60,61,62]) were included in the quantitative analysis.
Basic characteristics of the studies
A total of 20,395 patients were included. The main characteristics, demographics, and MRI information for the MRI-inclusive nomograms and clinical nomograms are presented in Tables 1 and 2, respectively. According to the TRIPOD statement (Type 1a: development only; Type 1b: development and validation using resampling; Type 2a: random split-sample development and validation; Type 3: development and validation using separate data; Type 4: validation only) [14], seventeen (35%) studies were Type 1a, eleven (23%) studies were Type 1b, four (8.3%) studies were Type 2a, one (2%) is Type 2b, six (12.5%) studies were Type 3, and nine (19%) studies were Type 4. For all included studies, the range of mean/median patient age was 61.5–70.2 years old; the range of mean/median serum PSA level was 5.75–60 ng/ml; and the range of pathological EPE rate was 0.16–0.73. Sixteen studies assessed the diagnostic performance for side-specific EPE, and the remaining 32 studies for whole-gland EPE. For quantitative analysis, three studies reported multiple different nomograms [55, 60, 61], and we only extracted the highest one into the analysis.
Studies quality assessment
According to TRIPOD items (Fig. 2), the total adherence rate was 64.7% (857/1325) after excluding 290 inapplicable items. None of the studies met Item 8 (sample size estimation). In addition, 5 items were poorly reported (< 50% adherence): title (Item 1), abstract (Item 2), blind assessment of outcome (6b), blind assessment of predictors (Item 7b), specify participants and outcome numbers (Item 14a), and availability of supplementary resources (Item 21).
The risk of bias and applicability concerns of all studies were assessed by PROBAST (Fig. 3). For the 3 domains of participants, outcomes and analysis, there were 27 (56.3%), 4 (8.3%), and 35 (72.9%) studies had a high risk of bias, respectively. The main contributing factors to this assessment were as follows: (1) the data source of 27 (56.3%) studies was a retrospective cohort, and 6 studies did not report; (2) nineteen (39.6%) studies lacked information on whether predictor assessment was made without knowledge of outcome data; (3) four studies (8.3%) determined outcomes with knowledge of predictor(s), and most studies did not report information on outcomes determined or did not report the time interval between predictor assessment and outcome determination; and (4) the sample sizes of 18 (37.5%) studies were unreasonable (events per variable < 20), sixteen studies used univariable analysis, and 20 (41.7%) studies did not perform calibration assessment or internal validation.
Qualitative analysis results
A summary of the qualitative analysis is presented in Supplementary Table S1, a total of 22 studies head-to-head compared the diagnostic performance of the MRI-inclusive nomogram with that of the traditional clinical nomogram. The ranges of AUCs for MRI-inclusive nomograms and clinical nomograms were 0.62–0.94 and 0.59–0.86, respectively. Twelve of the 22 studies showed that the MRI-inclusive nomogram significantly outperformed the traditional clinical nomogram (all p < 0.05). Two studies showed that the MRI + MSKCC/Partin nomogram provided no additional risk discrimination over the clinical nomogram alone (p > 0.05) [11, 12]. The remaining 8 studies also suggested that the AUC increased when MRI was added to clinical nomograms, but did not provide statistical significance.
Main statistical analysis results
Considering overfitting of performance in development models commonly existed, we therefore included validation cohorts only into meta-analysis in order to reduce overoptimism estimates. As shown in Table 3, for MRI-inclusive nomograms, a total of 13 validation cohorts [29, 38,39,40,41,42,43,44,45, 47, 48, 64] showed a pooled AUC of 0.80 (95% CI: 0.76, 0.83) for EPE prediction. For clinical nomograms, a total of 11 independent validation cohorts showed a pooled AUC of 0.75 (95% CI: 0.71, 0.79). No significant funnel plot asymmetry was observed for studies with MRI-inclusive nomogram (Fig. 4a, P= 0.17); however, significant funnel plot asymmetry was observed for studies with clinical nomogram (Fig. 4b, P= 0.02). The pooled sensitivities, specificities, and AUCs estimated by the HSROC curve (Fig. 5) of MRI-inclusive and clinical nomograms for predicting EPE are shown in Table 3. The forest plots of validation cohorts for MRI-inclusive and clinical nomograms are presented in Supplementary Figures S1- S4. In the subgroup analysis, eight external validations of the Partin table [50, 51, 53,54,55, 59, 61, 62] showed a pooled AUC of 0.72(95% CI: 0.68, 0.76), and 5 external validations of the MSKCC nomogram [55, 58, 60,61,62] showed a pooled AUC of 0.79(95% CI: 0.75, 0.82).
