Abstract
Background
Primary antibody deficiencies (PAD) are characterized by a heterogeneous clinical presentation and low prevalence, contributing to a median diagnostic delay of 3–10 years. This increases the risk of morbidity and mortality from undiagnosed PAD, which may be prevented with adequate therapy. To reduce the diagnostic delay of PAD, we developed a screening algorithm using primary care electronic health record (EHR) data to identify patients at risk of PAD. This screening algorithm can be used as an aid to notify general practitioners when further laboratory evaluation of immunoglobulins should be considered, thereby facilitating a timely diagnosis of PAD.
Methods
Candidate components for the algorithm were based on a broad range of presenting signs and symptoms of PAD that are available in primary care EHRs. The decision on inclusion and weight of the components in the algorithm was based on the prevalence of these components among PAD patients and control groups, as well as clinical rationale.
Results
We analyzed the primary care EHRs of 30 PAD patients, 26 primary care immunodeficiency patients and 58,223 control patients. The median diagnostic delay of PAD patients was 9.5 years. Several candidate components showed a clear difference in prevalence between PAD patients and controls, most notably the mean number of antibiotic prescriptions in the 4 years prior to diagnosis (5.14 vs. 0.48). The final algorithm included antibiotic prescriptions, diagnostic codes for respiratory tract and other infections, gastro-intestinal complaints, auto-immune symptoms, malignancies and lymphoproliferative symptoms, as well as laboratory values and visits to the general practitioner.
Conclusions
In this study, we developed a screening algorithm based on a broad range of presenting signs and symptoms of PAD, which is suitable to implement in primary care. It has the potential to considerably reduce diagnostic delay in PAD, and will be validated in a prospective study.
Trial registration The consecutive prospective study is registered at clinicaltrials.gov under NCT05310604
Similar content being viewed by others
Background
Primary antibody deficiencies (PAD) are characterized by an inability to produce clinically effective immunoglobulin responses and represent the majority of all primary immunodeficiency (PID) disorders [1,2,3]. PAD encompass a heterogeneous group of diseases such as common variable immunodeficiency (CVID), X-linked agammaglobulinemia (XLA), immunoglobulin (Ig) G subclass deficiency and specific antibody deficiency (SpAD) [4]. The estimated prevalence of PAD varies widely from 1:700 to 1:25.000, in part due to the suspected large number of undiagnosed patients [5,6,7,8].
The onset of symptoms of PAD is most commonly in the second to fourth decade of life [9,10,11,12]. The clinical presentation is heterogeneous, including increased susceptibility for respiratory tract and gastro-intestinal infections, auto-immune symptoms, lymphoproliferative disease and an increased risk of certain malignancies [4, 13,14,15]. Owing to the wide variety in presenting symptoms and the rarity of PAD, diagnosis can be challenging. This is evident by the reported mean diagnostic delay of 6–12 years (median 3–10 years), which has not significantly improved over the past 5 decades [13, 16,17,18]. This diagnostic delay may increase the risk of morbidity and mortality, as effective therapies and prophylaxes are available [18,19,20,21]. A timely diagnosis may also lead to substantial health care cost savings, even when correcting for the cost of treatment [22]. Thus, reducing the diagnostic delay of PAD is of key interest [18].
To this end, several ‘Early warning signs’ sets have previously been developed. However, these do not include the full spectrum of presenting symptoms of PAD, show suboptimal diagnostic performance, and are often used in a non-automated manner [23,24,25,26,27,28]. As manual screening systems depend on the awareness of an individual physician, these are suboptimal for rare diseases with heterogeneous presentations. Therefore, we aimed to develop a screening algorithm that encompasses an extended spectrum of PAD signs and symptoms based on electronic health record (EHR) data, that can easily be automated. Such an algorithm may be used as an aid to notify general practitioners (GPs) when further laboratory evaluation of immunoglobulins should be considered, thereby facilitating a timely diagnosis of PAD.
Primary care may be the optimal setting for such an algorithm for several reasons. Firstly, a patient will initially present with their symptoms in primary care, especially in countries where the GP has a gatekeeper function to secondary care. Therefore, screening in primary care could allow detection of PAD patients in an earlier phase when compared to screening in secondary care. Secondly, primary care EHRs encompass a comprehensive overview of the complaints for which a patient has sought medical care. In contrast, in secondary care usually only the symptoms for which a patient has been referred are registered structurally (i.e. in diagnostic codes). For example, if a patient is only referred for respiratory tract infections, relevant gastro-intestinal or auto-immune symptoms could be missed. Thus, focusing on primary care allows screening for a broad range of presenting signs and symptoms of PAD.
