Abstract
Autism spectrum disorder (ASD) is a group of complex neurodevelopmental disorders with diverse clinical manifestations and symptoms. In the last 10 years, there have been significant advances in understanding the genetic basis for ASD, critically supported through the establishment of ASD bio-collections and application in research. Here, we summarise a selection of major ASD bio-collections and their associated findings. Collectively, these include mapping ASD candidate genes, assessing the nature and frequency of gene mutations and their association with ASD clinical subgroups, insights into related molecular pathways such as the synapses, chromatin remodelling, transcription and ASD-related brain regions. We also briefly review emerging studies on the use of induced pluripotent stem cells (iPSCs) to potentially model ASD in culture. These provide deeper insight into ASD progression during development and could generate human cell models for drug screening. Finally, we provide perspectives concerning the utilities of ASD bio-collections and limitations, and highlight considerations in setting up a new bio-collection for ASD research.
Similar content being viewed by others
Background
Autism spectrum disorder (ASD) is a group of early onset and heterogeneous neurodevelopmental disorders affecting males (1/42) more often than females (1/189) [1]. The prevalence of ASD has risen rapidly; from 0.5/1000 people in early epidemiological studies of 1960–1970 [2, 3] to 1/68 children of school age according to recent data from the Centre for Disease Control [1].
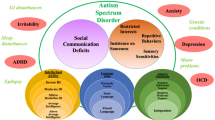
ASD is characterised by atypical development of social behaviour, communication deficits and the presence of repetitive and stereotyped behaviours [4]. It is highly clinically heterogeneous and accompanied by commonly occurring comorbidities that are not core to the disorder but frequently disabling. Communication deficit also persists in social communication disorder (SCD), and the new diagnosis of SCD (DSM-5) makes it possible to distinguish ASD from SCD individuals. The severity may vary across a range of parameters including ASD symptoms, IQ and comorbid behaviours [4]. For example, 70% ASD patients will have at least 1 comorbid psychiatric disorder [5], such as social anxiety, depression and bipolar disorder [6]. In addition, ASD is frequently associated with epilepsy, gastrointestinal and immune disorders [7].
ASD is a highly heritable complex polygenic condition. Estimated heritability based on family and twin studies are 50–80% [8, 9]. It is strongly linked to genetic factors involving the development and function of the nervous system [10], mitochondrial function [11], the immune system [12] and epigenetic regulations [13]. Genetic risk is attributed to rare copy number variants (CNV) and single nucleotide variants (SNV) acting on the background of common genetic variation (reviewed by [14]). High throughput genome sequencing technologies have facilitated genomic discovery, and advanced bioinformatics methodologies have enabled investigation of protein-protein interactions [15, 16] and functionally related pathways [17, 18]. The pathway to gene discovery has required large-scale international collaborative efforts based on the assembly of large bio-collections that are now publicly available and the subject of this review. In parallel to bio-collections, large-scale patient registries have provided epidemiological data that illustrate the course and prognosis of ASD and are helping to identify environmental factors influencing the aetiology [19,20,21,22]. Despite the advances, significant gaps in our knowledge of the aetiology remain and effective treatments for core ASD symptoms are elusive. The genetic and clinical heterogeneity of ASD means that further advancement will require larger bio-collections coupled with rich clinical data, ideally longitudinally to obtain a clear picture of the disorder both on the molecular and physiological levels.
Autism bio-collections
A bio-collection is a large set of biologically characterised samples, such as blood or tissue collected from a group of individuals who typically have a specific medical condition. Bio-collections are useful as a dedicated resource to generate clinical and scientific data for the analysis of medical conditions on a large scale [23], as well as to create functional disease models to explore the biology of clinical conditions. Large-scale bio-collections and associated comprehensive data that can aid the interrogation of the relationship between the genotype and phenotype effects at the individual and group levels can address the issue of heterogeneity. The purpose of this review is to provide a summary of the publicly available ASD bio-collections, to highlight the impact of these on ASD research and to identify new directions for ASD bio-collection for future research purposes.
Methods and search criteria
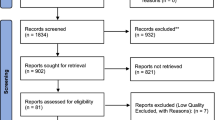
A literature search was conducted amongst published studies from Jan 2001 to Nov 2016 on electronic databases of Web of Science, EBSCO, PubMed, Science Direct, MEDLINE, Wiley Online Library. The search terms included “biobank”, “registry”, “collection”, “autism” and citation of bio-collections. A total of 263 studies from ASD bio-collections have been included in the tables and references of this review (Tables 2, 3, 4, 5 and 6).
Inclusion criteria
This review included (a) studies using original samples of human tissues in ASD bio-collections; (b) studies using bio-samples extracted from systematically collected bio-resources (i.e. DNA, RNA, protein) for investigating the risk or influence of ASD; (c) the population studies involving participants of autism, Asperger and pervasive developmental disorder not otherwise specified (PDD-NOS); (d) studies published in peer-reviewed journals and (e) in English.
Exclusion criteria
Studies were excluded (a) if they did not mention the collection(s) in the research data, references, acknowledgements or supplementary materials; (b) if the bio-samples were not derived from a systematic sample collection; and (d) if studies only concerned animal models of ASD without using ASD bio-collections or data.
We focus largely on studies from five bio-collections, four providing DNA, cell lines and metabolites, the Autism Genetic Resource Exchange (AGRE), Simons Simplex Collection (SSC), The Danish Newborn Screening Biobank (DNSB) and The Autism Simplex Collection (TASC) one providing brain tissue, Autism BrainNet (formerly the Autism Tissue Program (ATP)).We also included two emerging bio-collections that have fewer or no publications released yet, but could be of significant impact in the future. They are the Autism Inpatient Collection (AIC) [24] and the Autism Spectrum Stem Cell Resource [25]. An overview of the bio-collections and their website links can be found in Table 1.
Results
Autism Genetic Resource Exchange (AGRE)
AGRE was established in 1997 by the Cure Autism Now (CAN) Foundation and the Human Biological Data Interchange (HBDI). Samples are provided by families with children affected by ASD and are coupled with anonymously coded clinical diagnostic data, such as Autism Diagnostic Interview–Revised (ADI–R) and Autism Diagnostic Observational Schedule (ADOS). Additional clinical data include photographic dysmorphology, neurological and physical examination, and family and medical history. AGRE is currently managed by Autism Speaks. It contains over 2500 families and the resource has contributed to high profile genetic discoveries relating to ASD (Table 2). Samples are housed at the National Institute of Mental Health repository at Rutgers’ University in the form of immortalised cell lines, DNA and serum samples which can be accessed by researchers through applications [20].
The AGRE resource has been used extensively in genomics studies in ASD. Approaches have included gene-mapping such as genome-wide linkage and association studies in addition to studies of chromosomal structure, particularly the identification of copy number variants. Important ASD chromosomal regions identified include microdeletions and microduplications of 16p11.2 [26, 27], rearrangements and microdeletion/duplication of 15q13.2q13.3 [28,29,30,31], common variants in the 5p14.1 region [32, 33], Neurexins and 11p12–p13 [34].
It has also helped in identification of recurrent candidate genes, such as MECP2 [35,36,37], PTEN [38, 39], EN2 [40,41,42], RELN [40,44,45,, 43–46], RORA [47], MET [48,49,50], NGLN3-4 [51], BZRAP1 [28], SLC6A4 [40, 52] GABA receptors [32,54,, 43, 53–55], CACNA1G [56] and the sodium channel genes SCN1A, SCN2A and SCN3A [57].
These studies particularly highlighted an important role of de novo and large inherited copy number variations (CNVs), which are detected in 10% of sporadic ASD [58], which has been widely replicated in other bio-collections [59,60,61,62,63,64,65,66,67,68,69,70,71]. The use of AGRE combined with other AGP resources have uncovered SHANK2, SYNGAP1, DLGAP2 and the X-linked DDX53-PTCHD1 locus as novel ASD genes, as well as pathways of cellular proliferation, signalling, neuronal projection and motility [72]. AGRE samples formed a replication set in a separate analysis highlighting CNVs of neuronal cell adhesion and ubiquitin pathway in ASD [73].
AGRE lymphoblastoid cells enabled studies into shared ubiquitin and neuronal gene expression in lymphoblastoid cells and brain [73, 74], gluthathione metabolism, oxidative stress [75, 76] and stress response [77], microRNAs and their use in ASD profiling [78, 79], CYFIP1 dosage effect on mTOR regulation [80], and changes in methylation patterns of RORA and BLC2 and their effects on apoptosis, cellular differentiation, inflammation and neural development [47].
The AGRE collection was also used to establish genetic methodologies and bioinformatic tools. This included using mismatch repair to detect amplicons in ASD [81], using multiplex ligation-dependent probe amplification (MPLA) to improve detection of microduplications and microdeletions [82], and incorporating disease symptoms to improve linkage detection in genetic data [83] and analysis of genetic loci to search for candidate genes [84].
Simons Simplex Collection (SSC)
The SSC is a genetic and clinical repository, which contains material derived from 2600 families. Whereas the AGRE contains multiplex families and trios, The SSC ascertained “simplex” ASD families defined as families where only one child has ASD and at least one other typically developing sibling. DNA is available for both parents, the affected child and an unaffected sibling. Thus the SSC samples are particularly valuable in evaluating parental inheritance. Samples were collected at multiple sites and were stored as immortalised cell lines at Rutgers University Cell and DNA Repository (RUCDR). Each sample was verified for parentage, gender and Fragile X mutation. In-depth clinical phenotypes were characterised for all participants to support genotype-phenotype analyses. These included data on diagnostic status, medical and psychiatric comorbidity, family history and medication use for the affected person. Broader ASD phenotype measures were collected for unaffected family members.
The SSC has become a vast resource of ASD and contributed significantly to numerous Whole exome sequencing studies of ASD in the past ~7 years (Table 3). The main findings showed that de novo mutations were frequently enriched in ASD patients [60]. Whole-genome sequencing results showed a significant enrichment of de novo and private disruptive mutations in putative regulatory regions of previously identified ASD risk genes. It also identified novel risk factors of CANX, SAE1 and PIK3CA with small CNVs and exon-specific SNPs, which were overlooked in previous CNV studies or exon sequencing [85]. It has also been observed that many de novo mutations were of paternal origin (4:1) and positively correlated with paternal age, [65]. The disruptive mutations were located in genes involve in transcription regulation, chromatin remodelling and synapse formation [86, 87].
The SSC has enabled detection of the ultra-rare “recurrent” CNVs. This included duplications of 7q11.23, 15q11.2 (NIPA) and 16p13.11, and deletion/duplication of 16p11.2, 16p13.2 (USP7), 1q21.1, 2p16.3, 7q31.1, 15q13.2–q13.3, 16p13.3, 20q13.33 and 22q11.21 [60]. The SSC also helped identify recurrent gene mutations in ASD include CHD8, NTNG1, GRIN2B, SCN1A and LAMC3, which are important for transcriptional regulation, neuronal differentiation and function [87].
CHD8 was further evaluated as an ASD candidate gene in children with developmental delay or ASD, and 15 independent mutations were identified and enriched in a subset of ASD with altered brain size, distinct facial features and gastrointestinal complaints. Disruption of CDH8 in zebra fish recapitulated some of the patient phenotypes including increased head size and impaired gastrointestinal motility [88]. CHD8 is shown to control expression of other high-confidence de novo ASD risk genes such as DYRK1A, GRIN2B and POGZ [89]. Mutation of DYRK1A was strongly linked to a subset of ASD patients with seizures at infancy, hypertonia, intellectual disability, microencephaly, dysmorphic facial features and impaired speech [71, 89]. POGZ gene which plays a role in cell cycle progression is also found to contribute to a subset of ASD with varying developmental delay, vision problems, motor coordination impairment, tendency of obesity, microcephaly, hyperactivity and feeding problems [90].
Danish Newborn Screening (NBS) Biobank
The NBS Biobank has a large collection of dried blood spot samples (DBSS), which are taken from new-borns 5–7 days after birth. They are sent to the New-born Screening lab at the Statens Serum Institute for analysis, and stored at −20 °C in a separate freezing facility at the NBS Biobank. Prior to collection, parents are informed via leaflets about the biobank, with focus on what the samples will be used for (documentation, testing and retesting, research, etc.). Participants can opt out of storage at any time via a letter to the department. For security, both the clinical data and biological samples are linked via a unique number, kept in separate buildings, and are accessible by authorised personnel only [91]. The advantage of the NBS resources is that it provides a large amount of non-ASD controls as well as Danish ASD samples.
In the past 30 years the NBS Bio-collection has accumulated samples from 2.2 million individuals, around 65,000–70,000 samples per year from Denmark, Greenland and the Faroe Islands. Most recently this resource has been included under the Danish iPsych consortium with the Psychiatric Genomics Consortium (PGC), added 8–12 k samples to the PGC analysis and significantly increased its power to detect common genetic effects for ASD, which have been recently published [92].
DBSS were also used to examine metabolites. A group led by Abdallah carried out a series of studies on Danish collections (Table 4) to examine the potential role of cytokines and chemokines involved in signalling and immune response of ASD. Initially using amniotic fluid from the Danish Birth Cohort (DBC) collection [93, 94], they followed up with DBSS from new-borns crossed referenced from that cohort [95, 96]; they detected an imbalance of cytokines amongst ASD subjects compared to the controls. Most of the chemicals were lower than normal, such as Th-1 and Th-2 like cytokines involved in proliferation, priming and activation of these cell types, whereas a small number of cytokines displayed increased expression in ASD. The abnormal levels of these chemicals could lead to a hypoactive or “inactive” immune system in the brain, making it more susceptible to infection-related ASD. However, when chemokine levels were examined in amniotic fluid, no concrete relationship could be established.
The Autism Simplex Collection (TASC)
TASC is a trio-based international bio-collection that was assembled in collaboration with the Autism Genome Project and funded by Autism Speaks [97]. Trios, comprised of both parents and a child affected with ASD with no known medical or genetic cause. Collection of samples took place between 2008 and 2010 across 13 sites; 9 in North America and 4 in Europe. Management, storage and distribution of TASC data are handled by the Centre for Collaborative Genetic Studies on Mental Disorders (CCGSMD) [97]. Samples are housed at the NIMH and AGRE repositories both of which are located at Rutgers University.
So far, TASC has been used for GWAS studies [66] and CNV studies [72, 98, 99] and WES Studies [16, 100, 101]. In addition, TASC has also been used in WGS as part of the MSSNG project, which is discussed below
Autism Inpatient Collection (AIC)
The AIC is a bio-collection for ASD research based on those on the serve end of the spectrum with severe language impairment, intellectual disability and self-injurious behaviour. This collection was founded on the basis that this segment of ASD patients are largely unrepresented in current studies. Bio-samples are initially recruited from 147 patients, and ongoing recruitment is estimated at 400 per year. Psychiatric, clinical and phenotypic data are collected in addition to blood samples for the creation of lymphoblastoid cell lines by RUCDR. Amongst this collection, over half are non-verbal, over 40% have intellectual disability and a quarter exhibit self-injurious behaviour [24]. This collection has yet to be used in any genetics-based studies. The fact that many patients are on the severe end of the spectrum makes it a welcome addition, and it opens opportunities to explore this under-represented group.