Univariable meta-regression analysis
Meta-regression analysis was performed for all validation cohorts to identify the source of pooled heterogeneity (Table 4). For MRI-inclusive nomograms, the heterogeneity of pooled sensitivity was found in EPE-basing and AI/radiomics-based, and the sensitivity of nomograms which predicted side-specific EPE was higher than predicting whole-gland EPE (p = 0.01). The source of heterogeneity of pooled specificity did not be found from EPE basing, pathological EPE rate, slice thickness, MRI time, or AI-based predictors (all p > 0.05). For clinical nomograms, the pooled specificity of the MSKCC nomogram was significantly higher than that of the Partin table (p < 0.001); there was no significant difference of pooled sensitivity between the Partin table and MSKCC nomogram.
Evaluation of clinical utility
Fagan plots were drawn to calculate the posttest probabilities of all validation cohorts for MRI-inclusive nomogram and clinical nomogram respectively (Fig. 6). Within 50% as the pretest probability, for MRI-inclusive nomograms and clinical nomograms, the positive posttest probabilities were 72% and 70%, respectively; the negative posttest probabilities were 25% and 31%, respectively; and the positive likelihood ratios were 3 and 2, respectively.
Discussion
This present meta-analysis first systematically evaluated the diagnostic performance of preoperative MRI-inclusive nomograms and traditional clinical nomograms for predicting pathological EPE of PCa. Our meta-analysis based on validation cohorts showed that both MRI-inclusive nomograms and traditional clinical nomograms had moderate AUCs (0.72–0.80) for predicting EPE. We found that the pooled specificity of MSKCC nomograms was higher than that of Partin table. Our study provides baseline summative estimates for EPE prediction in PCa as well as provide comparative estimates for future study designs. These findings will be useful in the assessment of preoperative risk stratification and individual treatment strategies for PCa patients.
There are multitudinous studies that tried to head-to-head compare the diagnostic performance of the MRI-inclusive nomogram and traditional clinical nomogram for EPE prediction and suggested that MRI combined clinical nomogram can improve the diagnostic performance of traditional clinical nomogram. However, in our systematic review of 22 studies, we found that most of these studies were TRIPOD 1 type, which means they were limited to developing model. As we know, the overfitting of prediction model generally exists in development cohorts, particularly in a small data set. And the degree of overfitting can vary widely depending on the type of internal validation techniques employed, such as cross-validation or bootstrapping, leading to biased or over-optimistic performance results [15]. Therefore, these conclusions need to be validated in more external validation cohorts to make them more reliable. However, what predictors or how to add MRI variables into clinical nomograms was still not well-defined.
Compared with a previous meta-analysis by de Rooij et al. [7], which reported that MRI had poor and heterogeneous sensitivity (0.57) for EPE diagnosis, our results may provide evidence that MRI combined clinical nomogram had relatively higher sensitivity (0.76). Reasons for the low and variable sensitivity of MRI for EPE diagnosis may be as follows. First, image acquisition protocol can largely influence image quality. Moreover, connective tissue hyperplasia reaction or inflammation may change the appearance of prostatic capsule, subjectivity of assessing some qualitative features like bulging and irregularity of prostatic capsule could not be ruled out, and radiologic interpretation may be influenced by the specialization and experience of radiologists [65]. Thus, its accuracy for EPE diagnosis on MRI evaluation remains challenging. To overcome this issue, radiologists have been devoted to standardize the EPE reporting to improve its precision and repeatability, and several EPE grading systems have been proposed in recent years with relatively good performance [8, 9, 66]. Moreover, several scholars have reported that combined MRI-EPE grade with traditional clinical nomograms can significantly improve the diagnostic accuracy [9, 25]. Therefore, we suggested that more external validation studies for the MRI-EPE grade combined clinical nomograms should be carried on in the future research. Second, it was realized that sensitivity for detecting EPE on MRI can never match pathology, where the standard is microscopy [67]. However, few studies have focused on the prediction of microscopic EPE to date. Thus, improvements are needed for both the clinic and imaging to further investigate the predictive efficiency for detecting microscopic EPE.