The aim of the current study is to develop a screening algorithm to identify patients with an increased risk of PAD in a primary care setting. This algorithm could be applied to notify GPs of patients at risk of PAD, for whom further laboratory investigation of immunoglobulins and/or consultation of an immunologist is indicated.
Methods
The algorithm for the early detection of PAD was developed based on primary care EHR data of PAD patients and control groups. We focused on data that are available as structured data in the EHRs of primary care facilities in the Netherlands. Almost all inhabitants of the Netherlands have an EHR with a public primary care physician, and the primary care physician has a gatekeeper function to secondary care, signifying that in non-urgent cases referral from the GP is necessary to access hospital care [29]. Available structured data included age, diagnostic ICPC codes (International Classification of Primary Care), ATC codes (Anatomical Therapeutic Chemical) for medication prescriptions and (requests for) laboratory assessments.
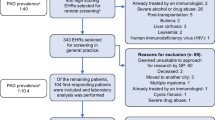
The algorithm was developed in 3 steps, see Fig. 1.
Identification of candidate components of the algorithm
The hallmark clinical features of PAD are recurrent respiratory tract infections (RTI), including otitis media, sinusitis and pneumonia [12, 21, 30]. Gastro-intestinal complaints, such as chronic diarrhea and parasitic infections, also occur frequently in certain PAD [12, 31,32,33,34]. In addition, it is estimated that around 30% of PAD patients develop auto-immune disorders, e.g. idiopathic thrombocytopenic purpura, auto-immune hemolytic anemia or rheumatoid arthritis [35,36,37]. Finally, certain PAD can also present with lymphoproliferative symptoms and are associated with an increased risk of non-Hodgkin lymphoma and gastric cancer [38,39,40,41]. All diagnostic ICPC codes related to these presenting symptoms of PAD were considered as candidate components of the algorithm.
Since PAD patients often present with recurrent infections that require antibiotic treatment, we also considered the type and number of antibiotic prescriptions for inclusion in the algorithm. We considered antibiotics registered in the Netherlands for the treatment of respiratory tract or parasitic intestinal infections [42]. As antibiotics for upper RTIs are frequently prescribed in the early years of childhood, we only took prescriptions from the age of 6 years into account [43].
As PAD, by definition, can be characterized by a reduced level of one or more (subtypes of) immunoglobulins, we considered these laboratory results for inclusion in the algorithm. Since immunoglobulin diagnostics may not be requested commonly in primary care, we also considered calculated globulin. Calculated globulin is a measure based on total protein and albumin levels, which can indicate hypogammaglobulinemia [44, 45].
A previous study reported that a higher number of consultations at the GP, as well as a higher number of requests for lung function tests and blood tests for infection, were statistically significantly associated with an increased odds of CVID diagnosis [46]. Therefore, we also considered these factors for inclusion in the algorithm.
In contrast to PAD, secondary antibody deficiencies are the consequence of an underlying disease, such as hematological malignancy. Diagnoses known to be causes of secondary antibody deficiencies (e.g. leukemia, multiple myeloma, HIV) were added as exclusion criteria [47,48,49]. In the case that a diagnosis could be both a cause of secondary antibody deficiency as well a complication of PAD (e.g. non-Hodgkin lymphoma), it was labelled as ‘‘ambiguous diagnosis’’. For these diagnoses, the EHRs were screened up to the moment of the ambiguous diagnosis. This approach allows detection of patients for whom the ambiguous diagnosis is the consequence of an underlying PAD, whilst still excluding patients with a secondary antibody deficiency. Immunosuppressant medication was not added as an exclusion criterion, as these may also be prescribed for auto-immune symptoms that are caused by an underlying PAD. Table 1 shows an overview of the candidate components of the algorithm.
Collection of EHR data of PAD patients and control groups
To gain insight in the prevalence of the candidate algorithm components among PAD patients and control groups, we used two data sources. First, we collected the primary care EHR data of 30 patients from different age groups with a variety of PAD diagnoses from an academic hospital in the Netherlands (the University Medical Center Utrecht). Patients signed informed consent and research agreements were signed by their GPs. Second, we collected primary care EHR data from the pseudonymized database of the Julius General Practitioner Network (JHN), a research collaboration of GPs in the region of Utrecht, Netherlands [50]. Owing to privacy regulations from the JHN database, we only had access to the metadata (e.g. means, medians and proportions) concerning the candidate components of the algorithm for several patient groups of interest, defined based on registered diagnostic ICPC codes. The groups of interest included PAD patients as well as several control groups. Because no specific ICPC code for PAD exists, we selected the first group based on the code for immunodeficiency (T99.01). This group approximates PAD patients, as causes of secondary immunodeficiencies were excluded based on the exclusion criteria, and because PAD represents the majority of the remaining PIDs. Therefore, this group of ‘primary care immunodeficiency’ patients was considered to be of interest, in addition to the group of confirmed PAD-patients from the academic hospital.