Autism Tissue Program (ATP)/Autism BrainNet
The Autism Tissue Program, now the Autism Brain Network, is a post-mortem ASD brain collection coordinated by a network of parents, caregivers, physicians and pathologists. Brain samples are preserved in formalin and/or in −80 °C freezers to maximise the potential studies. In some cases, both hemispheres are fixed in formalin when there is freezing capacity or if the post-mortem interval exceeds 24 h. Corresponding clinical data include age, sex, ethnicity, diagnosis, brain size, cause of death, post-mortem interval and preservation method for the left and right hemisphere of the brain. Due to the rarity of the sample, a thorough application procedure assesses scope, scale and feasibility of proposed projects prior to access of tissue, with the expectation that data, images and presentations generated by research on the samples are provided back to the Autism Brain Network 3 months after formal release of publications [102].
Brain pathology and molecular mechanisms have been the focus of studies using the ATP resource (Table 5) although many studies looking at brain anatomy and cell morphology employed samples from this collection, molecular and genetic studies are the primary focus of this review. Such studies included transcriptomics [103,104,105], epigenetics [29,107,108,109,110,111,112,113,114,, 106–115] and alternative splicing [116, 117]. A key discovery was the identification of convergent molecular pathology linking to neuronal, glial and immune genes [105] in a transcriptomics study that investigated the gene co-expression network between autistic and control brains. This led to the proposal of abnormal cortical patterning as an underlying mechanism due to attenuated differential expression in frontal and temporal cortices in ASD brains.
A recent study showed reduced Vitamin B12 in ASD brains [118] where the ATP made a very large contribution. Post-mortem examination of brain tissue ranging from foetal to the elderly subjects also showed a marked decline of the brain vitamin B12 with age, together with lower activity of methionine synthase in the elderly, but the differences were more pronounced in ASD and schizophrenia subjects when compared to controls. Acetylation is an important post-translational modification in the field of epigenetics. ATP also made a significant contribution to a large-scale histone acetylome-wide association study (HAWAS) using the prefrontal cortex, cerebellum and temporal cortex in ASD patients and controls. Despite their heterogeneity, 68% of syndromic and idiopathic ASD cases shared a common acetylome signature at >5000 cis-regulatory elements in the prefrontal cortex and temporal cortex. Aberrant acetylome was found to be associated with synaptic transmission, ion transport, epilepsy, behavioural abnormality, chemokinesis, histone deacetylation and immunity [113].
The ATP sample was used in a methylation study that investigated differential methylation in CpG loci in three brain regions: temporal cortex, dorsolateral prefrontal cortex and cerebellum. Differential methylation of four genes (PRRT1, C11orf21/TSPAN32, ZFP57 and SDHAP3) was detected. PRRT1, C11orf21/TSPAN32 were hypomethylated while the latter two were hypermethylated [109]. A further investigation in Brodmann’s area also found a pattern of hypomethylation of a number of genes including C11orf21/TSPAN32 that are implicated in immune function and synaptic pruning [111]. These hypomethylated genes correlated with those showing overexpression by Voineagu.
The methylation studies have further uncovered dysregulation of OXTR and SHANK3 genes in ASD. OXTR gene encoding oxytoxcin receptor was significantly hypermethylated in the peripheral blood cells and temporal cortex of ASD, highlighting a reduced oxytocin signalling in the aetiology of ASD [108] and a therapeutic target of ASD. Differential methylation of the SHANK3 gene was detected between ASD and control brains. They found that when three 5′ CpG islands of the gene were examined, they observed altered methylation also changed SHANK3 splicing, with specific SHANK3 isoforms expressed in ASD [114].
This is echoed by a recent study, which reveals a dynamic microexon regulation associated with the remodelling of protein-interaction networks during neurogenesis. The neural microexons are frequently dysregulated in the brains of ASD, which is associated with reduced expression of SRRM4 [116]. The neuronal-specific splicing factor A2BP1/FOX1 and A2BP1-dependent splicing of alternative exons are also dysregulated in ASD brain [105].
Replication studies and pooling resources
Research data from one bio-collection is not always replicable in another sample set. Therefore, cross-validation between different bio-collections will not only minimise false positive, but also identify the common risk factors and subset-specific factors. For example, a genome-wide survey was carried out to test trans-generational effects of mother-child interactions, and the AGRE and SSC samples were used to replicate the original findings of 16 ASD risk genes (PCDH9, FOXP1, GABRB3, NRXN1, RELN, MACROD2, FHIT, RORA, CNTN4, CNTNAP2, FAM135B, LAMA1, NFIA, NLGN4X, RAPGEF4 and SDK1) involving urea transport and neural development. The results from the AGRE and SSC cohorts did not match the original study and showed fewer associations. When post-correction of the statistics was applied, the results lost their significance [119]. This could partially be due to the differences in the array design with different coverage of SNPs and/or different methodologies.
The meta-analysis of five data sets including the AGRE and SSC demonstrates that females have a greater tolerance to CNV burden. This leads to a speculation that the maternal tolerance of the CNVs can result in decreased foetal loss amongst females compared to males, and that ASD-specific CNV burden contributes to high sibling occurrence. What is interesting about this study is that the results for high CNV burden in females are consistent throughout each data set. This is an example showing how multiple bio-collections can give a clearer picture in a combined study where individual studies may be ambiguous [120, 121].
Many major studies on the genetics of ASD have also been accomplished as a result of the collaborations amongst the institutions (Tables 2, 3, 4, 5 and 6). An effort was made to evaluate the association of Fragile X Mental Retardation 2 locus (AFF2) with ASD using joint resources from AGRE (127 males) and SSC (75 males). AFF2 encodes an RNA-binding protein, which is silenced in Fragile X. The study found that 2.5% of ASD males carry highly conserved missense mutations on AFF2 gene which was significantly enriched in ASD patients, when compared to >5000 unaffected controls [122]. A WES was published recently, which sequenced the exomes of over 20,000 individuals, including those from the SSC and Swedish registries. The study identified 107 candidate genes, and reinforced ASD pathways of synaptic formation, chromatin remodelling and gene transcription. This study detected mutations in genes involved in calcium- (CACNA2D3, CACNA1D) and sodium-gated channels (SCN2A) which were related to neuronal function, and in genes involved in post-translational methylation (SUV420H1, KMT2C, ASH1L, SETD5, WHSC1) and demethylation (KDM4B, KDM3A, KDM5B, KDM6B) of lysine residues on histones which provided molecular basis linking to neuronal excitation and epigenetic changes in ASD [86].
Multiple bio-collections were employed to investigate SHANK1, 2 and 3, which are scaffolding proteins implicated in ASD. They devised a genetic screen and meta-analysis on patients and controls including cohorts from the AGRE, SSC and Swedish twin registry. In total, ~1% of all patients in the study had a mutation in this group of genes. The mutations in SHANK3 had the highest frequency (0.69%) in patients with ASD and profound intellectual disability. SHANK1 (0.04%) and SHANK2 (0.17%) mutations occurred less frequently and were present in individuals with ASD and normal IQ, and ASD with moderate intellectual disability [123].
Recently Autism, Speaks, in coordination with Google and Genome Canada, have launched another initiative; MSSNG (https://www.mss.ng/). The objective of the MSSNG project is whole genome sequencing of 10000 genomes of families affected by ASD. This incorporates AGRE along with other bio-collections to sequence the entire genomes of families with autistic children, and as of the summer of 2016, it has reached the halfway goal of 5000 genomes out of 10000, with the contribution of the AGRE (1746) and TASC (458). Two studies have been published from this initiative. In the first study, genomes from 200 families were sequenced [124]. The findings revealed many of the de novo mutations (75%) from fathers, which increased dramatically with paternal age. Clustered de novo mutations however were mostly maternal origin, and located near CNV regions subject to high mutation. The ASD genomes were enriched with damaging de novo mutations, of which 15.6% were non-coding and 22.5% genic non-coding, respectively. Many of the mutations affected regulatory regions that are targeted by DNase 1 or involved in exon skipping [124]. The second study [125] featured 5205 sequenced genomes with clinical data, where an average of 73.8 de novo single nucleotide variants and 12.6 insertions/deletions/CNVs were detected per ASD patient. Eighteen new genes were also discovered (CIC, CNOT3, DIP2C, MED13, PAX5, PHF3, SMARCC2, SRSF11, UBN2, DYNC1H1, AGAP2, ADCY3, CLASP1, MYO5A, TAF6, PCDH11X, KIAA2022 and FAM47A) that were not reported in ASD previously. These data clearly demonstrate that ASD is associated with multiple risk factors, and within an ASD individual, and multiple genetic alterations may be present. The Whole genome sequencing is therefore a powerful tool to detect genetic changes at all levels. Resources like MSSNG are valuable, and pooling of ASD bio-collections are essential for identification of the common and subgroup-specific pathways and drug targets of such a multi-factorial disease of ASD which involves hundreds of risk factors.
Stem cell research and autism spectrum stem cell resource
A major impediment to recent drug discovery particularly in the field of neuroscience is the lack of human cell models. The iPSC technology developed by Nobel Laureate Shinya Yamanaka has provided an excellent opportunity [126]. Fibroblasts from patients’ biopsy can be converted into iPSCs with defined transcription factors, which resemble embryonic stem cells and can become most cell types in our body. Therefore, patient-derived iPSCs may be used to investigate disease pathology, progression and mechanisms to create human disease models for drug screening and testing [127, 128].
The SSC has also commenced efforts to create iPSC lines from idiopathic ASD patients who have large head circumference but unknown gene association [129]. The iPSCs were grown into organoids to mimic cortical development, and ASD organoids were shown to display a disproportionate ratio of inhibitory: excitatory neurons. The cortical gene FOXG1 was overexpressed in ASD organoids, and this overexpression correlated with the severity of ASD and their head size [129]. This study has demonstrated a proof-of-concept to model ASD in culture stem cells.
The Children’s Hospital in Orange County California has set up a bio-collection dedicated to this task, the ASD Stem Cell Resource. ASD patients were screened and accepted based on the following criteria: ASD patients if they have no other conditions (i.e. trauma, stroke, seizure disorders) affecting the central nervous system other than ASD; if they have no features of other known genetic conditions (e.g. tuberous sclerosis); Fragile X patients if they are genotypically confirmed for the CGG repeat number of the FMR1 mutation; idiopathic autism patients who are negative for FMR1 mutation and chromosomal abnormality; if they possess an IQ of 40 or greater, and if they are 8-year-old or above. Skin punches and blood were collected in one location (MIND Institute), and fibroblasts were cultured and stored at the Hospital. The collection has been organised into seven groups; unaffected controls, Fragile X without ASD, Fragile X with ASD, permutations without ASD, permutations with ASD, ASD (not meeting full criteria for idiopathic status) and idiopathic ASD.
As of 2014, this resource was composed of iPSCs from 200 unaffected donors and patients. The collection includes fibroblasts, blood, iPSCs, iPSC-derived neuronal and glial cells. The first study published using this bio-collection was the iPSC models of Fragile X syndrome [130]. The Fragile X patient fibroblasts were used to derive iPSCs and differentiate into neurons for transcriptomic analysis. The neuronal differentiation genes (WNT1, BMP4, POU3F4, TFAP2C, PAX3) were shown to be upregulated, whereas potassium channel genes (KCNA1, KCNC3, KCNG2, KCNIP4, KCNJ3, KCNK9, KCNT1) were downregulated in Fragile X iPSC-derived neurons. The temporal regulation of SHANK1 and NNAT genes were also altered, with reduced SHANK1 mRNA and increased NNAT mRNA in patient cells. While the stem cell collection is relatively new, it has great potential to facilitate brain cell culture in vitro, which would otherwise not be feasible by using post mortem brain tissue.
Discussion
It is clear from the studies reviewed here that large ASD bio-collections have had an undisputable impact on progressing genomic discovery in ASD, leading to enhanced understanding of ASD neurobiology. While many studies used private collections as sources for tissue and data, large and well characterised samples from the collections reviewed have supported the discovery of small genetic effects, e.g. in GWAS and rare genetic mutations such as pathogenic CNV and SNV but it is clear, as highlighted for other neurodevelopmental disorders such as Schizophrenia that larger samples are required. Both genetic and phenotypic heterogeneity are impediments to gene discovery. Large bio-collections aim to reduce these effects but challenges remain. Each of the bio-collections reviewed has its own strengths and limitations.
Phenotypic and genotypic heterogeneity
Some of the bio-collections, e.g. SSC, AGRE, TASC, reduced phenotypic heterogeneity through the use of research gold standards for ASD diagnosis, ADI-R and ADOS. Different versions of these instruments based on the timeline when these data have been collected have been used. IQ measurement is more complex to calculate due to the broad range of IQ commonly included within bio-collections. Differences also exist in the clinical profile of subjects included in the different collections with some samples, e.g. SSC, comprised of more individuals with higher cognitive functioning relative to AGRE, TASC or AIC. Medical and psychiatric comorbidities [7] have greater recognition but are not as systematically evaluated in each of the collections. Differences in ascertainment are also relevant. The SSC focused on simplex autism, i.e. families where only one child was affected to maximise the detection of rare variants. Consequently, the relative contribution of common genetic risk within the SSC sample appears reduced. In contrast to autism specific bio-collections, the DNSB, provides a large population-based sample with clinical diagnosis that can maximise power within GWAS studies to detect common genetic variation but does not provide in-depth clinical data for phenotype-genotype analyses. This was evident in the studies on amniotic fluid and DBSS where different diagnostic criteria would have been applied at the time of the subjects’ diagnoses, meaning one criteria would have excluded subjects(ICD8) whereas another would not (ICD10) [95, 96] [93, 94].
Throughout the studies listed here, there is an imbalance of ethnicities of bio-collections, as many of the studies rely heavily on Caucasian/European descent, which has been pointed out in some journals [131] and should consider diverse family structures [132], which can otherwise lead to population stratification [133]. Fortunately, efforts are underway to explore genetics of ASD in other countries such as China [134] and Brazil [135], which will reinforce many of the earlier findings covered in this review.
Samples
Large collections providing DNA for genomics studies have been advantageous; however, as studies move beyond the scope of genetics into transcriptomics, epigenomics and proteomics, a wider variety of sample types will be required. Serum will be valuable for investigating circulating metabolites and proteins that are expressed peripherally, including chemokines [93, 95], cytokines [94, 96], neurotropins [136], MMPs [137] and hormones [138]; however, this may not be the most appropriate tissue to investigate brain relevant ASD genes and proteins. DBSS, which can be useful for WES [139, 140], methylation [141] and gene expression [142], would not be as useful as fresh drawn blood for WGS, as DBSS-derived DNA would need to be amplified prior to use for analysis, potentially causing bias.