In particular, although our regression analysis did not find significant diagnostic superiority of nomogram which using AI/radiomics-based MRI predictors in EPE prediction, this may be due to the limited statistic power given the small number of studies on AI/radiomics-based MRI predictors (6 contingency tables from 4 studies [39, 42, 43, 45]). Moreover, the AI/radiomics-based MRI predictors of these 4 studies were heterogeneous, involving peritumoral region radiomic features [39], tumor radiomic signatures extracted from ADC [42], the ResNeXt network model [43], and the self-defined radiomic score [45]. Thus, more studies are needed to evaluate the optimal and repeatable radiomic characteristics of multiple MR sequences for EPE diagnosis. Many studies have proposed that artificial intelligence, including radiomics and deep learning, is a promising solution to diagnosis and stage PCa [68,69,70]. However, the combination of AI-based MRI characteristics and clinical variables to predict EPE has not yet been fully explored.
In our meta-regression analysis, no significant difference in sensitivity was observed between the Partin tables and MSKCC nomogram. The specificity of the MSKCC nomogram was significantly higher than that of the Partin table (0.77 vs. 0.60). One difference between the MSKCC nomogram and the Partin table is the addition of the biopsy-positive core ratio. It has been reported that using continuous risk variables in nomograms rather than binary variables can substantially improve predictive accuracy [71], which may be a probable explanation for why the MSKCC nomogram had a better diagnostic performance. In general, the predictive accuracy for EPE of all clinical nomograms was relatively low and diverse (AUC: 0.60–0.86). Several molecular markers have been discovered for predicting EPE (e.g., interleukin-6 soluble receptor, transforming growth factor-b1, GRE), incorporating these new markers into traditional nomograms may improve the prediction ability of disease progression [71, 72]. Therefore, in the era of PSA screening, as the composition of patients with localized disease increases, nomograms need to be periodically updated, and new biomarkers need to be added to reflect these population changes and to improve the prediction accuracy [73].
Some limitations existed in our study. First, the number of eligible studies included was small, and a large number of studies were excluded due to a lack of data to calculate TP, FN, FP, and TN. Second, there was significant heterogeneity in patient populations, clinical characteristics, and MRI practice, which increased the risk of intrinsic bias and led to significant heterogeneity (I2). Third, we could not explain the heterogeneity completely because many studies did not report sufficient information for all characteristics. Finally, there are no established MRI assessment criteria for EPE; therefore, the imaging predictors varied widely. To overcome these issues, we suggest that studies should be devoted to establishing a standardized MRI-EPE evaluation system, which would be helpful to improve diagnostic accuracy and repeatability. Moreover, nomograms need to be updated, and new biomarkers need to be added to further improve the diagnostic performance. In addition, no studies have focused on predicting microscopic EPE, which is the most relevant for RP purposes, as overt EPE is presumably easily found on MRI; thus, more studies should be carried out to evaluate microscopic EPE in the future.
Conclusion
This systematic review and meta-analysis first summatively evaluated the diagnostic accuracy of preoperative MRI-inclusive nomograms and traditional clinical nomograms in predicting pathological EPE of PCa. Both MRI-inclusive nomograms and traditional clinical nomograms had moderate AUCs (0.72–0.80) for predicting EPE. MRI combined clinical predictors can improve diagnostic value to MRI alone, which could aid urologists in making decision protocols for local PCa patient treatment.