The selected control groups were ‘patients from the general GP population’, ‘patients with upper RTIs’, ‘patients with chronic obstructive pulmonary disease (COPD) or asthma’, ‘patients with inflammatory bowel disease (IBD)’ and ‘patients with a malignancy’. These groups were selected as they were expected to have overlapping presenting symptoms with PAD patients, but should be distinguished from potential PAD patients by the algorithm in order to prevent false-positives. For all control groups we applied the exclusion criteria, and excluded patients that had an ICPC code for immunodeficiency (T99.01). The meta-data of all available patients in the JHN-database that met the criteria for the control groups were utilized.
From both the academic hospital and the primary care database we selected patients aged 12–70 years, as PAD most commonly presents in the second to fourth decade of life [9,10,11,12]. In addition, as recurrent infections and antibiotic prescriptions are common in younger children, other cut-off values are to be expected for this age group. Lastly, our algorithm was developed in line with the study design of a subsequent external validation study, where high-risk patients will be invited for laboratory analysis of immunoglobulins. Considering the medical ethical guidelines, this validation study is only feasible in patients aged ≥ 12 years.
A distinct ‘censoring date’ was selected for different patient groups. All available EHR data before the censoring date were extracted. The censoring date indicates the date up to which time the EHR data were extracted. For most patients, this was the date of data-extraction, November 18, 2021. For the PAD and primary care immunodeficiency patients, we extracted the EHR data both pre- and post-diagnosis. The censoring date could therefore be the date of PAD diagnosis (pre-diagnosis), or the date of data-extraction (post-diagnosis). When deciding which components were to be maintained in the algorithm, pre-diagnosis data were used because our aim is to identify patients before diagnosis. For patients with an ambiguous diagnosis, the date of this specific diagnosis was defined as their censoring date.
To determine the validity of the diagnostic ICPC codes, we compared the presenting symptoms registered as diagnostic ICPC codes in the primary care EHR of the PAD patients with the presenting symptoms registered as free text in the secondary care EHR of the academic hospital and determined their concordance.
Selection of components and weights of the algorithm
The decision to include a candidate component in the algorithm and the corresponding weight was based on the results of analyses of the corresponding EHR data in combination with clinical rationale. To estimate the ability of the presence of individual diagnostic ICPC codes to differentiate PAD patients from the control group, we calculated the Youden’s index. Youden’s index is a summary measure of diagnostic quality based on sensitivity and specificity, where a higher index indicates that the component has a better discriminatory ability [51]. The sensitivity of the presence of a component was calculated for the PAD patients and primary care immunodeficiency patients, and the mean of the sensitivities the was used to determine Youden’s index. Specificity was calculated from the general GP population control group. The weight of the diagnostic ICPC codes was based on the discriminative value as expressed by Youden’s index in combination with clinical rationale.
The diagnostic ICPC codes included in the algorithm were grouped according to the following categories: ‘respiratory tract infections’, ‘gastro-intestinal complaints’, ‘other infections’, ‘auto-immune symptoms’ and ‘malignancies, lymphoproliferative and other symptoms’. For each of these groups, we determined whether the entire EHR before the censoring date should be screened for these diagnostic codes, or only the 10 years before the censoring date. This decision was based on clinical rationale and on the discriminative value as expressed by Youden’s index.
For the ATC codes, we calculated the mean number of antibiotic prescriptions per year in the 10 years before the censoring date. The decision on the inclusion and weight of individual antibiotics was based on both clinical rationale and the difference in the mean number of prescriptions between the control groups and immunodeficiency patients.
For immunoglobulin levels and calculated globulin, we determined the mean laboratory values in the EHR in the 10 years before the censoring date. Lastly, regarding the number of consultations at the GP and the number of requests for lung function tests/blood tests for infection, we calculated the mean and median number of visits and requests in the year before the censoring date for both the PAD/primary care immunodeficiency groups and the control groups. To determine the optimal cut-off point for the number of lung function tests, blood tests for infection and number of visits to the GP, we calculated the Youden’s index for several cut-off points spread around the median values in PAD patients.
Ethical approval for this study was received from the Medical Research Ethics Committee Utrecht, protocol number 19–748. Statistical analysis was done with the base package in R version 4.0.2.
Results
Patient characteristics
Of the 30 included PAD patients from the academic hospital, 12 were diagnosed with CVID, 8 with IgG subclass deficiency, 4 with unclassified antibody deficiency, 3 with IgA and IgG subclass deficiency, 2 with selective IgA deficiency and 1 patient was diagnosed with SpAD. The mean age at the time of data extraction was 29.8 years, with a large variation (standard deviation of 14). The PAD patients thus represented a wide range of diagnoses and ages. The mean delay from symptom onset to diagnosis of these patients was 12.4 years [SD 12.2, median 9.5 (3.0–19.5)]. The patient characteristics of the PAD patients from the academic hospital, of the primary care immunodeficiency patients and the control groups (all aged 12–70 years) are shown in Table 2.