However, human brain tissue is a rare resource; brain tissue is very difficult to access due to its scarcity, and the preservation methods used may limit studies being carried out. Also, the types of brain cells are dependent on brain tissue being used; neuronal tissue in grey matter or glial tissue in white matter. Many of the studies listed in the Autism BrainNet, for example, utilised certain sections of the brain; and the most commonly used sections are the prefrontal cortex, temporal cortex, Brodmann’s area, cerebellum and cingulate gyrus. While findings from these sections have been of crucial importance, a capacity to model the entire brain and to observe progression of ASD development would be ideal, and patient’s somatic cells can now be converted to iPSCs and then into disease cell types.
IPSCs have been used as disease models for Fragile X syndrome [143,144,145] and Rett syndrome [146], and iPSCs have been generated from patents with deletions in SHANK3 [147] which are implicated in a number of neurodevelopmental disorders. The three-dimensional culture is developed and iPSCs can also be used to create mini-organoids, which can come very close to mimicking aspects of brain development [129, 148]. In addition to the brain cell types discussed earlier [129, 149], the iPSCs could be used to generate other cell types implicated in ASD co-morbidities, such as the gut [88, 150] and the blood brain barrier [151, 152].
Fibroblasts are the first cell type used to make iPSCs from mice [126] and humans [153] and remain as the most popular cell type for generating neural stem cells, neurons or iPSCs. Fibroblasts are easier to reprogram than many other somatic cells, and the reprogramming efficiency is between 0.1–1% depending on the reprogramming method [154]. They require basic culture media and proliferate rapidly, so large numbers of fibroblasts can be generated in a short period. Unlike keratinocytes they require trained medical personnel to obtain skin biopsies, which could be distressing to some ASD patients. Low passages of fibroblasts are required for reprogramming as higher passages dramatically reduce reprogramming efficiency and increase genomic instability [155].In addition to their use for IPSCs, fibroblasts can be used to investigate amino acid transport, and ASD fibroblasts were found to have greater affinity for transporting alanine, but less affinity for tyrosine—a key component for the synthesis of the neurotransmitter dopamine [156]. Fibroblasts can be used as a proxy to investigate transport across the blood-brain barrier [156, 157] and to investigate calcium signalling [158, 159].
Keratinocytes can also be used for generating IPSCs [160]. Collection is less invasive than skin biopsy and can be carried out by non-medical personnel. The hair samples are easy to transport and culture and transformed cells are easier to identify and isolate. Similar to fibroblasts, keratinocytes are reprogrammed at low passages and fewer methods have been employed to reprogram keratinocytes than fibroblasts. The lentiviral, retroviral and episomal reprogramming were tried successfully [155, 161, 162], and keratinocytes were shown to have high reprogramming efficiency of 1–2%. The major challenge is the reproducibility of keratinocyte growth, and it often requires repeated rounds of hair plucking from a same donor.
Organization
There are many generic articles and white papers for biobanks available, including consensus best-practice recommendations. For those who may wish to start their own bio-collections, we have listed a few articles in Table 7 for further reading on topics pertaining to collection, management, sustainability and quality control. In addition, links to international guidelines can be found here (http://www.oecd.org/sti/biotech/guidelinesforhumanbiobanksandgeneticresearchdatabaseshbgrds.htm; http://www.isber.org/?page=BPR; https://biospecimens.cancer.gov/practices/). However, even when using best practice guidelines, the storage and use of bio samples will be subject to the laws where the facilities are located, and will vary from country to country [163].
Participation and ethics
Stakeholders can have a considerable influence on how a bio-collection operates and how a bio-collection can be set up, managed and monitored [164]. In addition to researchers, clinicians and parents in bio-collections of ASD research, autistic stakeholders should be included as part of the stakeholder group, which could help guide and inform how research is carried out. A recent survey [165] was carried out amongst researcher-community engagement on ASD research in the UK. A high dissatisfaction and level of disengagement was expressed by parents and patients, who felt that research outcomes made little or no difference to their day-to-day lives and that they were not communicated, not involved or valued. Patients also felt that they did not receive follow-up and researchers were unapproachable and driven by data collection. Establishment and sustainability of a good stakeholder engagement are essential in ASD research and in biobanking. This will not only help guide research to subjects that matter to the community, but also the future of the biobank. One initiative, such as SPARK (Simons Foundation Powering Autism Research for Knowledge) is underway to encourage ASD communities in the USA to participate in ASD research. While such a goal is laudable, it is crucial that participants are engaged in the entire process. They are not just the suppliers of bio-collections for research and data collection, but also make an input into research areas, which directly impinge on the quality of their life. Meanwhile, regular public events to update research progress and challenges to the stakeholder community may help win their understanding, appreciation and continuous support.
The ethics and obtainment of consent are significant factors for bio-collection research. The main considerations include what information shall be given to potential donors regarding the protocol and its implications of the research, how consent should be obtained [166] or what shall be done if consent was not clearly given [167]. It is also a matter of debate whether the consent should be “broad” and if the patient shall consent to a framework of research; if ethical review of each project shall be carried out by independent committees, and what are the strategies to inform and renew consent if there is significant deviation of framework; where shall the consent be revisited and renewed for every new study [168]; how the data will be protected and accessed [169, 170]; and how the findings will be communicated [171]. The latter is especially important if findings are of clinical significance to certain donors or it may affect their health or well-being [167]. These are the issues that each ethical application faces in making the application.
For people with ASD, it can be very complicated. Parents will give consent for their children if they want to donate samples for the bio-collection, but there is a question of adults who may not have the ability to give consent or to fully understand the implications. It is also important to clearly communicate what this research will mean for the patient and the family, including findings that may be of pathological as well as clinical significance. Liu and Scott have commented on how the discoveries made in ASD research can be distorted by media. If parents/patients are misled to believe that a cure will come out a few years down the road, this may lead to disappointment and make them reluctant to participate in further research. Liu and Scott pointed out that the Neurodiversity Movement group (high-functioning autists) would have issues with certain research. They will not participate in research if they feel it may threaten or undermine people with ASD [128]. They prefer investment on services and therapies, rather than on genetic studies which may result in prevention of autistics being born [172,173,174], and the idea of curing autism is a complicated topic of debate [175].
For iPSC research, it was suggested to educate participants on the current state of research, to clearly explain the benefits and risks of biopsy donation and to consult the ASD community on research focus of an ASD bio-collection and on distribution of the cell lines [128]. For clinical trials of stem cells, stem cell counsellors shall inform participants the benefits and risks of enrolling in stem cell trials and to safeguard them from the dangers of stem cell tourism. Such an approach should also be considered for ASD-related studies [176].
Conclusions
In conclusion, bio-collections have been shown as valuable resources and enabled large-scale studies on ASD. The recent genetic studies have begun to reveal de novo mutations on major cellular pathways [17, 177]. There is also emerging evidence that ASD continuum contains subgroups with discrete mutations in specific genes such as CDH8 [88], DYRK1A [71] and POGZ [90] and gene mutations like NRXN1 [28, 60, 73, 178, 179] and SHANKs [72, 98, 114, 123] recurring in broad populations. There is a vast amount of clinical and biological information available in these bio-collections, and the data are in the need for concrete guidelines on ethics and governance. The communication and trust shall be maintained between the researchers and families who have given biological and personal information. Finally, the availability of iPSC resources dedicated to idiopathic and syndromic forms of ASD could be a tremendous boon to the research community and such models are anticipated to be complementary with animal models and to speed up the development of therapeutic interventions for ASD. They could open up the possibilities of functional studies of ASD on a large scale and could become a future model for other iPSC bio-collections to be set up worldwide.
Abbreviations
- ADI-R:
-
Autism Diagnostic Interview–Revised
- ADOS:
-
Autistic Diagnostic Observation Schedule
- AGP:
-
Autism Genome Project
- AGRE:
-
Autism Genetic Resource Exchange
- AIC:
-
Autism Inpatient Collection
- ASD:
-
Autism spectrum disorders
- ATP:
-
Autism tissue program
- CAN:
-
Cure Autism Now Foundation
- CCGSMD:
-
Centre for Collaborative Genetic Studies on Mental Disorders
- CNV:
-
Copy number variation
- DBC:
-
Danish Birth Cohort
- DBSS:
-
Dried blood spot samples
- DNSB:
-
Danish Newborn Screening Biobank
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorders
- GSH:
-
Glutathione
- GWAS:
-
Genome-wide association study
- HAWAS:
-
Histone acetylome-wide association study
- HBDI:
-
Human Biological Data Interchange
- ICD:
-
International Statistical Classification of Diseases and Related Health Problems
- iPSC:
-
Induced pluripotent stem cells
- MMP:
-
Matrix metalloproteinase
- MPLA:
-
Multiplex ligation-dependent probe amplification
- PDD-NOS:
-
Pervasive developmental disorder not otherwise specified
- RUCDR:
-
Rutgers University Cell and DNA Repository
- SCD:
-
Social (pragmatic) communication disorder
- SNP:
-
Single nucleotide polymorphism
- SNV:
-
Single nucleotide variation
- SSC:
-
Simons Simplex Collection
- TASC:
-
Autism Simplex Collection
- WES:
-
Whole exome sequencing study
References
Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi:10.15585/mmwr.ss6503a1.
Charman T. The prevalence of autism spectrum disorders. Recent evidence and future challenges. Eur Child Adolesc Psychiatry. 2002;11:249–56. doi:10.1007/s00787-002-0297-8.
Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–58. doi:10.1146/annurev.publhealth.28.021406.144007.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington: American Psychiatric Association; 2013. doi:10.1176/appi.books.9780890425596.
Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–9.
Mazzone L, Ruta L, Reale L. Psychiatric comorbidities in asperger syndrome and high functioning autism: diagnostic challenges. Ann Gen Psychiatry. 2012;11:16. doi:10.1186/1744-859X-11-16.
Chen M-H, Su T-P, Chen Y-S, Hsu J-W, Huang K-L, Chang W-H, et al. Comorbidity of allergic and autoimmune diseases in patients with autism spectrum disorder: A nationwide population-based study. Res Autism Spectr Disord. 2013;7:205–12. doi:10.1016/j.rasd.2012.08.008.
Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–55. doi:10.1038/nrg2346.
Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57:585–95. doi:10.1111/jcpp.12499.
Persico AM, Napolioni V. Autism genetics. Behav Brain Res. 2013;251:95–112. doi:10.1016/j.bbr.2013.06.012.
Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012;17:290–314. doi:10.1038/mp.2010.136.
Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–92. doi:10.1016/j.bbi.2011.08.007.
Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15 Spec No 2:R138–50. doi:10.1093/hmg/ddl213.
Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16:551–63.
Sakai Y, Shaw CA, Dawson BC, Dugas DV, Al-Mohtaseb Z, Hill DE, et al. Protein interactome reveals converging molecular pathways among autism disorders. Sci Transl Med. 2011;3:86ra49. doi:10.1126/scitranslmed.3002166.
Liu L, Lei J, Sanders SJ, Willsey AJ, Kou Y, Cicek AE, et al. DAWN: a framework to identify autism genes and subnetworks using gene expression and genetics. Mol Autism. 2014;5:22. doi:10.1186/2040-2392-5-22.
Oron O, Elliott E. Delineating the Common Biological Pathways Perturbed by ASD’s Genetic Etiology: Lessons from Network-Based Studies. Int J Mol Sci. 2017;18:828.
Wen Y, Alshikho MJ, Herbert MR. Pathway Network Analyses for Autism Reveal Multisystem Involvement, Major Overlaps with Other Diseases and Convergence upon MAPK and Calcium Signaling. PLoS ONE. 2016;11:e0153329. doi:10.1371/journal.pone.0153329.
Fischbach GD, Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 2010;68:192–5. doi:10.1016/j.neuron.2010.10.006.
Geschwind DH, Sowinski J, Lord C, Iversen P, Shestack J, Jones P, et al. The Autism Genetic Resource Exchange: A Resource for the Study of Autism and Related Neuropsychiatric Conditions. Am J Hum Genet. 2001;69:463–6. doi:10.1086/321292.
Stoltenberg C, Schjølberg S, Bresnahan M, Hornig M, Hirtz D, Dahl C, et al. The Autism Birth Cohort: a paradigm for gene-environment-timing research. Mol Psychiatry. 2010;15:676–80. doi:10.1038/mp.2009.143.
Al-Jawahiri R, Milne E. Resources available for autism research in the big data era: a systematic review. PeerJ. 2017;5:e2880. doi:10.7717/peerj.2880.
Lochmüller H, Schneiderat P. Biobanking in rare disorders. Adv Exp Med Biol. 2010;686:105–13. doi:10.1007/978-90-481-9485-8_7.
Siegel M, Smith KA, Mazefsky C, Gabriels RL, Erickson C, Kaplan D, et al. The autism inpatient collection: methods and preliminary sample description. Mol Autism. 2015;6:61. doi:10.1186/s13229-015-0054-8.
Brick DJ, Nethercott HE, Montesano S, Banuelos MG, Stover AE, Schutte SS, et al. The autism spectrum disorders stem cell resource at children’s hospital of orange county: implications for disease modeling and drug discovery. Stem Cells Transl Med. 2014;3:1275–86. doi:10.5966/sctm.2014-0073.
Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–38. doi:10.1093/hmg/ddm376.
Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–75. doi:10.1056/NEJMoa075974.
Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, Sonnenblick LI, et al. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5:e1000536. doi:10.1371/journal.pgen.1000536.
Jiang Y-H, Sahoo T, Michaelis RC, Bercovich D, Bressler J, Kashork CD, et al. A mixed epigenetic/genetic model for oligogenic inheritance of autism with a limited role for UBE3A. Am J Med Genet A. 2004;131:1–10. doi:10.1002/ajmg.a.30297.
Miller DT, Shen Y, Weiss LA, Korn J, Anselm I, Bridgemohan C, et al. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J Med Genet. 2009;46:242–8. doi:10.1136/jmg.2008.059907.
Sahoo T, Shaw CA, Young AS, Whitehouse NL, Schroer RJ, Stevenson RE, et al. Array-based comparative genomic hybridization analysis of recurrent chromosome 15q rearrangements. Am J Med Genet A. 2005;139A:106–13. doi:10.1002/ajmg.a.31000.
Ma DQ, Whitehead PL, Menold MM, Martin ER, Ashley-Koch AE, Mei H, et al. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77:377–88. doi:10.1086/433195.
Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459:528–33. doi:10.1038/nature07999.
Autism Genome Project Consortium, Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–28. doi:10.1038/ng1985.
Abuhatzira L, Shamir A, Schones DE, Schäffer AA, Bustin M. The chromatin-binding protein HMGN1 regulates the expression of methyl CpG-binding protein 2 (MECP2) and affects the behavior of mice. J Biol Chem. 2011;286:42051–62. doi:10.1074/jbc.M111.300541.
Harvey CG, Menon SD, Stachowiak B, Noor A, Proctor A, Mensah AK, et al. Sequence variants within exon 1 of MECP2 occur in females with mental retardation. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:355–60. doi:10.1002/ajmg.b.30425.