Abbreviations
- AUC:
-
Area under the curve
- CAPRA:
-
Cancer of the Prostate Risk Assessment
- EPE:
-
Extraprostatic extension
- FN:
-
False negative
- FP:
-
False positive
- HSROC:
-
Hierarchical summary receiver operating characteristic
- MSKCC:
-
Memorial Sloan Kettering Cancer Center
- PCa:
-
Prostate cancer
- TN:
-
True negative
- TP:
-
True positive
References
Jeong BC, Chalfin HJ, Lee SB et al (2015) The relationship between the extent of extraprostatic extension and survival following radical prostatectomy. Eur Urol 67:342–346
Yossepowitch O, Briganti A, Eastham JA et al (2014) Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol 65:303–313
Eifler JB, Feng Z, Lin BM et al (2013) An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int 111:22–29
Cooperberg MR, Pasta DJ, Elkin EP et al (2005) The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol 173:1938–1942
Ohori M, Kattan MW, Koh H et al (2004) Predicting the presence and side of extracapsular extension: a nomogram for staging prostate cancer. J Urol. 171:1844–1849; discussion 1849
Turkbey B, Rosenkrantz AB, Haider MA et al (2019) Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 76:340–351
de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM (2016) Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol 70:233–245
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757
Mehralivand S, Shih JH, Harmon S et al (2019) A grading system for the assessment of risk of extraprostatic extension of prostate cancer at multiparametric MRI. Radiology 290:709–719
Caglic I, Kovac V, Barrett T (2019) Multiparametric MRI - local staging of prostate cancer and beyond. Radiol Oncol 53:159–170
Jansen BHE, Nieuwenhuijzen JA, Oprea-Lager DE et al (2019) Adding multiparametric MRI to the MSKCC and Partin nomograms for primary prostate cancer: Improving local tumor staging? Urol Oncol 37:181.e181–181.e186
Weaver JK, Kim EH, Vetter JM et al (2018) Prostate magnetic resonance imaging provides limited incremental value over the Memorial Sloan Kettering Cancer Center preradical prostatectomy nomogram. Urology 113:119–128
Salameh JP, Bossuyt PM, McGrath TA et al (2020) Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ 370:m2632
Collins GS, Reitsma JB, Altman DG, Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 350:g7594
Wolff RF, Moons KGM, Riley RD et al (2019) PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 170:51–58
Jackson D, Turner R (2017) Power analysis for random-effects meta-analysis. Res Synth Methods 8:290–302
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Rutter CM, Gatsonis CA (2001) A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 20:2865–2884
Chen Y, Yu W, Fan Y et al (2017) Development and comparison of a Chinese nomogram adding multi-parametric MRI information for predicting extracapsular extension of prostate cancer. Oncotarget 8:22095–22103
Diamand R, Ploussard G, Roumiguié M et al (2021) External validation of a multiparametric magnetic resonance imaging-based nomogram for the prediction of extracapsular extension and seminal vesicle invasion in prostate cancer patients undergoing radical prostatectomy. Eur Urol 79:180–185
Feng TS, Sharif-Afshar AR, Wu J et al (2015) Multiparametric MRI improves accuracy of clinical nomograms for predicting extracapsular extension of prostate cancer. Urology 86:332–337
Losnegård A, Reisæter LAR, Halvorsen OJ et al (2020) Magnetic resonance radiomics for prediction of extraprostatic extension in non-favorable intermediate- and high-risk prostate cancer patients. Acta Radiol 61:1570–1579
Martini A, Gupta A, Lewis SC et al (2018) Development and internal validation of a side-specific, multiparametric magnetic resonance imaging-based nomogram for the prediction of extracapsular extension of prostate cancer. BJU Int 122:1025–1033
Morlacco A, Sharma V, Viers BR et al (2017) The incremental role of magnetic resonance imaging for prostate cancer staging before radical prostatectomy. Eur Urol 71:701–704