Diagnostic ICPC codes
Table 3 shows the concordance between the presenting symptoms registered as diagnostic ICPC codes in the primary care EHRs and the symptoms registered in free text in the secondary care EHRs. Concordance was assessed by calculating the percentage of patients for whom the presence or non-presence of a symptom was equal between the diagnostic codes in the primary care EHR and the free text in the secondary care EHR. For almost all presenting symptoms, apart from gastro-intestinal complaints, concordance between the diagnostic primary care ICPC codes and the free text in secondary care EHRs was high.
The selection of diagnostic codes and their attributed weight was based on the Youden’s index (Additional file 1: Table S1) in combination with evidence from literature and clinical expertise of PAD specialists in the University Medical Center Utrecht. The diagnostic codes included in the final algorithm are shown in Table 5.
Antibiotic prescriptions
Figure 2A shows the mean number of antibiotic prescriptions per year in the 10 years before the censoring date for the different patient groups. PAD patients and primary care immunodeficiency patients had a higher mean number of antibiotic prescriptions than all control groups. In the pre-diagnosis groups this effect appeared to be most pronounced from 4 years before the diagnosis. Figure 2B shows the mean total number of antibiotic prescriptions in the 4 years before the censoring date, where the immunodeficiency and PAD groups had a higher mean compared to the control groups (5.14 in PAD vs. 0.48 in the general population). It was therefore decided to include antibiotic prescriptions from the past 4 years in the algorithm, see Table 5. The mean number of antibiotic prescriptions per individual antibiotic is shown in Additional file 2: Table S2. The weight of the individual antibiotics was based on the difference in means between immunodeficiency and control groups in combination with clinical rationale. For example, if the difference in means was < 0.05, we generally attributed a weight of 1, rather than a weight of 2, for each antibiotic prescription.
A Mean number of antibiotic prescriptions per year in the 10 years before the censoring date. B Mean total number of antibiotic prescriptions in 4 years before the censoring date. AB antibiotic, COPD chronic obstructive pulmonary disease, IBD inflammatory bowel disease, immunodef. immunodeficiency patients from primary care, no. number, PAD primary antibody deficiencies patients from an academic hospital, RTI respiratory tract infection
Laboratory results and number of requests
Table 4 shows the mean laboratory values of the immunoglobulins and calculated globulin in the EHR in the 10 years before the censoring date. Immunoglobulins were only requested for a very small proportion of patients from the control groups. Calculated globulin was requested more often than immunoglobulins in these control groups, although still for a very small proportion. For example, calculated globulin was requested only for 0.38% of the general GP population. For the PAD and primary care immunodeficiency patients, immunoglobulins and calculated globulin were not requested pre-diagnosis. Although these parameters are thus not likely to apply to many patients, a reduced level of immunoglobulins or calculated globulin was still added to the algorithm based on the strong clinical relevance. Although it could be expected that a PAD diagnosis is quickly made after immunoglobulin testing, patients may be missed by the GP, thus making this a relevant component to add to the screening algorithm.
Based on previous research by Ilkjær et al. [46] we also considered the number of requests of leukocytes, C-reactive protein and lung function tests by the GP. These however did not appear to have a discriminatory value when comparing the general population with immunodeficiency patients, see Additional file 3: Fig. S1. These components were thus not added to the algorithm.
Visits to the general practitioner
Figure 3 shows the median number of visits to the GP in the year before the censoring date. The immunodeficiency groups showed a higher median number of visits to the GP compared to the control groups. For example, PAD patients pre-diagnosis had a median of 6 visits per year, compared to 2 visits per year in the general population. These data were not available for the PAD patients from the academic hospital. Based on Youden’s index the cut-off value for the algorithm was set to ≥ 6 visits to the GP in a year.
Median number of visits to the general practitioner in the year before the censoring date. Both physical and electronic visits are included. COPD chronic obstructive pulmonary disease, GP general practitioner, IBD inflammatory bowel disease, immunodef. immunodeficiency patients from primary care, no. number, RTI respiratory tract infection
Algorithm
The components and corresponding weights of the final PAD screening algorithm are shown in Table 5. The categories of the algorithm include ‘Antibiotics’, ‘Respiratory tract infections’, ‘Gastro-intestinal complaints’, ‘Other infections’, ‘Auto-immune symptoms’, ‘Malignancies, lymphoproliferative and other symptoms’, ‘Laboratory values’ and ‘Visits to general practitioner’.