Loat CS, Curran S, Lewis CM, Duvall J, Geschwind D, Bolton P, et al. Methyl-CpG-binding protein 2 polymorphisms and vulnerability to autism. Genes Brain Behav. 2008;7:754–60. doi:10.1111/j.1601-183X.2008.00414.x.
Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–21. doi:10.1136/jmg.2004.024646.
Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, et al. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:484–91. doi:10.1002/ajmg.b.30493.
Bartlett CW, Gharani N, Millonig JH, Brzustowicz LM. Three autism candidate genes: a synthesis of human genetic analysis with other disciplines. Int J Dev Neurosci. 2005;23:221–34. doi:10.1016/j.ijdevneu.2004.10.004.
Benayed R, Choi J, Matteson PG, Gharani N, Kamdar S, Brzustowicz LM, et al. Autism-associated haplotype affects the regulation of the homeobox gene, ENGRAILED 2. Biol Psychiatry. 2009;66:911–7. doi:10.1016/j.biopsych.2009.05.027.
Gharani N, Benayed R, Mancuso V, Brzustowicz LM, Millonig JH. Association of the homeobox transcription factor, ENGRAILED 2, 3, with autism spectrum disorder. Mol Psychiatry. 2004;9:474–84. doi:10.1038/sj.mp.4001498.
Ashley-Koch AE, Mei H, Jaworski J, Ma DQ, Ritchie MD, Menold MM, et al. An analysis paradigm for investigating multi-locus effects in complex disease: examination of three GABA receptor subunit genes on 15q11-q13 as risk factors for autistic disorder. Ann Hum Genet. 2006;70(Pt 3):281–92. doi:10.1111/j.1469-1809.2006.00253.x.
Serajee FJ, Zhong H, Mahbubul Huq AHM. Association of Reelin gene polymorphisms with autism. Genomics. 2006;87:75–83. doi:10.1016/j.ygeno.2005.09.008.
Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, et al. Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry. 2005;10:563–71. doi:10.1038/sj.mp.4001614.
Zhang H, Liu X, Zhang C, Mundo E, Macciardi F, Grayson DR, et al. Reelin gene alleles and susceptibility to autism spectrum disorders. Mol Psychiatry. 2002;7:1012–7. doi:10.1038/sj.mp.4001124.
Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J. 2010;24:3036–51. doi:10.1096/fj.10-154484.
Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–9. doi:10.1073/pnas.0605296103.
Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008;1:159–68. doi:10.1002/aur.27.
Campbell DB, Warren D, Sutcliffe JS, Lee EB, Levitt P. Association of MET with social and communication phenotypes in individuals with autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:438–46. doi:10.1002/ajmg.b.30998.
Talebizadeh Z, Lam DY, Theodoro MF, Bittel DC, Lushington GH, Butler MG. Novel splice isoforms for NLGN3 and NLGN4 with possible implications in autism. J Med Genet. 2006;43:e21. doi:10.1136/jmg.2005.036897.
McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, et al. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:104–12. doi:10.1002/ajmg.b.20151.
Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, et al. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–6. doi:10.1038/sj.mp.4001011.
Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, et al. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–74. doi:10.1007/s10048-006-0045-1.
McCauley JL, Olson LM, Delahanty R, Amin T, Nurmi EL, Organ EL, et al. A linkage disequilibrium map of the 1-Mb 15q12 GABA(A) receptor subunit cluster and association to autism. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:51–9. doi:10.1002/ajmg.b.30038.
Strom SP, Stone JL, Ten Bosch JR, Merriman B, Cantor RM, Geschwind DH, et al. High-density SNP association study of the 17q21 chromosomal region linked to autism identifies CACNA1G as a novel candidate gene. Mol Psychiatry. 2010;15:996–1005. doi:10.1038/mp.2009.41.
Weiss LA, Escayg A, Kearney JA, Trudeau M, MacDonald BT, Mori M, et al. Sodium channels SCN1A, SCN2A and SCN3A in familial autism. Mol Psychiatry. 2003;8:186–94. doi:10.1038/sj.mp.4001241.
Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–9. doi:10.1126/science.1138659.
Dong S, Walker MF, Carriero NJ, DiCola M, Willsey AJ, Ye AY, et al. De novo insertions and deletions of predominantly paternal origin are associated with autism spectrum disorder. Cell Rep. 2014;9:16–23. doi:10.1016/j.celrep.2014.08.068.
Levy D, Ronemus M, Yamrom B, Lee Y, Leotta A, Kendall J, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–97. doi:10.1016/j.neuron.2011.05.015.
Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–99. doi:10.1016/j.neuron.2012.04.009.
Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21. doi:10.1038/nature13908.
Luo R, Sanders SJ, Tian Y, Voineagu I, Huang N, Chu SH, et al. Genome-wide transcriptome profiling reveals the functional impact of rare de novo and recurrent CNVs in autism spectrum disorders. Am J Hum Genet. 2012;91:38–55. doi:10.1016/j.ajhg.2012.05.011.
O’Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, Girirajan S, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–9. doi:10.1038/ng.835.
O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–50. doi:10.1038/nature10989.
O’Roak BJ, Stessman HA, Boyle EA, Witherspoon KT, Martin B, Lee C, et al. Recurrent de novo mutations implicate novel genes underlying simplex autism risk. Nat Commun. 2014;5:5595. doi:10.1038/ncomms6595.
Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH, et al. Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proc Natl Acad Sci U S A. 2014;111:15161–5. doi:10.1073/pnas.1409204111.
Samocha KE, Robinson EB, Sanders SJ, Stevens C, Sabo A, McGrath LM, et al. A framework for the interpretation of de novo mutation in human disease. Nat Genet. 2014;46:944–50. doi:10.1038/ng.3050.
Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–85. doi:10.1016/j.neuron.2011.05.002.
Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–41. doi:10.1038/nature10945.
Van Bon BWM, Coe BP, Bernier R, Green C, Gerdts J, Witherspoon K, et al. Disruptive de novo mutations of DYRK1A lead to a syndromic form of autism and ID. Mol Psychiatry. 2016;21:126–32. doi:10.1038/mp.2015.5.
Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–72. doi:10.1038/nature09146.
Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–73. doi:10.1038/nature07953.
Baron CA, Liu SY, Hicks C, Gregg JP. Utilization of lymphoblastoid cell lines as a system for the molecular modeling of autism. J Autism Dev Disord. 2006;36:973–82. doi:10.1007/s10803-006-0134-x.
Bowers K, Li Q, Bressler J, Avramopoulos D, Newschaffer C, Fallin MD. Glutathione pathway gene variation and risk of autism spectrum disorders. J Neurodev Disord. 2011;3:132–43. doi:10.1007/s11689-011-9077-4.
James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23:2374–83. doi:10.1096/fj.08-128926.
Main PAE, Thomas P, Esterman A, Fenech MF. Necrosis is increased in lymphoblastoid cell lines from children with autism compared with their non-autistic siblings under conditions of oxidative and nitrosative stress. Mutagenesis. 2013;28:475–84. doi:10.1093/mutage/get025.
Talebizadeh Z, Butler MG, Theodoro MF. Feasibility and relevance of examining lymphoblastoid cell lines to study role of microRNAs in autism. Autism Res. 2008;1:240–50. doi:10.1002/aur.33.
Sarachana T, Zhou R, Chen G, Manji HK, Hu VW. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010;2:23. doi:10.1186/gm144.
Oguro-Ando A, Rosensweig C, Herman E, Nishimura Y, Werling D, Bill BR, et al. Increased CYFIP1 dosage alters cellular and dendritic morphology and dysregulates mTOR. Mol Psychiatry. 2015;20:1069–78. doi:10.1038/mp.2014.124.
Faham M, Zheng J, Moorhead M, Fakhrai-Rad H, Namsaraev E, Wong K, et al. Multiplexed variation scanning for 1,000 amplicons in hundreds of patients using mismatch repair detection (MRD) on tag arrays. Proc Natl Acad Sci U S A. 2005;102:14717–22. doi:10.1073/pnas.0506677102.
Cai G, Edelmann L, Goldsmith JE, Cohen N, Nakamine A, Reichert JG, et al. Multiplex ligation-dependent probe amplification for genetic screening in autism spectrum disorders: efficient identification of known microduplications and identification of a novel microduplication in ASMT. BMC Med Genomics. 2008;1:50. doi:10.1186/1755-8794-1-50.
Bureau A, Labbe A, Croteau J, Mérette C. Using disease symptoms to improve detection of linkage under genetic heterogeneity. Genet Epidemiol. 2008;32:476–86. doi:10.1002/gepi.20320.
Yonan AL, Palmer AA, Smith KC, Feldman I, Lee HK, Yonan JM, et al. Bioinformatic analysis of autism positional candidate genes using biological databases and computational gene network prediction. Genes Brain Behav. 2003;2:303–20.
Turner TN, Hormozdiari F, Duyzend MH, McClymont SA, Hook PW, Iossifov I, et al. Genome Sequencing of Autism-Affected Families Reveals Disruption of Putative Noncoding Regulatory DNA. Am J Hum Genet. 2016;98:58–74. doi:10.1016/j.ajhg.2015.11.023.
De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–15. doi:10.1038/nature13772.
O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–22. doi:10.1126/science.1227764.
Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158:263–76. doi:10.1016/j.cell.2014.06.017.
Cotney J, Muhle RA, Sanders SJ, Liu L, Willsey AJ, Niu W, et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat Commun. 2015;6:6404. doi:10.1038/ncomms7404.
Stessman HAF, Willemsen MH, Fenckova M, Penn O, Hoischen A, Xiong B, et al. Disruption of POGZ Is Associated with Intellectual Disability and Autism Spectrum Disorders. Am J Hum Genet. 2016;98:541–52. doi:10.1016/j.ajhg.2016.02.004.
Nørgaard-Pedersen B, Hougaard DM. Storage policies and use of the Danish Newborn Screening Biobank. J Inherit Metab Dis. 2007;30:530–6. doi:10.1007/s10545-007-0631-x.
The Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol Autism. 2017;8:21. doi:10.1186/s13229-017-0137-9.
Abdallah MW, Larsen N, Grove J, Nørgaard-Pedersen B, Thorsen P, Mortensen EL, et al. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a Danish Historic Birth Cohort. Brain Behav Immun. 2012;26:170–6. doi:10.1016/j.bbi.2011.09.003.
Abdallah MW, Larsen N, Grove J, Nørgaard-Pedersen B, Thorsen P, Mortensen EL, et al. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J Biol Psychiatry. 2013;14:528–38. doi:10.3109/15622975.2011.639803.
Abdallah MW, Larsen N, Grove J, Bonefeld-Jørgensen EC, Nørgaard-Pedersen B, Hougaard DM, et al. Neonatal chemokine levels and risk of autism spectrum disorders: findings from a Danish historic birth cohort follow-up study. Cytokine. 2013;61:370–6. doi:10.1016/j.cyto.2012.11.015.
Abdallah MW, Larsen N, Mortensen EL, Atladóttir HÓ, Nørgaard-Pedersen B, Bonefeld-Jørgensen EC, et al. Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol. 2012;252:75–82. doi:10.1016/j.jneuroim.2012.07.013.
Buxbaum JD, Bolshakova N, Brownfeld JM, Anney RJ, Bender P, Bernier R, et al. The Autism Simplex Collection: an international, expertly phenotyped autism sample for genetic and phenotypic analyses. Mol Autism. 2014;5:34. doi:10.1186/2040-2392-5-34.
Anney R, Klei L, Pinto D, Regan R, Conroy J, Magalhaes TR, et al. A genome-wide scan for common alleles affecting risk for autism. Hum Mol Genet. 2010;19:4072–82. doi:10.1093/hmg/ddq307.
Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–94. doi:10.1016/j.ajhg.2014.03.018.
Liu L, Sabo A, Neale BM, Nagaswamy U, Stevens C, Lim E, et al. Analysis of rare, exonic variation amongst subjects with autism spectrum disorders and population controls. PLoS Genet. 2013;9:e1003443. doi:10.1371/journal.pgen.1003443.
Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–5. doi:10.1038/nature11011.
Haroutunian V, Pickett J. Autism brain tissue banking. Brain Pathol. 2007;17:412–21. doi:10.1111/j.1750-3639.2007.00097.x.
Ginsberg MR, Rubin RA, Falcone T, Ting AH, Natowicz MR. Brain transcriptional and epigenetic associations with autism. PLoS ONE. 2012;7:e44736. doi:10.1371/journal.pone.0044736.
Gupta S, Ellis SE, Ashar FN, Moes A, Bader JS, Zhan J, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat Commun. 2014;5:5748. doi:10.1038/ncomms6748.
Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–4. doi:10.1038/nature10110.
Ben-David E, Shohat S, Shifman S. Allelic expression analysis in the brain suggests a role for heterogeneous insults affecting epigenetic processes in autism spectrum disorders. Hum Mol Genet. 2014;23:4111–24. doi:10.1093/hmg/ddu128.
Eran A, Li JB, Vatalaro K, McCarthy J, Rahimov F, Collins C, et al. Comparative RNA editing in autistic and neurotypical cerebella. Mol Psychiatry. 2013;18:1041–8. doi:10.1038/mp.2012.118.
Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi:10.1186/1741-7015-7-62.
Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP. Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry. 2014;19:862–71. doi:10.1038/mp.2013.114.
Mor M, Nardone S, Sams DS, Elliott E. Hypomethylation of miR-142 promoter and upregulation of microRNAs that target the oxytocin receptor gene in the autism prefrontal cortex. Mol Autism. 2015;6:46. doi:10.1186/s13229-015-0040-1.
Nardone S, Sams DS, Reuveni E, Getselter D, Oron O, Karpuj M, et al. DNA methylation analysis of the autistic brain reveals multiple dysregulated biological pathways. Transl Psychiatry. 2014;4:e433. doi:10.1038/tp.2014.70.
Shulha HP, Cheung I, Whittle C, Wang J, Virgil D, Lin CL, et al. Epigenetic signatures of autism: trimethylated H3K4 landscapes in prefrontal neurons. Arch Gen Psychiatry. 2012;69:314–24. doi:10.1001/archgenpsychiatry.2011.151.
Sun W, Poschmann J, Cruz-Herrera Del Rosario R, Parikshak NN, Hajan HS, Kumar V, et al. Histone Acetylome-wide Association Study of Autism Spectrum Disorder. Cell. 2016;167:1385–97. doi:10.1016/j.cell.2016.10.031. e11.
Zhu L, Wang X, Li X-L, Towers A, Cao X, Wang P, et al. Epigenetic dysregulation of SHANK3 in brain tissues from individuals with autism spectrum disorders. Hum Mol Genet. 2014;23:1563–78. doi:10.1093/hmg/ddt547.
Zhubi A, Chen Y, Dong E, Cook EH, Guidotti A, Grayson DR. Increased binding of MeCP2 to the GAD1 and RELN promoters may be mediated by an enrichment of 5-hmC in autism spectrum disorder (ASD) cerebellum. Transl Psychiatry. 2014;4:e349. doi:10.1038/tp.2013.123.