Nyarangi-Dix J, Wiesenfarth M, Bonekamp D et al (2020) Combined clinical parameters and multiparametric magnetic resonance imaging for the prediction of extraprostatic disease-a risk model for patient-tailored risk stratification when planning radical prostatectomy. Eur Urol Focus 6:1205–1212
Ravi C, Sanjeevan KV, Thomas A, Pooleri GK (2021) Development of an Indian nomogram for predicting extracapsular extension in prostate cancer. Indian J Urol 37:65–71
Rayn KN, Bloom JB, Gold SA et al (2018) Added value of multiparametric magnetic resonance imaging to clinical nomograms for predicting adverse pathology in prostate cancer. J Urol 200:1041–1047
Renard-Penna R, Rouprêt M, Comperat E et al (2013) Accuracy of high resolution (1.5 tesla) pelvic phased array magnetic resonance imaging (MRI) in staging prostate cancer in candidates for radical prostatectomy: results from a prospective study. Urol Oncol 31:448–454
Soeterik TFW, van Melick HHE, Dijksman LM et al (2020) Development and external validation of a novel nomogram to predict side-specific extraprostatic extension in patients with prostate cancer undergoing radical prostatectomy. Eur Urol Oncol. https://doi.org/10.1016/j.euo.2020.08.008
Wang L, Mullerad M, Chen HN et al (2004) Prostate cancer: incremental value of endorectal MR imaging findings for prediction of extracapsular extension. Radiology 232:133–139
Wibmer AG, Kattan MW, Alessandrino F et al (2021) International multi-site initiative to develop an MRI-inclusive nomogram for side-specific prediction of extraprostatic extension of prostate cancer. Cancers (Basel) 13(11):2627
Gandaglia G, Ploussard G, Valerio M et al (2020) The Key Combined Value of Multiparametric Magnetic Resonance Imaging, and Magnetic Resonance Imaging-targeted and Concomitant Systematic Biopsies for the Prediction of Adverse Pathological Features in Prostate Cancer Patients Undergoing Radical Prostatectomy. Eur Urol 77:733–741
Liu H, Tang K, Xia D et al (2020) Added value of biparametric MRI and TRUS-guided systematic biopsies to clinical parameters in predicting adverse pathology in prostate cancer. Cancer Manag Res 12:7761–7770
Sandeman K, Eineluoto JT, Pohjonen J et al (2020) Prostate MRI added to CAPRA, MSKCC and Partin cancer nomograms significantly enhances the prediction of adverse findings and biochemical recurrence after radical prostatectomy. PLoS One 15:e0235779
Blas L, Shiota M, Nagakawa S et al (2023) Validation of user-friendly models predicting extracapsular extension in prostate cancer patients. Asian J Urol 10:81–88
Diamand R, Roche JB, Lievore E et al (2023) External validation of models for prediction of side-specific extracapsular extension in prostate cancer patients undergoing radical prostatectomy. Eur Urol Focus 9:309–316
Sun YK, Yu Y, Xu G et al (2023) Added value of shear-wave elastography in the prediction of extracapsular extension and seminal vesicle invasion before radical prostatectomy. Asian J Androl 25:259–264
Alves JR, Muglia VF, Lucchesi FR et al (2020) Independent external validation of nomogram to predict extracapsular extension in patients with prostate cancer. Eur Radiol 30:5004–5010
Bai H, Xia W, Ji X et al (2021) Multiparametric magnetic resonance imaging-based peritumoral radiomics for preoperative prediction of the presence of extracapsular extension with prostate cancer. J Magn Reson Imaging 54:1222–1230
Giganti F, Coppola A, Ambrosi A et al (2016) Apparent diffusion coefficient in the evaluation of side-specific extracapsular extension in prostate cancer: Development and external validation of a nomogram of clinical use. Urol Oncol 34:291.e299–291.e217
Hara T, Nakanishi H, Nakagawa T et al (2013) Ability of preoperative 3.0-Tesla magnetic resonance imaging to predict the absence of side-specific extracapsular extension of prostate cancer. Int J Urol 20:993–999
He D, Wang X, Fu C et al (2021) MRI-based radiomics models to assess prostate cancer, extracapsular extension and positive surgical margins. Cancer Imaging 21:46