Discussion
In the current study we have developed a screening algorithm to identify patients at risk of PAD in a primary care setting, based on EHR data. The components of the algorithm encompass a broad range of presenting signs and symptoms of PAD, including antibiotic prescriptions, diagnostic ICPC codes, laboratory values and the number of visits to the GP. The presented algorithm can be used as an aid to notify GPs when further laboratory evaluation of immunoglobulins should be considered, and thus has the potential to considerably reduce the diagnostic delay of PAD.
Our results show a clear distinction in the number of antibiotic prescriptions when comparing PAD patients with relevant control groups. This effect was especially pronounced in the 4 years before PAD diagnosis. Furthermore, our results show a distinction regarding the number of GP consultations per year. This is in line with the main results from a previous study that compared primary care date of CVID patients with controls [46]. This study also found an increased odds ratio regarding the number of requests for lung function tests and blood tests for infection, a finding which could not be reproduced in the current study. However, the authors reported that a low effectiveness was expected of these measures as a screening tool, due to either a low procedure occurrence among cases or a relatively high occurrence of these procedures amongst controls. Lastly, our results show that serum immunoglobulin levels were very rarely requested by GPs. This could indicate that GPs do not often consider the PAD diagnosis, or that they are not yet aware of the added value of assessing immunoglobulin levels. The implementation of the algorithm presented in this study could aid GPs in when to consider requesting serum immunoglobulin levels, or calculated globulin as an alternative.
As reducing the diagnostic delay of PAD is of great clinical interest, several other studies have investigated screening possibilities for PAD and other PIDs. Several sets of early warning signs (EWS) have been developed by, for example, the Jeffrey Modell Foundation (JMF) and the European Society of Immunodeficiencies. However, these have been shown to have a poor performance, especially in adults [23,24,25,26, 28]. This could partially be because these warning signs focus almost exclusively on infectious complications, rather than, for instance, auto-immune symptoms. In addition, these manual screening systems are suboptimal for the detection of rare and heterogeneous diseases, because they depend on the awareness of an individual treating physician. For this reason, efforts have been made to develop automated algorithms for the early detection of PID such as the “SPIRIT software” of the JMF, the “PI Prob”, and a third which is currently in development [52,53,54]. Of these, only the “PI Prob” has been internally validated. These efforts are of great importance, however these algorithms have been developed mainly for secondary care, and mostly based on pediatric data. These may be less suitable for the recognition of PAD, as most patients present in adulthood [9,10,11,12]. Therefore, these pediatric and secondary care algorithms could be of complementary value to the primary care algorithm presented in this paper.
In this study, we focused specifically on the early detection of PAD in primary care. As mentioned, the advantage of primary care EHRs is that they include a comprehensive overview of medical complaints. In addition, patients often primarily present at the GP, thus allowing for an early recognition of high-risk patients. Focusing on PAD, rather than the full spectrum of PIDs, allows use of this algorithm in combination with serologic testing for immunoglobulins. Serologic testing for the full spectrum of PIDs would entail more expensive tests that are difficult to request and interpret by GPs, such as vaccination responses. By using the current algorithm to identify patients at risk for PAD for whom immunoglobulin analysis is indicated, it is both feasible and affordable to implement in primary care.
Because PAD are rare, our study was limited by a small sample size of PAD patients. In an effort to increase the sample size, we also analyzed primary care patients with a diagnostic code for immunodeficiency (T99.01). This group is expected to approximate PAD patients, as causes of secondary immunodeficiencies were excluded and because PAD represents the majority of the PIDs. Therefore, this group was considered to be of interest, in addition to the group of confirmed PAD patients. A second limitation was that we only had access to metadata (e.g. means/medians) from the JHN, due to privacy regulations. We could therefore only evaluate the diagnostic accuracy of individual components, rather than combinations of different components.
As we aimed to design an algorithm that can easily be automated, we focused on structured EHR data. The components of the algorithm are therefore inherently dependent on the available structured EHR data, such as diagnostic codes and medication prescriptions. Certain factors of possible interest, such as the number of hospitalizations, were unfortunately not structurally available in primary care. Furthermore, we did not have access to the data regarding the number of visits to the GP of the PAD patients from the academic hospital. These data were available for the primary care immunodeficiency patients. Although this is a limitation, it is important to note that the number of visits to the GP is only 1 of the 106 components of the algorithm.