Irimia M, Weatheritt RJ, Ellis JD, Parikshak NN, Gonatopoulos-Pournatzis T, Babor M, et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell. 2014;159:1511–23. doi:10.1016/j.cell.2014.11.035.
Muratore CR, Hodgson NW, Trivedi MS, Abdolmaleky HM, Persico AM, Lintas C, et al. Age-dependent decrease and alternative splicing of methionine synthase mRNA in human cerebral cortex and an accelerated decrease in autism. PLoS ONE. 2013;8:e56927. doi:10.1371/journal.pone.0056927.
Zhang Y, Hodgson NW, Trivedi MS, Abdolmaleky HM, Fournier M, Cuenod M, et al. Decreased brain levels of vitamin B12 in aging, autism and schizophrenia. PLoS ONE. 2016;11:e0146797. doi:10.1371/journal.pone.0146797.
Tsang KM, Croen LA, Torres AR, Kharrazi M, Delorenze GN, Windham GC, et al. A genome-wide survey of transgenerational genetic effects in autism. PLoS ONE. 2013;8:e76978. doi:10.1371/journal.pone.0076978.
Desachy G, Croen LA, Torres AR, Kharrazi M, Delorenze GN, Windham GC, et al. Increased female autosomal burden of rare copy number variants in human populations and in autism families. Mol Psychiatry. 2015;20:170–5. doi:10.1038/mp.2014.179.
Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S, et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet. 2014;94:415–25. doi:10.1016/j.ajhg.2014.02.001.
Mondal K, Ramachandran D, Patel VC, Hagen KR, Bose P, Cutler DJ, et al. Excess variants in AFF2 detected by massively parallel sequencing of males with autism spectrum disorder. Hum Mol Genet. 2012;21:4356–64. doi:10.1093/hmg/dds267.
Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10:e1004580. doi:10.1371/journal.pgen.1004580.
Yuen RKC, Merico D, Cao H, Pellecchia G, Alipanahi B, Thiruvahindrapuram B, et al. Genome-wide characteristics of de novo mutations in autism. npj. Genomic Med. 2016;1:160271–1602710. doi:10.1038/npjgenmed.2016.27.
C Yuen RK, Merico D, Bookman M, L Howe J, Thiruvahindrapuram B, Patel RV, et al. Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat Neurosci. 2017;20:602–11. doi:10.1038/nn.4524.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi:10.1016/j.cell.2006.07.024.
Dolmetsch R, Geschwind DH. The human brain in a dish: the promise of iPSC-derived neurons. Cell. 2011;145:831–4. doi:10.1016/j.cell.2011.05.034.
Liu EY, Scott CT. Great expectations: autism spectrum disorder and induced pluripotent stem cell technologies. Stem Cell Rev. 2014;10:145–50. doi:10.1007/s12015-014-9497-0.
Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell. 2015;162:375–90. doi:10.1016/j.cell.2015.06.034.
Lu P, Chen X, Feng Y, Zeng Q, Jiang C, Zhu X, et al. Integrated transcriptome analysis of human iPS cells derived from a fragile X syndrome patient during neuronal differentiation. Sci China Life Sci. 2016;59:1093–105. doi:10.1007/s11427-016-0194-6.
Pierce NP, O’Reilly MF, Sorrells AM, Fragale CL, White PJ, Aguilar JM, et al. Ethnicity reporting practices for empirical research in three autism-related journals. J Autism Dev Disord. 2014;44:1507–19. doi:10.1007/s10803-014-2041-x.
Hilton CL, Fitzgerald RT, Jackson KM, Maxim RA, Bosworth CC, Shattuck PT, et al. Brief report: Under-representation of African americans in autism genetic research: a rationale for inclusion of subjects representing diverse family structures. J Autism Dev Disord. 2010;40:633–9. doi:10.1007/s10803-009-0905-2.
Robinson EB, Howrigan D, Yang J, Ripke S, Anttila V, Duncan LE, et al. Response to “Predicting the diagnosis of autism spectrum disorder using gene pathway analysis”. Mol Psychiatry. 2014;19:859–61. doi:10.1038/mp.2013.125.
Wang T, Guo H, Xiong B, Stessman HAF, Wu H, Coe BP, et al. De novo genic mutations among a Chinese autism spectrum disorder cohort. Nat Commun. 2016;7:13316. doi:10.1038/ncomms13316.
Nascimento PP, Bossolani-Martins AL, Rosan DBA, Mattos LC, Brandão-Mattos C, Fett-Conte AC. Single nucleotide polymorphisms in the CNTNAP2 gene in Brazilian patients with autistic spectrum disorder. Genet Mol Res. 2016;15. doi:10.4238/gmr.15017422.
Abdallah MW, Mortensen EL, Greaves-Lord K, Larsen N, Bonefeld-Jørgensen EC, Nørgaard-Pedersen B, et al. Neonatal levels of neurotrophic factors and risk of autism spectrum disorders. Acta Psychiatr Scand. 2013;128:61–9. doi:10.1111/acps.12020.
Abdallah MW, Pearce BD, Larsen N, Greaves-Lord K, Nørgaard-Pedersen B, Hougaard DM, et al. Amniotic fluid MMP-9 and neurotrophins in autism spectrum disorders: an exploratory study. Autism Res. 2012;5:428–33. doi:10.1002/aur.1254.
Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015;20:369–76. doi:10.1038/mp.2014.48.
Hollegaard MV, Grauholm J, Nielsen R, Grove J, Mandrup S, Hougaard DM. Archived neonatal dried blood spot samples can be used for accurate whole genome and exome-targeted next-generation sequencing. Mol Genet Metab. 2013;110:65–72. doi:10.1016/j.ymgme.2013.06.004.
Poulsen JB, Lescai F, Grove J, Bækvad-Hansen M, Christiansen M, Hagen CM, et al. High-Quality Exome Sequencing of Whole-Genome Amplified Neonatal Dried Blood Spot DNA. PLoS ONE. 2016;11:e0153253. doi:10.1371/journal.pone.0153253.
Hollegaard MV, Grauholm J, Nørgaard-Pedersen B, Hougaard DM. DNA methylome profiling using neonatal dried blood spot samples: a proof-of-principle study. Mol Genet Metab. 2013;108:225–31. doi:10.1016/j.ymgme.2013.01.016.
Grauholm J, Khoo SK, Nickolov RZ, Poulsen JB, Bækvad-Hansen M, Hansen CS, et al. Gene expression profiling of archived dried blood spot samples from the Danish Neonatal Screening Biobank. Mol Genet Metab. 2015;116:119–24. doi:10.1016/j.ymgme.2015.06.011.
Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6:407–11. doi:10.1016/j.stem.2010.04.005.
Bar-Nur O, Caspi I, Benvenisty N. Molecular analysis of FMR1 reactivation in fragile-X induced pluripotent stem cells and their neuronal derivatives. J Mol Cell Biol. 2012;4:180–3. doi:10.1093/jmcb/mjs007.
Halevy T, Czech C, Benvenisty N. Molecular mechanisms regulating the defects in fragile X syndrome neurons derived from human pluripotent stem cells. Stem Cell Reports. 2015;4:37–46. doi:10.1016/j.stemcr.2014.10.015.
Marchetto MCN, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–39. doi:10.1016/j.cell.2010.10.016.
Cocks G, Curran S, Gami P, Uwanogho D, Jeffries AR, Kathuria A, et al. The utility of patient specific induced pluripotent stem cells for the modelling of Autistic Spectrum Disorders. Psychopharmacology (Berl). 2014;231:1079–88. doi:10.1007/s00213-013-3196-4.
Kelava I, Lancaster MA. Stem cell models of human brain development. Cell Stem Cell. 2016;18:736–48. doi:10.1016/j.stem.2016.05.022.
Griesi-Oliveira K, Acab A, Gupta AR, Sunaga DY, Chailangkarn T, Nicol X, et al. Modeling non-syndromic autism and the impact of TRPC6 disruption in human neurons. Mol Psychiatry. 2015;20:1350–65. doi:10.1038/mp.2014.141.
Mahe MM, Workman M, Trisno S, Poling H, Watson CL, Sundaram N, et al. Functional enteric nervous system in human small intestine derived from pluripotent stem cells. Neurogastroenterol Motil. 2016;28:6–6.
Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, et al. Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics. 2015;9:054124. doi:10.1063/1.4934713.
Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7:49. doi:10.1186/s13229-016-0110-z.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi:10.1016/j.cell.2007.11.019.
Rao MS, Malik N. Assessing iPSC reprogramming methods for their suitability in translational medicine. J Cell Biochem. 2012;113:3061–8.
Raab S, Klingenstein M, Liebau S, Linta L. A Comparative View on Human Somatic Cell Sources for iPSC Generation. Stem Cells Int. 2014;2014:768391. doi:10.1155/2014/768391.
Fernell E, Karagiannakis A, Edman G, Bjerkenstedt L, Wiesel F-A, Venizelos N. Aberrant amino acid transport in fibroblasts from children with autism. Neurosci Lett. 2007;418:82–6. doi:10.1016/j.neulet.2007.03.004.
Vumma R, Wiesel F-A, Flyckt L, Bjerkenstedt L, Venizelos N. Functional characterization of tyrosine transport in fibroblast cells from healthy controls. Neurosci Lett. 2008;434:56–60. doi:10.1016/j.neulet.2008.01.028.
Schmunk G, Boubion BJ, Smith IF, Parker I, Gargus JJ. Shared functional defect in IP3R-mediated calcium signaling in diverse monogenic autism syndromes. Transl Psychiatry. 2015;5:e643. doi:10.1038/tp.2015.123.
Schmunk G, Nguyen RL, Ferguson DL, Kumar K, Parker I, Gargus JJ. High-throughput screen detects calcium signaling dysfunction in typical sporadic autism spectrum disorder. Sci Rep. 2017;7:40740. doi:10.1038/srep40740.
Aasen T, Izpisúa Belmonte JC. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc. 2010;5:371–82. doi:10.1038/nprot.2009.241.
Streckfuss-Bömeke K, Wolf F, Azizian A, Stauske M, Tiburcy M, Wagner S, et al. Comparative study of human-induced pluripotent stem cells derived from bone marrow cells, hair keratinocytes, and skin fibroblasts. Eur Heart J. 2013;34:2618–29. doi:10.1093/eurheartj/ehs203.
Piao Y, Hung SS-C, Lim SY, Wong RC-B, Ko MSH. Efficient generation of integration-free human induced pluripotent stem cells from keratinocytes by simple transfection of episomal vectors. Stem Cells Transl Med. 2014;3:787–91. doi:10.5966/sctm.2013-0036.
Chalmers D, Nicol D, Kaye J, Bell J, Campbell AV, Ho CWL, et al. Has the biobank bubble burst? Withstanding the challenges for sustainable biobanking in the digital era. BMC Med Ethics. 2016;17:39. doi:10.1186/s12910-016-0124-2.
Bjugn R, Casati B. Stakeholder analysis: a useful tool for biobank planning. Biopreserv Biobank. 2012;10:239–44. doi:10.1089/bio.2011.0047.
Pellicano E, Dinsmore A, Charman T. Views on researcher-community engagement in autism research in the United Kingdom: a mixed-methods study. PLoS ONE. 2014;9:e109946. doi:10.1371/journal.pone.0109946.
Hansson MG. Ethics and biobanks. British Journal of Cancer. 2009;100:8–12.
Helgesson G, Dillner J, Carlson J, Bartram CR, Hansson MG. Ethical framework for previously collected biobank samples. Nat Biotechnol. 2007;25:973–6. doi:10.1038/nbt0907-973b.
Steinsbekk KS, Kåre Myskja B, Solberg B. Broad consent versus dynamic consent in biobank research: is passive participation an ethical problem? Eur J Hum Genet. 2013;21:897–902. doi:10.1038/ejhg.2012.282.
Knoppers BM, Isasi R. Stem cell banking: between traceability and identifiability. Genome Med. 2010;2:73. doi:10.1186/gm194.
Auray-Blais C, Patenaude J. A biobank management model applicable to biomedical research. BMC Med Ethics. 2006;7:E4. doi:10.1186/1472-6939-7-4.
Lemke AA, Wolf WA, Hebert-Beirne J, Smith ME. Public and biobank participant attitudes toward genetic research participation and data sharing. Public Health Genomics. 2010;13:368–77. doi:10.1159/000276767.
Ortega F. The Cerebral Subject and the Challenge of Neurodiversity. BioSocieties. 2009;4:425–45. doi:10.1017/S1745855209990287.
Pellicano E, Stears M. Bridging autism, science and society: moving toward an ethically informed approach to autism research. Autism Res. 2011;4:271–82. doi:10.1002/aur.201.
Kapp SK, Gillespie-Lynch K, Sherman LE, Hutman T. Deficit, difference, or both? Autism and neurodiversity. Dev Psychol. 2013;49:59–71. doi:10.1037/a0028353.
Barnes RE, McCabe H. Should we welcome a cure for autism? A survey of the arguments. Med Health Care Philos. 2012;15:255–69. doi:10.1007/s11019-011-9339-7.
Scott CT. The case for stem cell counselors. Stem Cell Rep. 2015;4:1–6. doi:10.1016/j.stemcr.2014.10.016.
Krumm N, O’Roak BJ, Shendure J, Eichler EE. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014;37:95–105. doi:10.1016/j.tins.2013.11.005.
Girirajan S, Dennis MY, Baker C, Malig M, Coe BP, Campbell CD, et al. Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am J Hum Genet. 2013;92:221–37. doi:10.1016/j.ajhg.2012.12.016.
Kim H-G, Kishikawa S, Higgins AW, Seong I-S, Donovan DJ, Shen Y, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi:10.1016/j.ajhg.2007.09.011.
Alarcón M, Cantor RM, Liu J, Gilliam TC, Geschwind DH. Autism Genetic Research Exchange Consortium. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Am J Hum Genet. 2002;70:60–71. doi:10.1086/338241.
Alarcón M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–9. doi:10.1016/j.ajhg.2007.09.005.
Alarcón M, Yonan AL, Gilliam TC, Cantor RM, Geschwind DH. Quantitative genome scan and Ordered-Subsets Analysis of autism endophenotypes support language QTLs. Mol Psychiatry. 2005;10:747–57. doi:10.1038/sj.mp.4001666.
Allen-Brady K, Cannon D, Robison R, McMahon WM, Coon H. A unified theory of autism revisited: linkage evidence points to chromosome X using a high-risk subset of AGRE families. Autism Res. 2010;3:47–52. doi:10.1002/aur.119.
Anitha A, Nakamura K, Yamada K, Suda S, Thanseem I, Tsujii M, et al. Genetic analyses of roundabout (ROBO) axon guidance receptors in autism. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1019–27. doi:10.1002/ajmg.b.30697.
Anitha A, Thanseem I, Nakamura K, Yamada K, Iwayama Y, Toyota T, et al. Protocadherin α (PCDHA) as a novel susceptibility gene for autism. J Psychiatry Neurosci. 2013;38:192–8. doi:10.1503/jpn.120058.