Hou Y, Zhang YH, Bao J et al (2021) Artificial intelligence is a promising prospect for the detection of prostate cancer extracapsular extension with mpMRI: a two-center comparative study. Eur J Nucl Med Mol Imaging 48:3805–3816
Lebacle C, Roudot-Thoraval F, Moktefi A, Bouanane M, De La Taille A, Salomon L (2017) Integration of MRI to clinical nomogram for predicting pathological stage before radical prostatectomy. World J Urol 35:1409–1415
Xu L, Zhang G, Zhao L et al (2020) Radiomics based on multiparametric magnetic resonance imaging to predict extraprostatic extension of prostate cancer. Front Oncol 10:940
Zapała P, Dybowski B, Bres-Niewada E et al (2019) Predicting side-specific prostate cancer extracapsular extension: a simple decision rule of PSA, biopsy, and MRI parameters. Int Urol Nephrol 51:1545–1552
Zapała P, Kozikowski M, Dybowski B, Zapała Ł, Dobruch J, Radziszewski P (2021) External validation of a magnetic resonance imaging-based algorithm for prediction of side-specific extracapsular extension in prostate cancer. Cent European J Urol 74:327–333
Chen X, Li W, Yang J et al (2023) Extracapsular extension of transitional zone prostate cancer miss-detected by multiparametric magnetic resonance imaging. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-023-04573-w
Guerra A, Alves FC, Maes K et al (2022) Early biomarkers of extracapsular extension of prostate cancer using MRI-derived semantic features. Cancer Imaging 22:74
Boyce S, Fan Y, Watson RW, Murphy TB (2013) Evaluation of prediction models for the staging of prostate cancer. BMC Med Inform Decis Mak 13:126
Dal Moro F, Prayer-Galetti T, Angelini L et al (2018) Study of diagnostic accuracy of Fagan’s two-step nomogram in increasing the value of predictive tools for prostate cancer: application of specific spatial distribution of positive/negative bioptic cores to predict extracapsular extension. Aging Clin Exp Res 30:1497–1504
Egawa S, Suyama K, Matsumoto K et al (1998) Improved predictability of extracapsular extension and seminal vesicle involvement based on clinical and biopsy findings in prostate cancer in Japanese men. Urology 52:433–440
Tsao CW, Liu CY, Cha TL et al (2014) Artificial neural network for predicting pathological stage of clinically localized prostate cancer in a Taiwanese population. J Chin Med Assoc 77:513–518
Song C, Kang T, Ro JY, Lee MS, Kim CS, Ahn H (2005) Nomograms for the prediction of pathologic stage of clinically localized prostate cancer in Korean men. J Korean Med Sci 20:262–266
Thalgott M, Düwel C, Rauscher I et al (2018) One-stop-shop whole-body (68)Ga-PSMA-11 PET/MRI compared with clinical nomograms for preoperative T and N staging of high-risk prostate cancer. J Nucl Med 59:1850–1856
Partin AW, Kattan MW, Subong EN et al (1997) Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. JAMA. 277:1445–1451
Sighinolfi MC, Assumma S, Cassani A et al (2023) Pre-operative prediction of extracapsular extension of prostate cancer: first external validation of the PRECE model on an independent dataset. Int Urol Nephrol 55:93–97
Majchrzak N, Cieśliński P, Głyda M, Karmelita-Katulska K (2021) Prostate magnetic resonance imaging analyses, clinical parameters, and preoperative nomograms in the prediction of extraprostatic extension. Clin Pract 11:763–774
Wang J, Wu CJ, Bao ML, Zhang J, Shi HB, Zhang YD (2018) Using support vector machine analysis to assess PartinMR: a new prediction model for organ-confined prostate cancer. J Magn Reson Imaging 48:499–506
Xu L, Zhang G, Zhang X et al (2021) External validation of the extraprostatic extension grade on MRI and its incremental value to clinical models for assessing extraprostatic cancer. Front Oncol 11:655093
Zanelli E, Giannarini G, Cereser L et al (2019) Head-to-head comparison between multiparametric MRI, the partin tables, memorial sloan kettering cancer center nomogram, and CAPRA score in predicting extraprostatic cancer in patients undergoing radical prostatectomy. J Magn Reson Imaging 50:1604–1613