Our algorithm is designed to detect adolescent and adult patients aged 12–70 years at risk of undiagnosed PAD in a primary care setting. We focused on patients aged ≥ 12 years, as other cut-off points are to be expected for younger patients (e.g. due to frequent infections and antibiotic prescriptions), and because most PADs present in adulthood [9,10,11,12]. Consequently, our algorithm may be less suitable to detect XLA patients, as these usually present with symptoms during the first few years of life. However, our main aim is to reduce the diagnostic delay of PAD, which is considerably lower for XLA patients (e.g. reported median of 1 year for XLA vs. 7.5 years for PAD in general [12]), and almost all cases of XLA are diagnosed before 5 years of age [55]. In addition, it has recently been suggested to add XLA to newborn screening, which is likely a more effective way to reduce diagnostic delay for this particular PAD [56]. Lastly, the diagnostic delay in our cohort was relatively high (median 9.5 years), which may be a consequence of our focus on a population aged 12–70 years, although the delay is within the ranges described in previous cohorts of PAD patients [12, 13].
A major strength of the current study is that our algorithm is based completely on structured EHR data, making it suitable to implement in an automated manner. As ICPC and ATC codes are used internationally, this algorithm could potentially also be applied in other countries. In a consecutive study, we will prospectively validate the algorithm by applying it to 60,000 primary care EHRs in the Netherlands. The highest scoring patients will undergo laboratory assessment of serum immunoglobulin levels, and referral to an immunologist if necessary. This prospective study will allow analysis of patient-level data and determine the optimal cut-off value for high-risk patients.
Conclusions
In conclusion, in this study we developed a screening algorithm based on presenting signs and symptoms of PAD that is suitable to implement in primary care. It has the potential to considerably reduce the diagnostic delay of PAD, and will be evaluated in a prospective study.
Data availability
Data are handled according to the FAIR principles. For access to the study data, an application can submitted to the corresponding author, which will be reviewed by the study team.
Abbreviations
- ATC:
-
Anatomical Therapeutic Chemical
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- CVID:
-
Common variable immunodeficiency
- EHR:
-
Electronic health record
- GP:
-
General practitioner
- IBD:
-
Inflammatory bowel disease
- Ig:
-
Immunoglobulin
- ICPC:
-
International Classification of Primary Care
- JHN:
-
Julius General Practitioner’s Network
- JMF:
-
Jeffrey Modell Foundation
- PAD:
-
Primary antibody deficiencies
- PID:
-
Primary immunodeficiency
- RTI:
-
Respiratory tract infection
- SpAD:
-
Specific antibody deficiency
- XLA:
-
X-linked agammaglobulinemia
References
Grimbacher B, ESID Registry Working Party. The European Society for Immunodeficiencies (ESID) registry 2014. Clin Exp Immunol. 2014;178:18–20.
Wood PM. Primary antibody deficiency syndromes. Curr Opin Hematol. 2010;17:356–61.
Chan HY, Yang YH, Yu HH, Chien YH, Chiang LL, Chiang BL. Clinical characteristics and outcomes of primary antibody deficiency: a 20-year follow-up study. J Formos Med Assoc. 2014;113:340–8.
Fried AJ, Bonilla FA. Pathogenesis, diagnosis, and management of primary antibody deficiencies and infections. Clin Microbiol Rev. 2009;22:396–414.
Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317:617–9.
Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007;27:497–502.
Bousfiha AA, Jeddane L, Ailal F, Benhsaien I, Mahlaoui N, Casanova JL, et al. Primary immunodeficiency diseases worldwide: more common than generally thought. J Clin Immunol. 2013;33:1–7.
Wood P, Stanworth S, Burton J, Jones A, Peckham DG, Green T, et al. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: a systematic review. Clin Exp Immunol. 2007;149(3):410–23.
Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematology Am Soc Hematol Educ Program. 2012;2012:301–5.
Wiesik-Szewczyk E, Jahnz-Rozyk K. From infections to autoimmunity: diagnostic challenges in common variable immunodeficiency. World J Clin Cases. 2020;8:3942–55.
Song J, Lleo A, Yang GX, Zhang W, Bowlus CL, Gershwin ME, et al. Common variable immunodeficiency and liver involvement. Clin Rev Allergy Immunol. 2018;55:340–51.
Slade CA, Bosco JJ, Giang TB, Kruse E, Stirling RG, Cameron PU, et al. Delayed diagnosis and complications of predominantly antibody deficiencies in a cohort of Australian adults. Front Immunol. 2018;14(9):694.
Maarschalk-Ellerbroek LJ, Hoepelman AIM, Van Montfrans JM, Ellerbroek PM. The spectrum of disease manifestations in patients with common variable immunodeficiency disorders and partial antibody deficiency in a university hospital. J Clin Immunol. 2012;32:901–21.
Aghamohammadi A, Pouladi N, Parvaneh N, Yeganeh M, Movahedi M, Gharagolou M, et al. Mortality and morbidity in common variable immunodeficiency. J Trop Pediatr. 2007;53:32–8.