Anitha A, Thanseem I, Nakamura K, Vasu MM, Yamada K, Ueki T, et al. Zinc finger protein 804A (ZNF804A) and verbal deficits in individuals with autism. J Psychiatry Neurosci. 2014;39:294–303. doi:10.1503/jpn.130126.
Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–4. doi:10.1016/j.ajhg.2007.09.015.
Babatz TD, Kumar RA, Sudi J, Dobyns WB, Christian SL. Copy number and sequence variants implicate APBA2 as an autism candidate gene. Autism Res. 2009;2:359–64. doi:10.1002/aur.107.
Bakkaloglu B, O’Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–73. doi:10.1016/j.ajhg.2007.09.017.
Baron CA, Tepper CG, Liu SY, Davis RR, Wang NJ, Schanen NC, et al. Genomic and functional profiling of duplicated chromosome 15 cell lines reveal regulatory alterations in UBE3A-associated ubiquitin-proteasome pathway processes. Hum Mol Genet. 2006;15:853–69. doi:10.1093/hmg/ddl004.
Baugher JD, Baugher BD, Shirley MD, Pevsner J. Sensitive and specific detection of mosaic chromosomal abnormalities using the Parent-of-Origin-based Detection (POD) method. BMC Genomics. 2013;14:367. doi:10.1186/1471-2164-14-367.
Benayed R, Gharani N, Rossman I, Mancuso V, Lazar G, Kamdar S, et al. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am J Hum Genet. 2005;77:851–68. doi:10.1086/497705.
Buxbaum JD, Silverman JM, Smith CJ, Kilifarski M, Reichert J, Hollander E, et al. Evidence for a susceptibility gene for autism on chromosome 2 and for genetic heterogeneity. Am J Hum Genet. 2001;68:1514–20. doi:10.1086/320588.
Buxbaum JD, Silverman J, Keddache M, Smith CJ, Hollander E, Ramoz N, et al. Linkage analysis for autism in a subset families with obsessive-compulsive behaviors: evidence for an autism susceptibility gene on chromosome 1 and further support for susceptibility genes on chromosome 6 and 19. Mol Psychiatry. 2004;9:144–50. doi:10.1038/sj.mp.4001465.
Cantor RM, Kono N, Duvall JA, Alvarez-Retuerto A, Stone JL, Alarcón M, et al. Replication of autism linkage: fine-mapping peak at 17q21. Am J Hum Genet. 2005;76:1050–6. doi:10.1086/430278.
Carayol J, Sacco R, Tores F, Rousseau F, Lewin P, Hager J, et al. Converging evidence for an association of ATP2B2 allelic variants with autism in male subjects. Biol Psychiatry. 2011;70:880–7. doi:10.1016/j.biopsych.2011.05.020.
Carayol J, Schellenberg GD, Dombroski B, Genin E, Rousseau F, Dawson G. Autism risk assessment in siblings of affected children using sex-specific genetic scores. Mol Autism. 2011;2:17. doi:10.1186/2040-2392-2-17.
Carayol J, Schellenberg GD, Dombroski B, Amiet C, Génin B, Fontaine K, et al. A scoring strategy combining statistics and functional genomics supports a possible role for common polygenic variation in autism. Front Genet. 2014;5:33. doi:10.3389/fgene.2014.00033.
Chang S-C, Pauls DL, Lange C, Sasanfar R, Santangelo SL. Common genetic variation in the GAD1 gene and the entire family of DLX homeobox genes and autism spectrum disorders. Am J Med Genet B Neuropsychiatr Genet. 2011;156:233–9. doi:10.1002/ajmg.b.31148.
Chang S-C, Pauls DL, Lange C, Sasanfar R, Santangelo SL. Sex-specific association of a common variant of the XG gene with autism spectrum disorders. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:742–50. doi:10.1002/ajmg.b.32165.
Chen GK, Kono N, Geschwind DH, Cantor RM. Quantitative trait locus analysis of nonverbal communication in autism spectrum disorder. Mol Psychiatry. 2006;11:214–20. doi:10.1038/sj.mp.4001753.
Cheslack-Postava K, Fallin MD, Avramopoulos D, Connors SL, Zimmerman AW, Eberhart CG, et al. beta2-Adrenergic receptor gene variants and risk for autism in the AGRE cohort. Mol Psychiatry. 2007;12:283–91. doi:10.1038/sj.mp.4001940.
Cheung J, Petek E, Nakabayashi K, Tsui LC, Vincent JB, Scherer SW. Identification of the human cortactin-binding protein-2 gene from the autism candidate region at 7q31. Genomics. 2001;78:7–11. doi:10.1006/geno.2001.6651.
Conciatori M, Stodgell CJ, Hyman SL, O’Bara M, Militerni R, Bravaccio C, et al. Association between the HOXA1 A218G polymorphism and increased head circumference in patients with autism. Biol Psychiatry. 2004;55:413–9. doi:10.1016/j.biopsych.2003.10.005.
Connolly JJ, Glessner JT, Hakonarson H. A genome-wide association study of autism incorporating autism diagnostic interview-revised, autism diagnostic observation schedule, and social responsiveness scale. Child Dev. 2013;84:17–33. doi:10.1111/j.1467-8624.2012.01838.x.
Connors SL, Crowell DE, Eberhart CG, Copeland J, Newschaffer CJ, Spence SJ, et al. beta2-adrenergic receptor activation and genetic polymorphisms in autism: data from dizygotic twins. J Child Neurol. 2005;20:876–84. doi:10.1177/08830738050200110401.
D’Amelio M, Ricci I, Sacco R, Liu X, D’Agruma L, Muscarella LA, et al. Paraoxonase gene variants are associated with autism in North America, but not in Italy: possible regional specificity in gene-environment interactions. Mol Psychiatry. 2005;10:1006–16. doi:10.1038/sj.mp.4001714.
Davis LK, Meyer KJ, Rudd DS, Librant AL, Epping EA, Sheffield VC, et al. Novel copy number variants in children with autism and additional developmental anomalies. J Neurodev Disord. 2009;1:292–301. doi:10.1007/s11689-009-9013-z.
Dennis MY, Nuttle X, Sudmant PH, Antonacci F, Graves TA, Nefedov M, et al. Evolution of human-specific neural SRGAP2 genes by incomplete segmental duplication. Cell. 2012;149:912–22. doi:10.1016/j.cell.2012.03.033.
Duvall J. A Quantitative Trait Locus Analysis of Social Responsiveness in Multiplex Autism Families. Am J Psychiatry. 2007;164:656. doi:10.1176/appi.ajp.164.4.656.
Yonan AL, Alarcón M, Cheng R, Magnusson PKE, Spence SJ, Palmer AA, et al. A Genomewide Screen of 345 Families for Autism-Susceptibility Loci. Am J Hum Genet. 2003;73:886–97. doi:10.1086/378778.
Fatemi SH, Stary JM, Egan EA. Reduced blood levels of reelin as a vulnerability factor in pathophysiology of autistic disorder. Cell Mol Neurobiol. 2002;22:139–52.
Cisternas FA, Vincent JB, Scherer SW, Ray PN. Cloning and characterization of human CADPS and CADPS2, new members of the Ca2 + −dependent activator for secretion protein family. Genomics. 2003;81:279–91. doi:10.1016/S0888-7543(02)00040-X.
Flax JF, Hare A, Azaro MA, Vieland VJ, Brzustowicz LM. Combined linkage and linkage disequilibrium analysis of a motor speech phenotype within families ascertained for autism risk loci. J Neurodev Disord. 2010;2:210–23. doi:10.1007/s11689-010-9063-2.
Fradin D, Cheslack-Postava K, Ladd-Acosta C, Newschaffer C, Chakravarti A, Arking DE, et al. Parent-of-origin effects in autism identified through genome-wide linkage analysis of 16,000 SNPs. PLoS ONE. 2010;5. doi:10.1371/journal.pone.0012513.
Girirajan S, Johnson RL, Tassone F, Balciuniene J, Katiyar N, Fox K, et al. Global increases in both common and rare copy number load associated with autism. Hum Mol Genet. 2013;22:2870–80. doi:10.1093/hmg/ddt136.
Goin-Kochel RP, Porter AE, Peters SU, Shinawi M, Sahoo T, Beaudet AL. The MTHFR 677C→T polymorphism and behaviors in children with autism: exploratory genotype-phenotype correlations. Autism Res. 2009;2:98–108. doi:10.1002/aur.70.
McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–7. doi:10.1038/ng.474.
Hamilton SP, Woo JM, Carlson EJ, Ghanem N, Ekker M, Rubenstein JLR. Analysis of four DLX homeobox genes in autistic probands. BMC Genet. 2005;6:52. doi:10.1186/1471-2156-6-52.
Hettinger JA, Liu X, Schwartz CE, Michaelis RC, Holden JJA. A DRD1 haplotype is associated with risk for autism spectrum disorders in male-only affected sib-pair families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:628–36. doi:10.1002/ajmg.b.30655.
Higashida H, Yokoyama S, Huang J-J, Liu L, Ma W-J, Akther S, et al. Social memory, amnesia, and autism: brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem Int. 2012;61:828–38. doi:10.1016/j.neuint.2012.01.030.
Munesue T, Yokoyama S, Nakamura K, Anitha A, Yamada K, Hayashi K, et al. Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci Res. 2010;67:181–91. doi:10.1016/j.neures.2010.03.004.
Hu VW, Frank BC, Heine S, Lee NH, Quackenbush J. Gene expression profiling of lymphoblastoid cell lines from monozygotic twins discordant in severity of autism reveals differential regulation of neurologically relevant genes. BMC Genomics. 2006;7:118. doi:10.1186/1471-2164-7-118.
Hu VW, Sarachana T, Kim KS, Nguyen A, Kulkarni S, Steinberg ME, et al. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2009;2:78–97. doi:10.1002/aur.73.
Hussman JP, Chung R-H, Griswold AJ, Jaworski JM, Salyakina D, Ma D, et al. A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism. Mol Autism. 2011;2:1. doi:10.1186/2040-2392-2-1.
Kistner-Griffin E, Brune CW, Davis LK, Sutcliffe JS, Cox NJ, Cook EH. Parent-of-origin effects of the serotonin transporter gene associated with autism. Am J Med Genet B Neuropsychiatr Genet. 2011;156:139–44. doi:10.1002/ajmg.b.31146.
Kumar RA, Sudi J, Babatz TD, Brune CW, Oswald D, Yen M, et al. A de novo 1p34.2 microdeletion identifies the synaptic vesicle gene RIMS3 as a novel candidate for autism. J Med Genet. 2010;47:81–90. doi:10.1136/jmg.2008.065821.
Lee L-C, Zachary AA, Leffell MS, Newschaffer CJ, Matteson KJ, Tyler JD, et al. HLA-DR4 in families with autism. Pediatr Neurol. 2006;35:303–7. doi:10.1016/j.pediatrneurol.2006.06.006.
Liu X, Malenfant P, Reesor C, Lee A, Hudson ML, Harvard C, et al. 2p15-p16.1 microdeletion syndrome: molecular characterization and association of the OTX1 and XPO1 genes with autism spectrum disorders. Eur J Hum Genet. 2011;19:1264–70. doi:10.1038/ejhg.2011.112.
Liu X, Novosedlik N, Wang A, Hudson ML, Cohen IL, Chudley AE, et al. The DLX1and DLX2 genes and susceptibility to autism spectrum disorders. Eur J Hum Genet. 2009;17:228–35. doi:10.1038/ejhg.2008.148.
Liu X, Solehdin F, Cohen IL, Gonzalez MG, Jenkins EC, Lewis MES, et al. Population- and family-based studies associate the MTHFR gene with idiopathic autism in simplex families. J Autism Dev Disord. 2011;41:938–44. doi:10.1007/s10803-010-1120-x.
Lu ATH, Cantor RM. Allowing for sex differences increases power in a GWAS of multiplex Autism families. Mol Psychiatry. 2012;17:215–22. doi:10.1038/mp.2010.127.
Lu AT-H, Dai X, Martinez-Agosto JA, Cantor RM. Support for calcium channel gene defects in autism spectrum disorders. Mol Autism. 2012;3:18. doi:10.1186/2040-2392-3-18.
Ma D, Salyakina D, Jaworski JM, Konidari I, Whitehead PL, Andersen AN, et al. A genome-wide association study of autism reveals a common novel risk locus at 5p14.1. Ann Hum Genet. 2009;73(Pt 3):263–73. doi:10.1111/j.1469-1809.2009.00523.x.
Malenfant P, Liu X, Hudson ML, Qiao Y, Hrynchak M, Riendeau N, et al. Association of GTF2i in the Williams-Beuren syndrome critical region with autism spectrum disorders. J Autism Dev Disord. 2012;42:1459–69. doi:10.1007/s10803-011-1389-4.
Martin CL, Duvall JA, Ilkin Y, Simon JS, Arreaza MG, Wilkes K, et al. Cytogenetic and molecular characterization of A2BP1/FOX1 as a candidate gene for autism. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:869–76. doi:10.1002/ajmg.b.30530.
Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–16. doi:10.1016/j.bbi.2007.12.007.
Maussion G, Carayol J, Lepagnol-Bestel A-M, Tores F, Loe-Mie Y, Milbreta U, et al. Convergent evidence identifying MAP/microtubule affinity-regulating kinase 1 (MARK1) as a susceptibility gene for autism. Hum Mol Genet. 2008;17:2541–51. doi:10.1093/hmg/ddn154.
McCauley JL, Li C, Jiang L, Olson LM, Crockett G, Gainer K, et al. Genome-wide and Ordered-Subset linkage analyses provide support for autism loci on 17q and 19p with evidence of phenotypic and interlocus genetic correlates. BMC Med Genet. 2005;6:1. doi:10.1186/1471-2350-6-1.
McInnes LA, Nakamine A, Pilorge M, Brandt T, Jiménez González P, Fallas M, et al. A large-scale survey of the novel 15q24 microdeletion syndrome in autism spectrum disorders identifies an atypical deletion that narrows the critical region. Mol Autism. 2010;1:5. doi:10.1186/2040-2392-1-5.
Meyer WK, Arbeithuber B, Ober C, Ebner T, Tiemann-Boege I, Hudson RR, et al. Evaluating the evidence for transmission distortion in human pedigrees. Genetics. 2012;191:215–32. doi:10.1534/genetics.112.139576.
Molloy CA, Keddache M, Martin LJ. Evidence for linkage on 21q and 7q in a subset of autism characterized by developmental regression. Mol Psychiatry. 2005;10:741–6. doi:10.1038/sj.mp.4001691.
Muscarella LA, Guarnieri V, Sacco R, Militerni R, Bravaccio C, Trillo S, et al. HOXA1 gene variants influence head growth rates in humans. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:388–90. doi:10.1002/ajmg.b.30469.