Xiang JY, Huang XS, Xu JX et al (2022) MRI extraprostatic extension grade: accuracy and clinical incremental value in the assessment of extraprostatic cancer. Biomed Res Int 2022:3203965
Patel VR, Sandri M, Grasso AAC et al (2018) A novel tool for predicting extracapsular extension during graded partial nerve sparing in radical prostatectomy. BJU Int 121:373–382
Soeterik TFW, van Melick HHE, Dijksman LM et al (2020) External validation of the Martini nomogram for prediction of side-specific extraprostatic extension of prostate cancer in patients undergoing robot-assisted radical prostatectomy. Urol Oncol 38:372–378
Wibmer A, Vargas HA, Donahue TF et al (2015) Diagnosis of extracapsular extension of prostate cancer on prostate MRI: impact of second-opinion readings by subspecialized genitourinary oncologic radiologists. AJR Am J Roentgenol 205:W73–78
Costa DN, Passoni NM, Leyendecker JR et al (2018) Diagnostic utility of a Likert scale versus qualitative descriptors and length of capsular contact for determining extraprostatic tumor extension at multiparametric prostate MRI. AJR Am J Roentgenol 210:1066–1072
Eberhardt SC (2019) Local staging of prostate cancer with MRI: a need for standardization. Radiology 290:720–721
Ma S, Xie H, Wang H et al (2019) MRI-based radiomics signature for the preoperative prediction of extracapsular extension of prostate cancer. J Magn Reson Imaging 50:1914–1925
Cuocolo R, Stanzione A, Faletti R et al (2021) MRI index lesion radiomics and machine learning for detection of extraprostatic extension of disease: a multicenter study. Eur Radiol 31:7575–7583
Gugliandolo SG, Pepa M, Isaksson LJ et al (2021) MRI-based radiomics signature for localized prostate cancer: a new clinical tool for cancer aggressiveness prediction? Sub-study of prospective phase II trial on ultra-hypofractionated radiotherapy (AIRC IG-13218). Eur Radiol 31:716–728
Kattan MW, Shariat SF, Andrews B et al (2003) The addition of interleukin-6 soluble receptor and transforming growth factor beta1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol 21:3573–3579
Lu LI, Zhang H, Pang J, Hou GL, Lu MH, Gao X (2016) ERG rearrangement as a novel marker for predicting the extra-prostatic extension of clinically localised prostate cancer. Oncol Lett 11:2532–2538
Turo R, Forster JA, West RM, Prescott S, Paul AB, Cross WR (2015) Do prostate cancer nomograms give accurate information when applied to European patients? Scand J Urol 49:16–24
Acknowledgements
Figure 1 Flow Diagram was obtained from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
Funding
This work was funded by the National Key Research and Development Project in China (No. 2017YFC0113405), and National Natural Science Foundation of China (82071877).
Author information
Authors and Affiliations
Contributions
M.Z. conceived and designed the study. M.Z. and J.G. contributed to the performed analysis and interpretation of data and drafted the manuscript. M.Z., J.G., F.H., L.Y., L.Z., and Y.Y. contributed to the literature research and interpretation of data and revised the manuscript. J.Z. and Y.Y. contributed to the clinical aspects as well as to the critical revision of the paper. J.Z. and Y.Y. contributed to the critical revision of the paper and was the guarantor of integrity of the entire study. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and finally approved the version of the manuscript to be published. All authors read and approved by the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary material. Supplementary Figures. Supplementary Tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, M., Gao, J., Han, F. et al. Diagnostic performance of prediction models for extraprostatic extension in prostate cancer: a systematic review and meta-analysis. Insights Imaging 14, 140 (2023). https://doi.org/10.1186/s13244-023-01486-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13244-023-01486-7