Jolles S. The variable in common variable immunodeficiency: a disease of complex phenotypes. J Allergy Clin Immunol Pract. 2013;1:545–56.
Dong J, Liang H, Wen D, Wang J. Adult common variable immunodeficiency. Am J Med Sci. 2016;351:239–43.
El-Helou SM, Biegner AK, Bode S, Ehl SR, Heeg M, Maccari ME, et al. The German national registry of primary immunodeficiencies (2012–2017). Front Immunol. 2019;10:1272.
Odnoletkova I, Kindle G, Quinti I, Grimbacher B, Knerr V, Gathmann B, et al. The burden of common variable immunodeficiency disorders: a retrospective analysis of the European Society for Immunodeficiency (ESID) registry data. Orphanet J Rare Dis. 2018;13:201.
Gathmann B, Mahlaoui N, Gérard L, Oksenhendler E, Warnatz K, Schulze I, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134(1):116–26.
Blore J, Haeney MR. Primary antibody deficiency and diagnostic delay. BMJ. 1989;298:516–7.
Seymour B, Miles J, Haeney M. Primary antibody deficiency and diagnostic delay. J Clin Pathol. 2005;58:546–7.
Modell V, Orange JS, Quinn J, Modell F. Global report on primary immunodeficiencies: 2018 update from the Jeffrey Modell Centers Network on disease classification, regional trends, treatment modalities, and physician reported outcomes. Immunol Res. 2018;66:367–80.
Reda SM, El-Ghoneimy DH, Afifi HM. Clinical predictors of primary immunodeficiency diseases in children. Allergy Asthma Immunol Res. 2013;5:88–95.
Arkwright PD, Gennery AR. Ten warning signs of primary immunodeficiency: a new paradigm is needed for the 21st century. Ann N Y Acad Sci. 2011;1238:7–14.
O’Sullivan MD, Cant AJ. The 10 warning signs: a time for a change? Curr Opin Allergy Clin Immunol. 2012;12:588–94.
Arslan S, Ucar R, Caliskaner AZ, Reisli I, Guner SN, Sayar EH, et al. How effective are the 6 European Society of Immunodeficiency warning signs for primary immunodeficiency disease? Ann Allergy Asthma Immunol. 2016;116:151-5.e1.
Lankisch P, Schiffner J, Ghosh S, Babor F, Borkhardt A, Laws HJ. The Duesseldorf warning signs for primary immunodeficiency: is it time to change the rules? J Clin Immunol. 2015;35:273–9.
Bjelac JA, Yonkof JR, Fernandez J. Differing performance of the warning signs for immunodeficiency in the diagnosis of pediatric versus adult patients in a two-center tertiary referral population. J Clin Immunol. 2019;39:90–8.
Netherlands Institute for Health Services Research, Nivel.nl Netherlands [updated 08–11–2022; cited 2022 28–11–2022]. https://www.nivel.nl/nl/nivel-zorgregistraties-eerste-lijn/cijfers-over-zorgverlening/cijfers-zorgverlening-huisartsen.
Thickett KM, Kumararatne DS, Banerjee AK, Dudley R, Stableforth DE. Common variable immune deficiency: respiratory manifestations, pulmonary function and high-resolution CT scan findings. QJM. 2002;95:655–62.
Heyworth MF. Immunological aspects of Giardia infections. Parasite. 2014;21:55.
Khodadad A, Aghamohammadi A, Parvaneh N, Rezaei N, Mahjoob F, Bashashati M, et al. Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci. 2007;52:2977–83.
Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48.
Lederman HM, Winkelstein JA. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore). 1985;64:145–56.
Knight AK, Cunningham-Rundles C. Inflammatory and autoimmune complications of common variable immune deficiency. Autoimmun Rev. 2006;5:156–9.
Agarwal S, Cunningham-Rundles C. Autoimmunity in common variable immunodeficiency. Ann Allergy Asthma Immunol. 2019;123:454–60.
Azizi G, Bagheri Y, Tavakol M, Askarimoghaddam F, Porrostami K, Rafiemanesh H, et al. The clinical and immunological features of patients with primary antibody deficiencies. Endocr Metab Immune Disord Drug Targets. 2018;18:537–45.
Mellemkjaer L, Hammarstrom L, Andersen V, Yuen J, Heilmann C, Barington T, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002;130:495–500.
Kinlen LJ, Webster AD, Bird AG, Haile R, Peto J, Soothill JF, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985;1:263–6.
Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27:308–16.
Graziano V, Pecoraro A, Mormile I, Quaremba G, Genovese A, Buccelli C, et al. Delay in diagnosis affects the clinical outcome in a cohort of cvid patients with marked reduction of iga serum levels. Clin Immunol. 2017;180:1–4.