Nabi R, Zhong H, Serajee FJ, Huq AHMM. No association between single nucleotide polymorphisms in DLX6 and Piccolo genes at 7q21-q22 and autism. Am J Med Genet B Neuropsychiatr Genet. 2003;119B:98–101. doi:10.1002/ajmg.b.10012.
Nabi R, Serajee FJ, Chugani DC, Zhong H, Huq AHMM. Association of tryptophan 2,3 dioxygenase gene polymorphism with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:63–8. doi:10.1002/ajmg.b.20147.
Nakamura K, Anitha A, Yamada K, Tsujii M, Iwayama Y, Hattori E, et al. Genetic and expression analyses reveal elevated expression of syntaxin 1A (STX1A) in high functioning autism. Int J Neuropsychopharmacol. 2008;11:1073–84. doi:10.1017/S1461145708009036.
Nicholas B, Rudrasingham V, Nash S, Kirov G, Owen MJ, Wimpory DC. Association of Per1 and Npas2 with autistic disorder: support for the clock genes/social timing hypothesis. Mol Psychiatry. 2007;12:581–92. doi:10.1038/sj.mp.4001953.
Nishimura K, Nakamura K, Anitha A, Yamada K, Tsujii M, Iwayama Y, et al. Genetic analyses of the brain-derived neurotrophic factor (BDNF) gene in autism. Biochem Biophys Res Commun. 2007;356:200–6. doi:10.1016/j.bbrc.2007.02.135.
Noor A, Whibley A, Marshall CR, Gianakopoulos PJ, Piton A, Carson AR, et al. Disruption at the PTCHD1 Locus on Xp22.11 in Autism spectrum disorder and intellectual disability. Sci Transl Med. 2010;2:49ra68. doi:10.1126/scitranslmed.3001267.
Petek E, Schwarzbraun T, Noor A, Patel M, Nakabayashi K, Choufani S, et al. Molecular and genomic studies of IMMP2L and mutation screening in autism and Tourette syndrome. Mol Genet Genomics. 2007;277:71–81. doi:10.1007/s00438-006-0173-1.
Philippi A, Roschmann E, Tores F, Lindenbaum P, Benajou A, Germain-Leclerc L, et al. Haplotypes in the gene encoding protein kinase c-beta (PRKCB1) on chromosome 16 are associated with autism. Mol Psychiatry. 2005;10:950–60. doi:10.1038/sj.mp.4001704.
Philippi A, Tores F, Carayol J, Rousseau F, Letexier M, Roschmann E, et al. Association of autism with polymorphisms in the paired-like homeodomain transcription factor 1 (PITX1) on chromosome 5q31: a candidate gene analysis. BMC Med Genet. 2007;8:74. doi:10.1186/1471-2350-8-74.
Rabionet R, Jaworski JM, Ashley-Koch AE, Martin ER, Sutcliffe JS, Haines JL, et al. Analysis of the autism chromosome 2 linkage region: GAD1 and other candidate genes. Neurosci Lett. 2004;372:209–14. doi:10.1016/j.neulet.2004.09.037.
Raiford KL, Shao Y, Allen IC, Martin ER, Menold MM, Wright HH, et al. No association between the APOE gene and autism. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:57–60. doi:10.1002/ajmg.b.20104.
Ramos PS, Sajuthi S, Langefeld CD, Walker SJ. Immune function genes CD99L2, JARID2 and TPO show association with autism spectrum disorder. Mol Autism. 2012;3:4. doi:10.1186/2040-2392-3-4.
Ramoz N, Cai G, Reichert JG, Silverman JM, Buxbaum JD. An analysis of candidate autism loci on chromosome 2q24-q33: evidence for association to the STK39 gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1152–8. doi:10.1002/ajmg.b.30739.
Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, et al. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry. 2004;161:662–9. doi:10.1176/appi.ajp.161.4.662.
Ramoz N, Cai G, Reichert JG, Corwin TE, Kryzak LA, Smith CJ, et al. Family-based association study of TPH1 and TPH2 polymorphisms in autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:861–7. doi:10.1002/ajmg.b.30356.
Ramoz N, Reichert JG, Corwin TE, Smith CJ, Silverman JM, Hollander E, et al. Lack of evidence for association of the serotonin transporter gene SLC6A4 with autism. Biol Psychiatry. 2006;60:186–91. doi:10.1016/j.biopsych.2006.01.009.
Alvarez Retuerto AI, Cantor RM, Gleeson JG, Ustaszewska A, Schackwitz WS, Pennacchio LA, et al. Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum Mol Genet. 2008;17:3887–96. doi:10.1093/hmg/ddn291.
Roberson EDO, Pevsner J. Visualization of shared genomic regions and meiotic recombination in high-density SNP data. PLoS ONE. 2009;4:e6711. doi:10.1371/journal.pone.0006711.
Russo AJ, Neville L, Wroge C. Low Serum Alpha-1 Antitrypsin (AAT) in Family Members of Individuals with Autism Correlates with PiMZ Genotype. Biomark Insights. 2009;4:45–56.
Sadakata T, Washida M, Iwayama Y, Shoji S, Sato Y, Ohkura T, et al. Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J Clin Invest. 2007;117:931–43. doi:10.1172/JCI29031.
Salyakina D, Ma DQ, Jaworski JM, Konidari I, Whitehead PL, Henson R, et al. Variants in several genomic regions associated with asperger disorder. Autism Res. 2010;3:303–10. doi:10.1002/aur.158.
Schwender H, Bowers K, Fallin MD, Ruczinski I. Importance measures for epistatic interactions in case-parent trios. Ann Hum Genet. 2011;75:122–32. doi:10.1111/j.1469-1809.2010.00623.x.
Serajee FJ, Mahbubul Huq AHM. Association of Y chromosome haplotypes with autism. J Child Neurol. 2009;24:1258–61. doi:10.1177/0883073809333530.
Serajee FJ, Nabi R, Zhong H, Mahbubul Huq AHM. Association of INPP1, PIK3CG, and TSC2 gene variants with autistic disorder: implications for phosphatidylinositol signalling in autism. J Med Genet. 2003;40:e119. doi:10.1136/jmg.40.11.e119.
Serajee FJ, Zhong H, Nabi R, Huq AHMM. The metabotropic glutamate receptor 8 gene at 7q31: partial duplication and possible association with autism. J Med Genet. 2003;40:e42. doi:10.1136/jmg.40.4.e42.
Serajee FJ, Nabi R, Zhong H, Huq M. Polymorphisms in xenobiotic metabolism genes and autism. J Child Neurol. 2004;19:413–7. doi:10.1177/088307380401900603.
Shi L, Zhang X, Golhar R, Otieno FG, He M, Hou C, et al. Whole-genome sequencing in an autism multiplex family. Mol Autism. 2013;4:8. doi:10.1186/2040-2392-4-8.
Shi J, Li P. An integrative segmentation method for detecting germline copy number variations in SNP arrays. Genet Epidemiol. 2012;36:373–83. doi:10.1002/gepi.21631.
Silverman JM, Buxbaum JD, Ramoz N, Schmeidler J, Reichenberg A, Hollander E, et al. Autism-related routines and rituals associated with a mitochondrial aspartate/glutamate carrier SLC25A12 polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2008;147:408–10. doi:10.1002/ajmg.b.30614.
Steinberg KM, Ramachandran D, Patel VC, Shetty AC, Cutler DJ, Zwick ME. Identification of rare X-linked neuroligin variants by massively parallel sequencing in males with autism spectrum disorder. Mol Autism. 2012;3:8. doi:10.1186/2040-2392-3-8.
Stone JL, Merriman B, Cantor RM, Geschwind DH, Nelson SF. High density SNP association study of a major autism linkage region on chromosome 17. Hum Mol Genet. 2007;16:704–15. doi:10.1093/hmg/ddm015.
Stone JL, Merriman B, Cantor RM, Yonan AL, Gilliam TC, Geschwind DH, et al. Evidence for sex-specific risk alleles in autism spectrum disorder. Am J Hum Genet. 2004;75:1117–23. doi:10.1086/426034.
Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, et al. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–79. doi:10.1086/432648.
Tierney E, Bukelis I, Thompson RE, Ahmed K, Aneja A, Kratz L, et al. Abnormalities of cholesterol metabolism in autism spectrum disorders. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:666–8. doi:10.1002/ajmg.b.30368.
Toyoda T, Nakamura K, Yamada K, Thanseem I, Anitha A, Suda S, et al. SNP analyses of growth factor genes EGF, TGFbeta-1, and HGF reveal haplotypic association of EGF with autism. Biochem Biophys Res Commun. 2007;360:715–20. doi:10.1016/j.bbrc.2007.06.051.
Vardarajan BN, Eran A, Jung JY, Kunkel LM, Wall DP. Haplotype structure enables prioritization of common markers and candidate genes in autism spectrum disorder. Transl Psychiatry. 2013;3:e262. doi:10.1038/tp.2013.38.
Vincent JB, Petek E, Thevarkunnel S, Kolozsvari D, Cheung J, Patel M, et al. The RAY1/ST7 Tumor-Suppressor Locus on Chromosome 7q31 Represents a Complex Multi-transcript System. Genomics. 2002;80:283–94. doi:10.1006/geno.2002.6835.
Vincent JB, Kolozsvari D, Roberts WS, Bolton PF, Gurling HMD, Scherer SW. Mutation screening of X-chromosomal neuroligin genes: no mutations in 196 autism probands. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:82–4. doi:10.1002/ajmg.b.30069.
Vincent JB, Thevarkunnel S, Kolozsvari D, Paterson AD, Roberts W, Scherer SW. Association and transmission analysis of the FMR1 IVS10 + 14C-T variant in autism. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:54–6. doi:10.1002/ajmg.b.20088.
Walker SJ, Segal J, Aschner M. Cultured lymphocytes from autistic children and non-autistic siblings up-regulate heat shock protein RNA in response to thimerosal challenge. Neurotoxicology. 2006;27:685–92. doi:10.1016/j.neuro.2006.06.003.
Weiss LA, Arking DE, Gene Discovery Project of Johns Hopkins & the Autism Consortium, Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–8. doi:10.1038/nature08490.
Weiss LA, Kosova G, Delahanty RJ, Jiang L, Cook EH, Ober C, et al. Variation in ITGB3 is associated with whole-blood serotonin level and autism susceptibility. Eur J Hum Genet. 2006;14:923–31. doi:10.1038/sj.ejhg.5201644.
Werling DM, Geschwind DH. Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins. Mol Autism. 2015;6:27. doi:10.1186/s13229-015-0004-5.
Werling DM, Lowe JK, Luo R, Cantor RM, Geschwind DH. Replication of linkage at chromosome 20p13 and identification of suggestive sex-differential risk loci for autism spectrum disorder. Mol Autism. 2014;5:13. doi:10.1186/2040-2392-5-13.
Yamagata T, Aradhya S, Mori M, Inoue K, Momoi MY, Nelson DL. The Human Secretin Gene: Fine Structure in 11p15.5 and Sequence Variation in Patients with Autism. Genomics. 2002;80:185–94. doi:10.1006/geno.2002.6814.
Yaspan BL, Bush WS, Torstenson ES, Ma D, Pericak-Vance MA, Ritchie MD, et al. Genetic analysis of biological pathway data through genomic randomization. Hum Genet. 2011;129:563–71. doi:10.1007/s00439-011-0956-2.
Ylisaukko-oja T, Alarcón M, Cantor RM, Auranen M, Vanhala R, Kempas E, et al. Search for autism loci by combined analysis of Autism Genetic Resource Exchange and Finnish families. Ann Neurol. 2006;59:145–55. doi:10.1002/ana.20722.
Liu J, Nyholt DR, Magnussen P, Parano E, Pavone P, Geschwind D, et al. A genomewide screen for autism susceptibility loci. Am J Hum Genet. 2001;69:327–40. doi:10.1086/321980.
Zandi PP, Kalaydjian A, Avramopoulos D, Shao H, Fallin MD, Newschaffer CJ. Rh and ABO maternal-fetal incompatibility and risk of autism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:643–7. doi:10.1002/ajmg.b.30391.
Zhao X, Leotta A, Kustanovich V, Lajonchere C, Geschwind DH, Law K, et al. A unified genetic theory for sporadic and inherited autism. Proc Natl Acad Sci U S A. 2007;104:12831–6. doi:10.1073/pnas.0705803104.
Zhong H. No association between the EN2 gene and autistic disorder. J Med Genet. 2003;40:4e–4. doi:10.1136/jmg.40.1.e4.
James SJ, Jill James S, Melnyk S, Jernigan S, Hubanks A, Rose S, et al. Abnormal transmethylation/transsulfuration metabolism and DNA hypomethylation among parents of children with autism. J Autism Dev Disord. 2008;38:1966–75. doi:10.1007/s10803-008-0591-5.
Ackerman S, Wenegrat J, Rettew D, Althoff R, Bernier R. No increase in autism-associated genetic events in children conceived by assisted reproduction. Fertil Steril. 2014;102:388–93. doi:10.1016/j.fertnstert.2014.04.020.
Bahl S, Chiang C, Beauchamp RL, Neale BM, Daly MJ, Gusella JF, et al. Lack of association of rare functional variants in TSC1/TSC2 genes with autism spectrum disorder. Mol Autism. 2013;4:5. doi:10.1186/2040-2392-4-5.
Ben-David E, Shifman S. Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol Psychiatry. 2013;18:1054–6. doi:10.1038/mp.2012.148.
Brand H, Collins RL, Hanscom C, Rosenfeld JA, Pillalamarri V, Stone MR, et al. Paired-Duplication Signatures Mark Cryptic Inversions and Other Complex Structural Variation. Am J Hum Genet. 2015;97:170–6. doi:10.1016/j.ajhg.2015.05.012.
Campbell MG, Kohane IS, Kong SW. Pathway-based outlier method reveals heterogeneous genomic structure of autism in blood transcriptome. BMC Med Genomics. 2013;6:34. doi:10.1186/1755-8794-6-34.
Celestino-Soper PBS, Shaw CA, Sanders SJ, Li J, Murtha MT, Ercan-Sencicek AG, et al. Use of array CGH to detect exonic copy number variants throughout the genome in autism families detects a novel deletion in TMLHE. Hum Mol Genet. 2011;20:4360–70. doi:10.1093/hmg/ddr363.
Celestino-Soper PBS, Violante S, Crawford EL, Luo R, Lionel AC, Delaby E, et al. A common X-linked inborn error of carnitine biosynthesis may be a risk factor for nondysmorphic autism. Proc Natl Acad Sci U S A. 2012;109:7974–81. doi:10.1073/pnas.1120210109.
Chang J, Gilman SR, Chiang AH, Sanders SJ, Vitkup D. Genotype to phenotype relationships in autism spectrum disorders. Nat Neurosci. 2015;18:191–8. doi:10.1038/nn.3907.
Chaste P, Klei L, Sanders SJ, Hus V, Murtha MT, Lowe JK, et al. A genome-wide association study of autism using the Simons Simplex Collection: Does reducing phenotypic heterogeneity in autism increase genetic homogeneity? Biol Psychiatry. 2015;77:775–84. doi:10.1016/j.biopsych.2014.09.017.