Zorginstituut Nederland. Farmacotherapeutisch Kompas. www.farmacotherapeutischkompas.nl.
Hobbs MR, Grant CC, Ritchie SR, Chelimo C, Morton SMB, Berry S, et al. Antibiotic consumption by New Zealand children: exposure is near universal by the age of 5 years. J Antimicrob Chemother. 2017;72:1832–40.
Holding S, Khan S, Sewell WA, Jolles S, Dore PC. Using calculated globulin fraction to reduce diagnostic delay in primary and secondary hypogammaglobulinaemias: results of a demonstration project. Ann Clin Biochem. 2015;52:319–26.
Holding S, Jolles S. Current screening approaches for antibody deficiency. Curr Opin Allergy Clin Immunol. 2015;15:547–55.
Ilkjær FV, Rasmussen LD, Martin-Iguacel R, Westh L, Katzenstein TL, Hansen ABE, et al. How to identify common variable immunodeficiency patients earlier: general practice patterns. J Clin Immunol. 2019;39:641–52.
Raje N, Dinakar C. Overview of immunodeficiency disorders. Immunol Allergy Clin N Am. 2015;35:599–623.
Srivastava S, Wood P. Secondary antibody deficiency—causes and approach to diagnosis. Clin Med (Lond). 2016;16:571–6.
Cunningham-Rundles C, Sidi P, Estrella L, Doucette J. Identifying undiagnosed primary immunodeficiency diseases in minority subjects by using computer sorting of diagnosis codes. J Allergy Clin Immunol. 2004;113:747–55.
Smeets HM, Kortekaas MF, Rutten FH, Bots ML, van der Kraan W, Daggelders G, et al. Routine primary care data for scientific research, quality of care programs and educational purposes: the Julius General Practitioners’ Network (JGPN). BMC Health Serv Res. 2018;18:735.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5.
Modell V, Gee B, Lewis DB, Orange JS, Roifman CM, Routes JM, et al. Global study of primary immunodeficiency diseases (PI)—diagnosis, treatment, and economic impact: an updated report from the Jeffrey Modell Foundation. Immunol Res. 2011;51:61–70.
Rider NL, Cahill G, Motazedi T, Wei L, Kurian A, Noroski LM, et al. PI Prob: a risk prediction and clinical guidance system for evaluating patients with recurrent infections. PLoS ONE. 2021;16: e0237285.
Kumar B, Zetumer S, Swee M, Endelman ELK, Suneja M, Davis B. Reducing delays in diagnosing primary immunodeficiency through the development and implementation of a clinical decision support tool: protocol for a quality improvement project. JMIR Res Protoc. 2022;11: e32635.
Lackey AE, Ahmad F. X-linked Agammaglobulinemia. StatPearls. Treasure Island: StatPearls Publishing; 2022.
Blom M, Bredius RGM, van der Burg M. Future perspectives of newborn screening for inborn errors of immunity. Int J Neonatal Screen. 2021;7:74.
Acknowledgements
We thank the Julius General Practitioner Network and Novivex for their collaboration in this project concerning the collection of EHR metadata. We thank Alfred Sachs for his clinical input.
Funding
This study was funded by Takeda Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
HL, PW, RB and MM developed the concept. MM analyzed the data and drafted the manuscript, which was critically revised by all authors. All authors have agreed to be accountable for their contributions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was received from the Medical Research Ethics Committee Utrecht, protocol number 19-748. Patients with primary antibody deficiency of the University Medical Center Utrecht provided written informed consent. The need for informed consent for the group-level primary care data of the Julius General Practitioner’s Network was waived, as this only concerns meta-level data (e.g. means and medians).
Consent for publication
Not applicable.
Competing interests
HL and JM received consulting fees for AdBoards with Takeda Pharmaceuticals. RB received personal fees from Takeda Pharmaceuticals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Youden’s index per ICPC code.
Additional file 2: Table S2.
Mean number of antibiotic prescriptions per ATCcode in the 4 years before the extraction date.
Additional file 3: Figure S1.
Data from the primary care electronic health record (EHR) on the number of diagnostic requests for leukocytes, C-reactive protein (CRP) and lung function tests. The censoring date is the date before which the EHR was screened. For most patients this is the date of data-extraction, November 2021. For immunodeficiency patients pre-diagnosis, the censoring date is the diagnosis date. For details on the censoring date, see “Methods” section.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Messelink, M.A., Berbers, R.M., van Montfrans, J.M. et al. Development of a primary care screening algorithm for the early detection of patients at risk of primary antibody deficiency. Allergy Asthma Clin Immunol 19, 44 (2023). https://doi.org/10.1186/s13223-023-00790-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13223-023-00790-7