Cheng Y, Quinn JF, Weiss LA. An eQTL mapping approach reveals that rare variants in the SEMA5A regulatory network impact autism risk. Hum Mol Genet. 2013;22:2960–72. doi:10.1093/hmg/ddt150.
Fang H, Wu Y, Narzisi G, O’Rawe JA, Barrón LTJ, Rosenbaum J, et al. Reducing INDEL calling errors in whole genome and exome sequencing data. Genome Med. 2014;6:89. doi:10.1186/s13073-014-0089-z.
Gamsiz ED, Viscidi EW, Frederick AM, Nagpal S, Sanders SJ, Murtha MT, et al. Intellectual disability is associated with increased runs of homozygosity in simplex autism. Am J Hum Genet. 2013;93:103–9. doi:10.1016/j.ajhg.2013.06.004.
Girirajan S, Brkanac Z, Coe BP, Baker C, Vives L, Vu TH, et al. Relative burden of large CNVs on a range of neurodevelopmental phenotypes. PLoS Genet. 2011;7:e1002334. doi:10.1371/journal.pgen.1002334.
Griswold AJ, Dueker ND, Van Booven D, Rantus JA, Jaworski JM, Slifer SH, et al. Targeted massively parallel sequencing of autism spectrum disorder-associated genes in a case control cohort reveals rare loss-of-function risk variants. Mol Autism. 2015;6:43. doi:10.1186/s13229-015-0034-z.
He X, Sanders SJ, Liu L, De Rubeis S, Lim ET, Sutcliffe JS, et al. Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes. PLoS Genet. 2013;9:e1003671. doi:10.1371/journal.pgen.1003671.
He Z, O’Roak BJ, Smith JD, Wang G, Hooker S, Santos-Cortez RLP, et al. Rare-variant extensions of the transmission disequilibrium test: application to autism exome sequence data. Am J Hum Genet. 2014;94:33–46. doi:10.1016/j.ajhg.2013.11.021.
Iossifov I, Levy D, Allen J, Ye K, Ronemus M, Lee Y-H, et al. Low load for disruptive mutations in autism genes and their biased transmission. Proc Natl Acad Sci U S A. 2015;112:E5600–7. doi:10.1073/pnas.1516376112.
Kim S-J, Silva RM, Flores CG, Jacob S, Guter S, Valcante G, et al. A quantitative association study of SLC25A12 and restricted repetitive behavior traits in autism spectrum disorders. Mol Autism. 2011;2:8. doi:10.1186/2040-2392-2-8.
Klei L, Sanders SJ, Murtha MT, Hus V, Lowe JK, Willsey AJ, et al. Common genetic variants, acting additively, are a major source of risk for autism. Mol Autism. 2012;3:9. doi:10.1186/2040-2392-3-9.
Kong SW, Collins CD, Shimizu-Motohashi Y, Holm IA, Campbell MG, Lee I-H, et al. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PLoS ONE. 2012;7:e49475. doi:10.1371/journal.pone.0049475.
Kong SW, Shimizu-Motohashi Y, Campbell MG, Lee IH, Collins CD, Brewster SJ, et al. Peripheral blood gene expression signature differentiates children with autism from unaffected siblings. Neurogenetics. 2013;14:143–52. doi:10.1007/s10048-013-0363-z.
Cross-Disorder Group of the Psychiatric Genomics Consortium, Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi:10.1038/ng.2711.
Lim ET, Raychaudhuri S, Sanders SJ, Stevens C, Sabo A, MacArthur DG, et al. Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron. 2013;77:235–42. doi:10.1016/j.neuron.2012.12.029.
Mazina V, Gerdts J, Trinh S, Ankenman K, Ward T, Dennis MY, et al. Epigenetics of autism-related impairment: copy number variation and maternal infection. J Dev Behav Pediatr. 2015;36:61–7. doi:10.1097/DBP.0000000000000126.
Merner ND, Chandler MR, Bourassa C, Liang B, Khanna AR, Dion P, et al. Regulatory domain or CpG site variation in SLC12A5, encoding the chloride transporter KCC2, in human autism and schizophrenia. Front Cell Neurosci. 2015;9:386. doi:10.3389/fncel.2015.00386.
Moreno-De-Luca D, SGENE Consortium, Mulle JG, Simons Simplex Collection Genetics Consortium, Kaminsky EB, Sanders SJ, et al. Deletion 17q12 is a recurrent copy number variant that confers high risk of autism and schizophrenia. Am J Hum Genet. 2010;87:618–30. doi:10.1016/j.ajhg.2010.10.004.
Moreno-De-Luca D, Sanders SJ, Willsey AJ, Mulle JG, Lowe JK, Geschwind DH, et al. Using large clinical data sets to infer pathogenicity for rare copy number variants in autism cohorts. Mol Psychiatry. 2013;18:1090–5. doi:10.1038/mp.2012.138.
Murdoch JD, Gupta AR, Sanders SJ, Walker MF, Keaney J, Fernandez TV, et al. No evidence for association of autism with rare heterozygous point mutations in Contactin-Associated Protein-Like 2 (CNTNAP2), or in Other Contactin-Associated Proteins or Contactins. PLoS Genet. 2015;11:e1004852. doi:10.1371/journal.pgen.1004852.
Narzisi G, O’Rawe JA, Iossifov I, Fang H, Lee Y-H, Wang Z, et al. Accurate de novo and transmitted indel detection in exome-capture data using microassembly. Nat Methods. 2014;11:1033–6. doi:10.1038/nmeth.3069.
Noh HJ, Ponting CP, Boulding HC, Meader S, Betancur C, Buxbaum JD, et al. Network topologies and convergent aetiologies arising from deletions and duplications observed in individuals with autism. PLoS Genet. 2013;9:e1003523. doi:10.1371/journal.pgen.1003523.
Peters SU, Hundley RJ, Wilson AK, Warren Z, Vehorn A, Carvalho CMB, et al. The behavioral phenotype in MECP2 duplication syndrome: a comparison with idiopathic autism. Autism Res. 2013;6:42–50. doi:10.1002/aur.1262.
Sagar A, Bishop JR, Tessman DC, Guter S, Martin CL, Cook EH. Co-occurrence of autism, childhood psychosis, and intellectual disability associated with a de novo 3q29 microdeletion. Am J Med Genet A. 2013;161A:845–9. doi:10.1002/ajmg.a.35754.
Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48:552–5. doi:10.1038/ng.3529.
Skafidas E, Testa R, Zantomio D, Chana G, Everall IP, Pantelis C. Predicting the diagnosis of autism spectrum disorder using gene pathway analysis. Mol Psychiatry. 2014;19:504–10. doi:10.1038/mp.2012.126.
Stanco A, Pla R, Vogt D, Chen Y, Mandal S, Walker J, et al. NPAS1 represses the generation of specific subtypes of cortical interneurons. Neuron. 2014;84:940–53. doi:10.1016/j.neuron.2014.10.040.
Wang Y, Picard M, Gu Z. Genetic evidence for elevated pathogenicity of mitochondrial DNA heteroplasmy in autism spectrum disorder. PLoS Genet. 2016;12:e1006391. doi:10.1371/journal.pgen.1006391.
Yi JJ, Berrios J, Newbern JM, Snider WD, Philpot BD, Hahn KM, et al. An Autism-Linked Mutation Disables Phosphorylation Control of UBE3A. Cell. 2015;162:795–807. doi:10.1016/j.cell.2015.06.045.
Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77:259–73. doi:10.1016/j.neuron.2012.11.002.
Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87:1215–33. doi:10.1016/j.neuron.2015.09.016.
Krumm N, Turner TN, Baker C, Vives L, Mohajeri K, Witherspoon K, et al. Excess of rare, inherited truncating mutations in autism. Nat Genet. 2015;47:582–8. doi:10.1038/ng.3303.
Krumm N, O’Roak BJ, Karakoc E, Mohajeri K, Nelson B, Vives L, et al. Transmission disequilibrium of small CNVs in simplex autism. Am J Hum Genet. 2013;93:595–606. doi:10.1016/j.ajhg.2013.07.024.
Anitha A, Nakamura K, Thanseem I, Yamada K, Iwayama Y, Toyota T, et al. Brain region-specific altered expression and association of mitochondria-related genes in autism. Mol Autism. 2012;3:12. doi:10.1186/2040-2392-3-12.
Anitha A, Nakamura K, Thanseem I, Matsuzaki H, Miyachi T, Tsujii M, et al. Downregulation of the expression of mitochondrial electron transport complex genes in autism brains. Brain Pathol. 2013;23:294–302. doi:10.1111/bpa.12002.
Chow ML, Pramparo T, Winn ME, Barnes CC, Li H-R, Weiss L, et al. Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages. PLoS Genet. 2012;8:e1002592. doi:10.1371/journal.pgen.1002592.
D’Gama AM, Pochareddy S, Li M, Jamuar SS, Reiff RE, Lam A-TN, et al. Targeted DNA Sequencing from Autism Spectrum Disorder Brains Implicates Multiple Genetic Mechanisms. Neuron. 2015;88:910–7. doi:10.1016/j.neuron.2015.11.009.
Fatemi SH, Folsom TD, Kneeland RE, Yousefi MK, Liesch SB, Thuras PD. Impairment of fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling and its downstream cognates ras-related C3 botulinum toxin substrate 1, amyloid beta A4 precursor protein, striatal-enriched protein tyrosine phosphatase, and homer 1, in autism: a postmortem study in cerebellar vermis and superior frontal cortex. Mol Autism. 2013;4:21. doi:10.1186/2040-2392-4-21.
Fatemi SH, Reutiman TJ, Folsom TD, Rustan OG, Rooney RJ, Thuras PD. Downregulation of GABAA receptor protein subunits α6, β2, δ, ε, γ2, θ, and ρ2 in superior frontal cortex of subjects with autism. J Autism Dev Disord. 2014;44:1833–45. doi:10.1007/s10803-014-2078-x.
Garcia KLP, Yu G, Nicolini C, Michalski B, Garzon DJ, Chiu VS, et al. Altered balance of proteolytic isoforms of pro-brain-derived neurotrophic factor in autism. J Neuropathol Exp Neurol. 2012;71:289–97. doi:10.1097/NEN.0b013e31824b27e4.
Hu VW, Sarachana T, Sherrard RM, Kocher KM. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol Autism. 2015;6:7. doi:10.1186/2040-2392-6-7.
Huang H-S, Cheung I, Akbarian S. RPP25 is developmentally regulated in prefrontal cortex and expressed at decreased levels in autism spectrum disorder. Autism Res. 2010;3:153–61. doi:10.1002/aur.141.
Kerin T, Ramanathan A, Rivas K, Grepo N, Coetzee GA, Campbell DB. A noncoding RNA antisense to moesin at 5p14.1 in autism. Sci Transl Med. 2012;4:128ra40. doi:10.1126/scitranslmed.3003479.
Nagarajan RP, Hogart AR, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1:e1–11.
Nicolini C, Ahn Y, Michalski B, Rho JM, Fahnestock M. Decreased mTOR signaling pathway in human idiopathic autism and in rats exposed to valproic acid. Acta Neuropathol Commun. 2015;3:3. doi:10.1186/s40478-015-0184-4.
Sarachana T, Hu VW. Genome-wide identification of transcriptional targets of RORA reveals direct regulation of multiple genes associated with autism spectrum disorder. Mol Autism. 2013;4:14. doi:10.1186/2040-2392-4-14.
Smith RM, Banks W, Hansen E, Sadee W, Herman GE. Family-based clinical associations and functional characterization of the serotonin 2A receptor gene (HTR2A) in autism spectrum disorder. Autism Res. 2014;7:459–67. doi:10.1002/aur.1383.
Stamova B, Ander BP, Barger N, Sharp FR, Schumann CM. Specific Regional and Age-Related Small Noncoding RNA Expression Patterns Within Superior Temporal Gyrus of Typical Human Brains Are Less Distinct in Autism Brains. J Child Neurol. 2015;30:1930–46. doi:10.1177/0883073815602067.
Thanseem I, Nakamura K, Anitha A, Suda S, Yamada K, Iwayama Y, et al. Association of transcription factor gene LMX1B with autism. PLoS ONE. 2011;6:e23738. doi:10.1371/journal.pone.0023738.
Werling DM, Parikshak NN, Geschwind DH. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat Commun. 2016;7:10717. doi:10.1038/ncomms10717.
Eicher JD, Gruen JR. Language impairment and dyslexia genes influence language skills in children with autism spectrum disorders. Autism Res. 2015;8:229–34. doi:10.1002/aur.1436.
Gockley J, Willsey AJ, Dong S, Dougherty JD, Constantino JN, Sanders SJ. The female protective effect in autism spectrum disorder is not mediated by a single genetic locus. Mol Autism. 2015;6:25. doi:10.1186/s13229-015-0014-3.
Turner TN, Sharma K, Oh EC, Liu YP, Collins RL, Sosa MX, et al. Loss of δ-catenin function in severe autism. Nature. 2015;520:51–6. doi:10.1038/nature14186.
Simeon-Dubach D, Watson P. Biobanking 3.0: evidence based and customer focused biobanking. Clin Biochem. 2014;47:300–8. doi:10.1016/j.clinbiochem.2013.12.018.
Beskow LM, Friedman JY, Hardy NC, Lin L, Weinfurt KP. Simplifying informed consent for biorepositories: stakeholder perspectives. Genet Med. 2010;12:567–72. doi:10.1097/GIM.0b013e3181ead64d.
Vaught J, Lockhart NC. The evolution of biobanking best practices. Clin Chim Acta. 2012;413:1569–75. doi:10.1016/j.cca.2012.04.030.
Stacey GN, Crook JM, Hei D, Ludwig T. Banking human induced pluripotent stem cells: lessons learned from embryonic stem cells? Cell Stem Cell. 2013;13:385–8. doi:10.1016/j.stem.2013.09.007.
Aoi T, Stacey G. Impact of National and International Stem Cell Banking Initiatives on progress in the field of cell therapy: IABS-JST Joint Workshop: Summary for Session 5. Biologicals. 2015;43:399–401. doi:10.1016/j.biologicals.2015.07.007.
Stover AE, Herculian S, Banuelos MG, Navarro SL, Jenkins MP, Schwartz PH. Culturing human pluripotent and neural stem cells in an enclosed cell culture system for basic and preclinical research. J Vis Exp. 2016. doi:10.3791/53685.
Acknowledgements
I would like to thank all authors who contributed to this manuscript
Funding
Not applicable.
Availability of data and materials
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Contributions
JR collected the literature and wrote the manuscript and tables. JLC authored the search criteria section. SS, LG and GL gave feedback and suggested changes which were incorporated into the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Reilly, J., Gallagher, L., Chen, J.L. et al. Bio-collections in autism research. Molecular Autism 8, 34 (2017). https://doi.org/10.1186/s13229-017-0154-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13229-017-0154-8